In Vitro Effect of Pitavastatin and Its Synergistic Activity with Isavuconazole against Acanthamoeba castellanii
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Activity of HMGR Inhibitors against Clinical Strains of A. castellanii
2.2. Effect of Combination of Pitavastatin and Isavuconazole
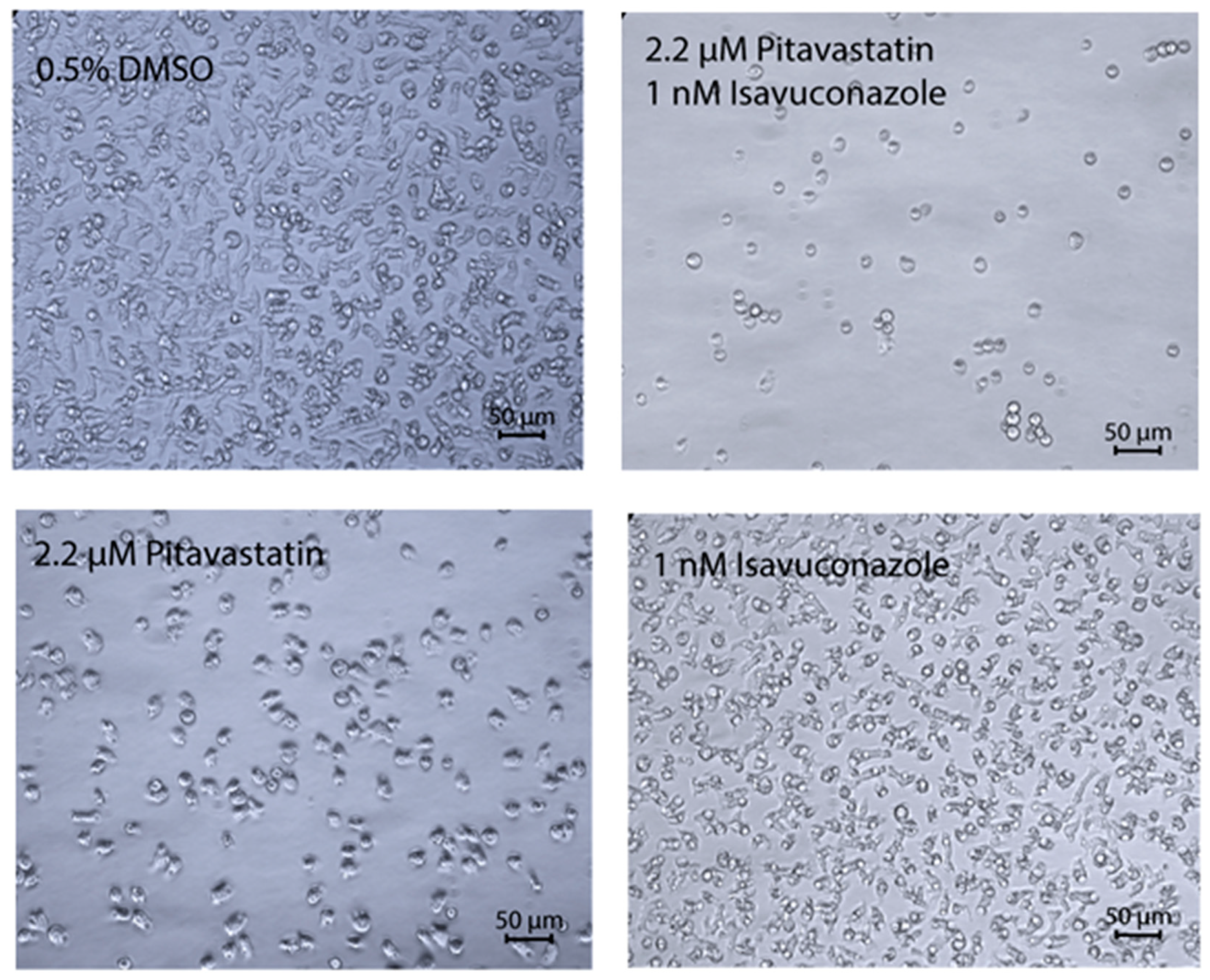
2.3. Microscopy Study to Determine the Effect of Combination of Pitavastatin and Isavuconazole
3. Materials and Methods
3.1. In Vitro Activity of HMGR Inhibitors against Clinical Strains of A. castellanii
3.2. Effect of Combination of Pitavastatin and Isavuconazole
3.3. Microscopy Study to Determine the Effect of Combination of Pitavastatin and Isavuconazole
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parmar, D.N.; Awwad, S.T.; Petroll, W.M.; Bowman, R.W.; McCulley, J.P.; Cavanagh, H.D. Tandem scanning confocal corneal microscopy in the diagnosis of suspected acanthamoeba keratitis. Ophthalmology 2006, 113, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Donzis, P.B.; Mondino, B.J.; Weissman, B.A.; Bruckner, D.A. Microbial contamination of contact lens care systems. Am. J. Ophthalmol. 1987, 104, 325–333. [Google Scholar] [CrossRef]
- Illingworth, C.D.; Cook, S.D.; Karabatsas, C.H.; Easty, D.L. Acanthamoeba keratitis: Risk factors and outcome. Br. J. Ophthalmol. 1995, 79, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, D.A.; Snow, K.K.; Dana, M.R. The epidemic of Acanthamoeba keratitis: Where do we stand? Cornea 1998, 17, 3–10. [Google Scholar] [CrossRef]
- Scruggs, B.A.; Quist, T.S.; Salinas, J.L.; Greiner, M.A. Notes from the Field: Acanthamoeba Keratitis Cases-Iowa, 2002–2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 448–449. [Google Scholar] [CrossRef]
- Sharma, S.; Garg, P.; Rao, G.N. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br. J. Ophthalmol. 2000, 84, 1103–1108. [Google Scholar] [CrossRef]
- Awwad, S.T.; Petroll, W.M.; McCulley, J.P.; Cavanagh, H.D. Updates in Acanthamoeba keratitis. Eye Contact Lens 2007, 33, 1–8. [Google Scholar] [CrossRef]
- Lee, G.A.; Gray, T.B.; Dart, J.K.; Pavesio, C.E.; Ficker, L.A.; Larkin, D.F.; Matheson, M.M. Acanthamoeba sclerokeratitis: Treatment with systemic immunosuppression. Ophthalmology 2002, 109, 1178–1182. [Google Scholar] [CrossRef]
- Gupta, D.; Panda, G.S.; Bakhshi, S. Successful treatment of acanthamoeba meningoencephalitis during induction therapy of childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2008, 50, 1292–1293. [Google Scholar] [CrossRef]
