Ticks and Tick-Borne Diseases in Cuba, Half a Century of Scientific Research
Abstract
1. Introduction
2. Tick Species in Cuba
3. Ecophysiology of Ticks in Cuba
4. TBDs in Humans
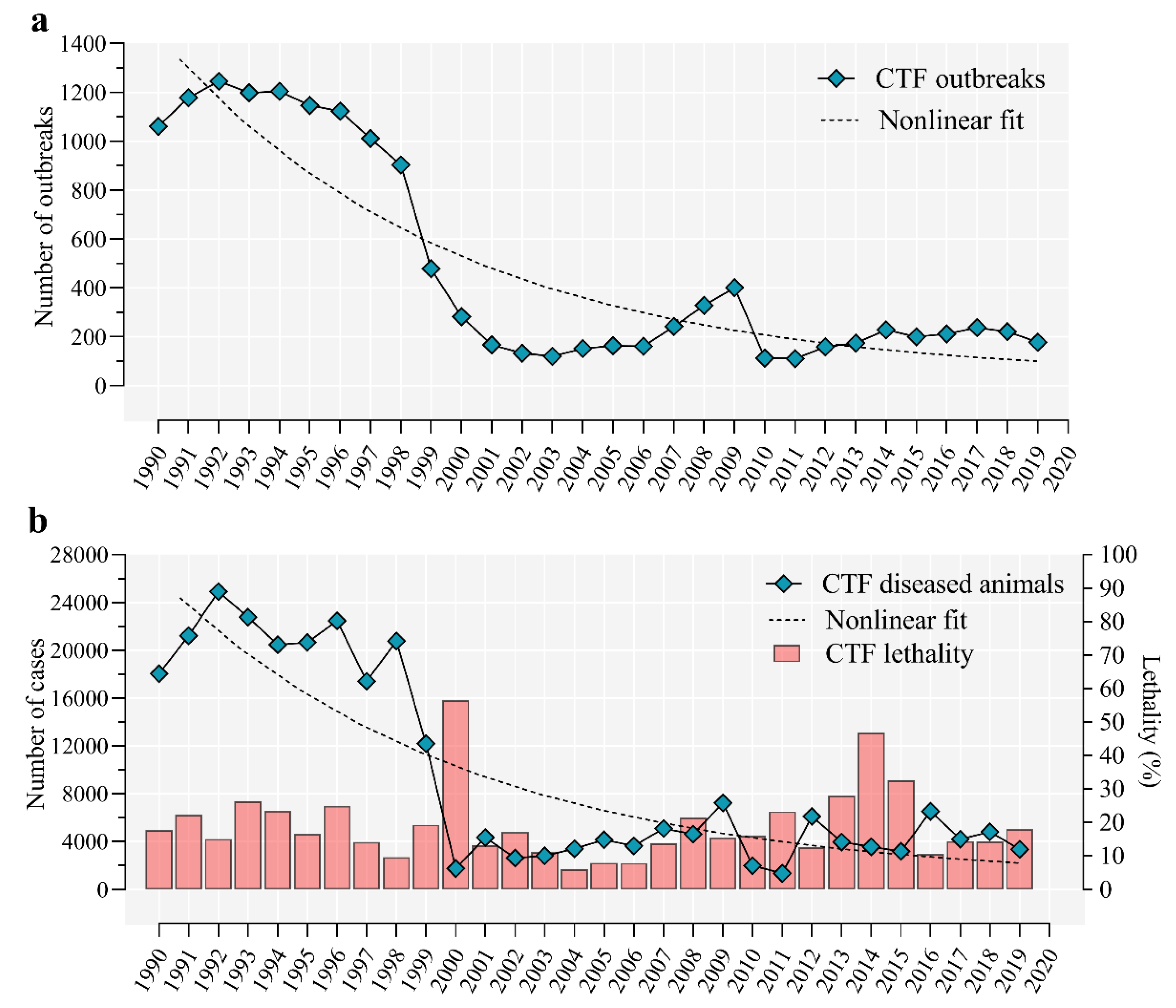
5. Epidemiology of Cattle Tick Fever in Cuba
6. Ticks and TBPs Infection in Water Buffalo
7. Ticks and TBPs Infection in Horses
8. Ticks and TBPs Infection in Dogs
9. TBPs Infection in Sheep and Goats
10. Development of Diagnostic Tools for TBPs in Cuba
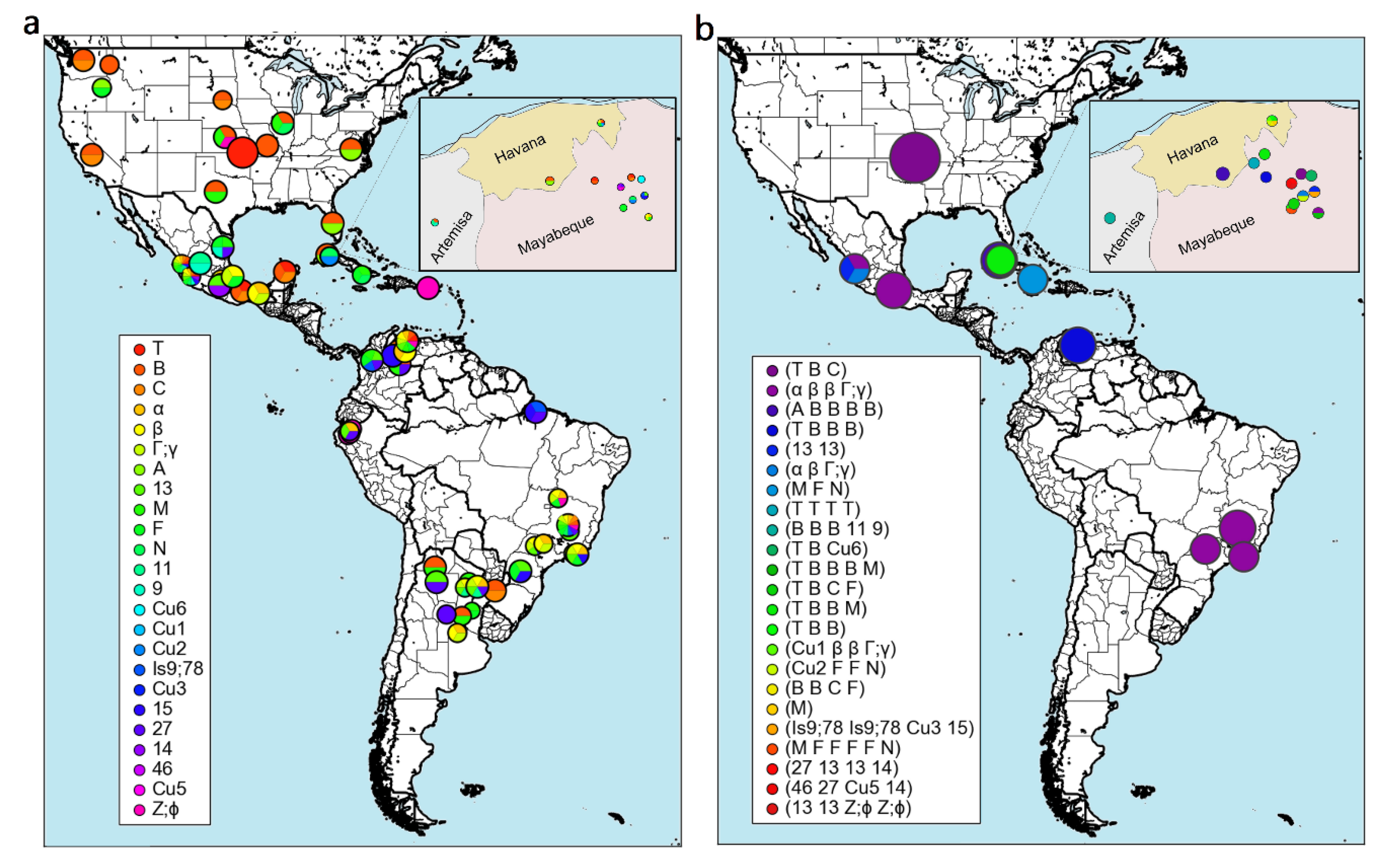
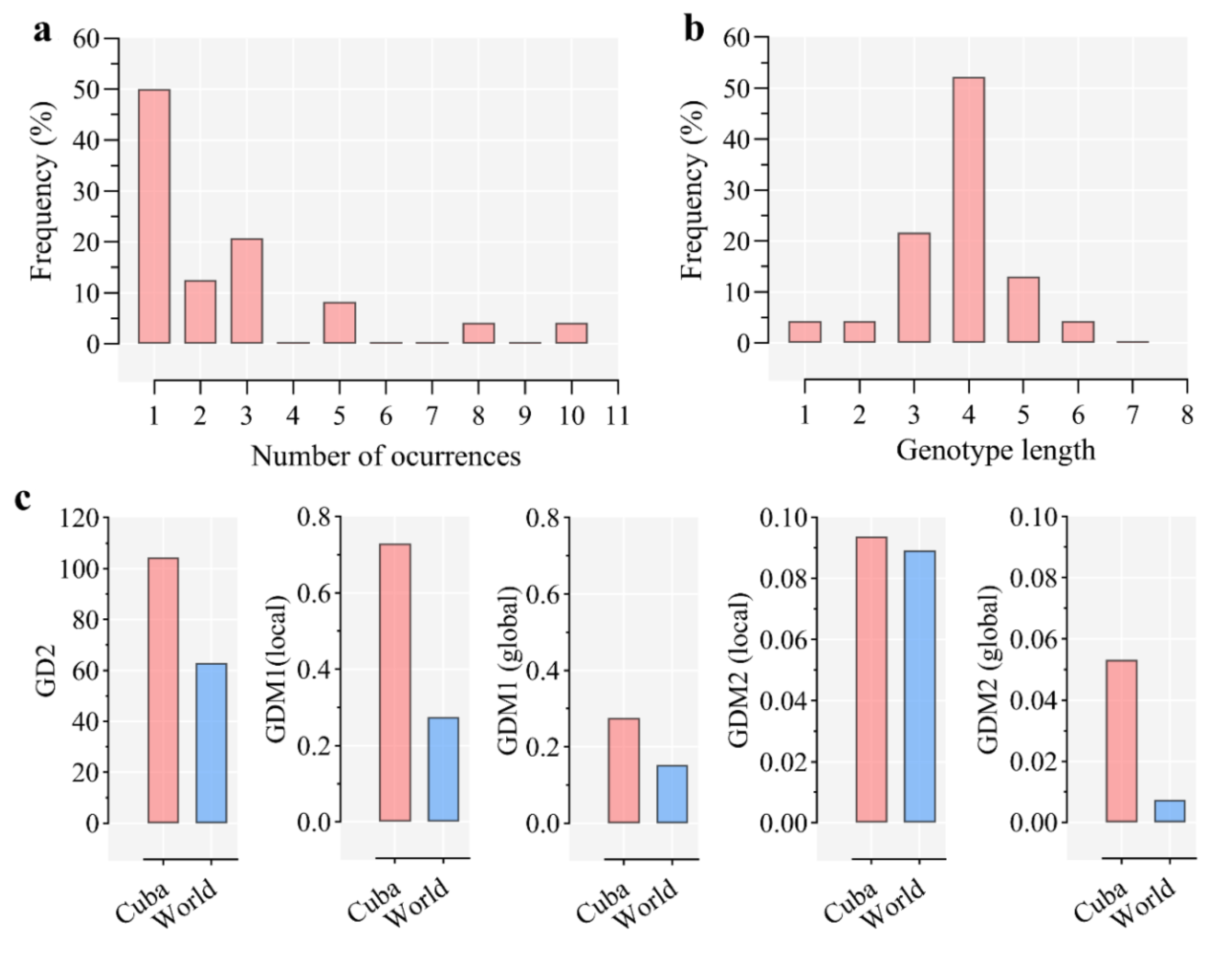
11. Genetic Variability of A. marginale and Other TBPs in Cuba
12. Applications of Biotechnology in the Control of ticks in Cuba
13. Programs for the Control of Ticks and TBDs in Cattle in Cuba
Author Contributions
Funding
Conflicts of Interest
References
- FAO; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2019; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- FAO; UNICEF; WFP; WHO. The