- Singhal, T.; Bajpai, A.; Kalra, V.; Kabra, S.K.; Samantaray, J.C.; Satpathy, G.; Gupta, A.K. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr. Infect. Dis. J. 2001, 20, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S. Amebic meningoencephalitides and keratitis: Challenges in diagnosis and treatment. Curr. Opin. Infect. Dis. 2010, 23, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Visvesvara, G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004, 34, 1001–1027. [Google Scholar] [CrossRef] [PubMed]
- Ledee, D.R.; Iovieno, A.; Miller, D.; Mandal, N.; Diaz, M.; Fell, J.; Fini, M.E.; Alfonso, E.C. Molecular identification of t4 and t5 genotypes in isolates from acanthamoeba keratitis patients. J. Clin. Microbiol. 2009, 47, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Debnath, A.; Calvet, C.M.; Jennings, G.; Zhou, W.; Aksenov, A.; Luth, M.R.; Abagyan, R.; Nes, W.D.; McKerrow, J.H.; Podust, L.M. CYP51 is an essential drug target for the treatment of primary amebic meningoencephalitis (PAM). PLoS Negl. Trop. Dis. 2017, 11, e0006104. [Google Scholar] [CrossRef]
- Thomson, S.; Rice, C.A.; Zhang, T.; Edrada-Ebel, R.; Henriquez, F.L.; Roberts, C.W. Characterisation of sterol biosynthesis and validation of 14alpha-demethylase as a drug target in Acanthamoeba. Sci. Rep. 2017, 7, 8247. [Google Scholar] [CrossRef]
- Zhou, W.; Debnath, A.; Jennings, G.; Hahn, H.J.; Vanderloop, B.H.; Chaudhuri, M.; Nes, W.D.; Podust, L.M. Enzymatic chokepoints and synergistic drug targets in the sterol biosynthesis pathway of Naegleria fowleri. PLoS Pathog. 2018, 14, e1007245. [Google Scholar] [CrossRef]
- Zhou, W.; Warrilow, A.G.S.; Thomas, C.D.; Ramos, E.; Parker, J.E.; Price, C.L.; Vanderloop, B.H.; Fisher, P.M.; Loftis, M.D.; Kelly, D.E.; et al. Functional importance for developmental regulation of sterol biosynthesis in Acanthamoeba castellanii. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1164–1178. [Google Scholar] [CrossRef]
- Lamb, D.C.; Warrilow, A.G.; Rolley, N.J.; Parker, J.E.; Nes, W.D.; Smith, S.N.; Kelly, D.E.; Kelly, S.L. Azole Antifungal Agents to Treat the Human Pathogens Acanthamoeba castellanii and Acanthamoeba polyphaga through Inhibition of Sterol 14alpha-Demethylase (CYP51). Antimicrob. Agents Chemother. 2015, 59, 4707–4713. [Google Scholar] [CrossRef]
- Shing, B.; Singh, S.; Podust, L.M.; McKerrow, J.H.; Debnath, A. The Antifungal Drug Isavuconazole Is both Amebicidal and Cysticidal against Acanthamoeba castellanii. Antimicrob. Agents Chemother. 2020, 64, e02223-19. [Google Scholar] [CrossRef]
- Edwards, P.A.; Ericsson, J. Sterols and isoprenoids: Signaling molecules derived from the cholesterol biosynthetic pathway. Annu. Rev. Biochem. 1999, 68, 157–185. [Google Scholar] [CrossRef] [PubMed]
- Gazzerro, P.; Proto, M.C.; Gangemi, G.; Malfitano, A.M.; Ciaglia, E.; Pisanti, S.; Santoro, A.; Laezza, C.; Bifulco, M. Pharmacological actions of statins: A critical appraisal in the management of cancer. Pharmacol. Rev. 2012, 64, 102–146. [Google Scholar] [CrossRef] [PubMed]
- Martin-Navarro, C.M.; Lopez-Arencibia, A.; Sifaoui, I.; Reyes-Batlle, M.; Valladares, B.; Martinez-Carretero, E.; Pinero, J.E.; Maciver, S.K.; Lorenzo-Morales, J. Statins and voriconazole induce programmed cell death in Acanthamoeba castellanii. Antimicrob. Agents Chemother. 2015, 59, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Martin-Navarro, C.M.; Lorenzo-Morales, J.; Machin, R.P.; Lopez-Arencibia, A.; Garcia-Castellano, J.M.; de Fuentes, I.; Loftus, B.; Maciver, S.K.; Valladares, B.; Pinero, J.E. Inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and application of statins as a novel effective therapeutic approach against Acanthamoeba infections. Antimicrob. Agents Chemother. 2013, 57, 375–381. [Google Scholar] [CrossRef]
- Chen, L.W.; Lin, C.S.; Tsai, M.C.; Shih, S.F.; Lim, Z.W.; Chen, S.J.; Tsui, P.F.; Ho, L.J.; Lai, J.H.; Liou, J.T. Pitavastatin Exerts Potent Anti-Inflammatory and Immunomodulatory Effects via the Suppression of AP-1 Signal Transduction in Human T Cells. Int. J. Mol. Sci. 2019, 20, 3534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Scialis, R.J.; Feng, B.; Leach, K. Detection of statin cytotoxicity is increased in cells expressing the OATP1B1 transporter. Toxicol. Sci. 2013, 134, 73–82. [Google Scholar] [CrossRef]
- Yu, S.; Chu, Y.; Li, G.; Ren, L.; Zhang, Q.; Wu, L. Statin Use and the Risk of Cataracts: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e004180. [Google Scholar] [CrossRef] [PubMed]
- de Macedo-Silva, S.T.; Visbal, G.; Urbina, J.A.; de Souza, W.; Rodrigues, J.C. Potent In Vitro Antiproliferative Synergism of Combinations of Ergosterol Biosynthesis Inhibitors against Leishmania amazonensis. Antimicrob. Agents Chemother. 2015, 59, 6402–6418. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Jenks, J.D.; Salzer, H.J.; Prattes, J.; Krause, R.; Buchheidt, D.; Hoenigl, M. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: Design, development, and place in therapy. Drug Des. Devel. Ther. 2018, 12, 1033–1044. [Google Scholar] [CrossRef]
- Rice, C.A.; Troth, E.V.; Russell, A.C.; Kyle, D.E. Discovery of Anti-Amoebic Inhibitors from Screening the MMV Pandemic Response Box on Balamuthia mandrillaris, Naegleria fowleri, and Acanthamoeba castellanii. Pathogens 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Tomlinson, B. Evaluation of the pharmacokinetics and drug interactions of the two recently developed statins, rosuvastatin and pitavastatin. Expert Opin. Drug Metab. Toxicol. 2014, 10, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y. Critical appraisal of the role of pitavastatin in treating dyslipidemias and achieving lipid goals. Vasc. Health Risk Manag. 2009, 5, 921–936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ansari, Z.; Miller, D.; Galor, A. Current Thoughts in Fungal Keratitis: Diagnosis and Treatment. Curr Fungal Infect. Rep. 2013, 7, 209–218. [Google Scholar] [CrossRef]
- Hariprasad, S.M.; Mieler, W.F.; Lin, T.K.; Sponsel, W.E.; Graybill, J.R. Voriconazole in the treatment of fungal eye infections: A review of current literature. Br. J. Ophthalmol. 2008, 92, 871–878. [Google Scholar] [CrossRef]
- Miceli, M.H.; Kauffman, C.A. Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent. Clin. Infect. Dis. 2015, 61, 1558–1565. [Google Scholar] [CrossRef]
- Fraunfelder, F.W.; Richards, A.B. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology 2008, 115, 2282–2285. [Google Scholar] [CrossRef]
- Terai, N.; Spoerl, E.; Fischer, S.; Hornykewycz, K.; Haustein, M.; Haentzschel, J.; Pillunat, L.E. Statins affect ocular microcirculation in patients with hypercholesterolaemia. Acta Ophthalmol. 2011, 89, e500–e504. [Google Scholar] [CrossRef]
- Parihar, S.P.; Hartley, M.A.; Hurdayal, R.; Guler, R.; Brombacher, F. Topical Simvastatin as Host-Directed Therapy against Severity of Cutaneous Leishmaniasis in Mice. Sci. Rep. 2016, 6, 33458. [Google Scholar] [CrossRef]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.; Hamed, M.I.; Sobreira, T.J.; Hedrick, V.E.; Paul, L.N.; Seleem, M.N. Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci. Rep. 2015, 5, 16407. [Google Scholar] [CrossRef]
- Gasper, S.R.; West, R.; Martinez, T.; Robbins, K.G.; McKernan, P.A.; Baindur, N.; Labroo, V.M.; Mundy, G.R. Topical Administration of Statins for Treatment of Bone Disorders. U.S. Patent 7,101,907, 5 September 2006. [Google Scholar]
- Diaz-Tome, V.; Luaces-Rodriguez, A.; Silva-Rodriguez, J.; Blanco-Dorado, S.; Garcia-Quintanilla, L.; Llovo-Taboada, J.; Blanco-Mendez, J.; Garcia-Otero, X.; Varela-Fernandez, R.; Herranz, M.; et al. Ophthalmic Econazole Hydrogels for the Treatment of Fungal Keratitis. J. Pharm. Sci. 2018, 107, 1342–1351. [Google Scholar] [CrossRef]
- Bae, S.H.; Park, J.H.; Choi, H.G.; Kim, H.; Kim, S.H. Imidazole Antifungal Drugs Inhibit the Cell Proliferation and Invasion of Human Breast Cancer Cells. Biomol. Ther. 2018, 26, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.G.; Igbavboa, U.; Muller, W.E.; Eckert, G.P. Statins, Bcl-2, and apoptosis: Cell death or cell protection? Mol. Neurobiol. 2013, 48, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Otri, A.M.; Mohammed, I.; Abedin, A.; Cao, Z.; Hopkinson, A.; Panjwani, N.; Dua, H.S. Antimicrobial peptides expression by ocular surface cells in response to Acanthamoeba castellanii: An in vitro study. Br. J. Ophthalmol. 2010, 94, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Debnath, A.; Nelson, A.T.; Silva-Olivares, A.; Shibayama, M.; Siegel, D.; McKerrow, J.H. In Vitro Efficacy of Ebselen and BAY 11-7082 Against Naegleria fowleri. Front. Microbiol. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
| HMGR Inhibitors | Strain | EC50 (µM) Mean ± SE |
|---|---|---|
| Fluvastatin | Ma | 11 ± 0.04 |
| Atorvastatin | Ma | 9.6 ± 0.04 |
| Simvastatin | Ma | 52.8 ± 0.2 |
| Rosuvastatin | Ma | 12.5 ± 2.3 |
| Pravastatin | Ma | Not active |
| Pitavastatin | Ma | 1.9 ± 0.03 |
| CDC:V240 | 1 ± 0.03 | |
| MEEI 0184 | 0.5 ± 0.01 | |
| Standards of Care | ||
| Chlorhexidine [20] | Ma | 2 ± 0.07 |
| CDC:V240 | 1.1 ± 0.1 | |
| MEEI 0184 | 1 ± 0.05 | |
| PHMB [20] | Ma | 7.2 ± 0.06 |
| CDC:V240 | 11.8 ± 0.02 | |
| MEEI 0184 | 4.6 ± 0.03 |
| Pitavastatin: Isavuconazole Ratio | % Growth Inhibition | Combination Index (CI) | Dose Reduction Index (DRI) | Dose Required to Achieve 97% Inhibition (µM) | ||
|---|---|---|---|---|---|---|
| Pitavastatin | Isavuconazole | Pitavastatin | Isavuconazole | |||
| 4000:1 | 97 | 0.2 ± 0.1 | 4.3 ± 0.2 | 4077.8 ± 2.1 | 2.2 ± 0.1 | 0.0005 ± 0.0001 |
| 2000:1 | 97 | 0.5 ± 0.1 | 2.6 ± 0.6 | 1630.6 ± 114 | 4.2 ± 0.4 | 0.002 ± 0.0001 |
| 32:1 | 97 | 0.7 ± 0.1 | 2.0 ± 0.5 | 20.0 ± 12.0 | 5.4 ± 0.6 | 0.2 ± 0.04 |
| 16:1 | 97 | 0.5 ± 0.1 | 2.8 ± 0.2 | 12.8 ± 3.8 | 3.3 ± 0.4 | 0.2 ± 0.01 |
| 8:1 | 97 | 0.4 ± 0.1 | 4.8 ± 0.5 | 11.5 ± 4.5 | 2.0 ± 0.4 | 0.3 ± 0.06 |
| 4:1 | 97 | 0.3 ± 0.1 | 9.2 ± 1.3 | 11.1 ± 5.6 | 1.1 ± 0.1 | 0.3 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, H.J.; Escrig, J.I.; Shing, B.; Debnath, A. In Vitro Effect of Pitavastatin and Its Synergistic Activity with Isavuconazole against Acanthamoeba castellanii. Pathogens 2020, 9, 681. https://doi.org/10.3390/pathogens9090681
Hahn HJ, Escrig JI, Shing B, Debnath A. In Vitro Effect of Pitavastatin and Its Synergistic Activity with Isavuconazole against Acanthamoeba castellanii. Pathogens. 2020; 9(9):681. https://doi.org/10.3390/pathogens9090681
Chicago/Turabian StyleHahn, Hye Jee, Jose Ignacio Escrig, Brian Shing, and Anjan Debnath. 2020. "In Vitro Effect of Pitavastatin and Its Synergistic Activity with Isavuconazole against Acanthamoeba castellanii" Pathogens 9, no. 9: 681. https://doi.org/10.3390/pathogens9090681
APA StyleHahn, H. J., Escrig, J. I., Shing, B., & Debnath, A. (2020). In Vitro Effect of Pitavastatin and Its Synergistic Activity with Isavuconazole against Acanthamoeba castellanii. Pathogens, 9(9), 681. https://doi.org/10.3390/pathogens9090681