Future of Food and Agriculture–Alternative Pathways to 2050; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- de la Fuente, J.; Contreras, M.; Kasaija, P.D.; Gortazar, C.; Ruiz-Fons, J.F.; Mateo, R.; Kabi, F. Towards a multidisciplinary approach to improve cattle health and production in Uganda. Vaccines 2019, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.L.; Jizhou, L.; Phipps, L.P.; Johnson, N. Emerging tick-borne viruses in the twenty-first century. Front. Cell. Infect. Microbiol. 2017, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Panahi, H.K.S.; Holmes, E.C.; Hudson, B.J.; Schloeffel, R.; Guillemin, G.J. Human tick-borne diseases in Australia. Front. Cell. Infect. Microbiol. 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Sanchez-Vicente, S.; Tagliafierro, T.; Coleman, J.L.; Benach, J.L.; Tokarz, R. Polymicrobial nature of tick-borne diseases. MBio 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Valdés, J.J. Are ticks venomous animals? Front. Zool. 2014, 11, 47. [Google Scholar] [CrossRef]
- de la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-cruz, A.; Domingos, A.G.; Estrada-peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-pathogen interactions and vector competence: Identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Fernández-Ruiz, N. A Retrospective assessment of temperature trends in Northern Europe reveals a deep impact on the life cycle of Ixodes ricinus (Acari: Ixodidae). Pathogens 2020, 9, 345. [Google Scholar] [CrossRef]
- Fernández-Ruiz, N.; Estrada-Peña, A. Could climate trends disrupt the contact rates between Ixodes ricinus (Acari, Ixodidae) and the reservoirs of Borrelia burgdorferi s.l.? PLoS ONE 2020, 15, e0233771. [Google Scholar] [CrossRef]
- Sagurova, I.; Ludwig, A.; Ogden, N.H.; Pelcat, Y.; Dueymes, G.; Gachon, P. Predicted northward expansion of the geographic range of the tick vector Amblyomma americanum in North America under future climate conditions. Environ. Health Perspect. 2019, 127, 107014. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by ixodes tick-borne pathogens: Ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Sánchez, A.A.; Meli, M.L.; Álvarez, D.O.; Fonseca-Rodríguez, O.; Cabezas-Cruz, A.; Hofmann-Lehmann, R.; Corona-González, B. Development and application of a multiplex TaqMan® real-time qPCR assay for the simultaneous detection of Anaplasma marginale and Theileria annulata and molecular characterization of Anaplasma marginale from cattle in Western Cuba. Ticks Tick-Borne Dis. 2019, 11, 101356. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.L. Global food security: The impact of veterinary parasites and parasitologists. Vet. Parasitol. 2013, 195, 233–248. [Google Scholar] [CrossRef]
- Barros-Battesti, D.; Reyes, M.H.; Onofrio, V.C.; Beati, L.; Famada, K.; Guglielmone, A.A. The ixodid ticks (Acari: Ixodidae) of Cuba. Syst. Appl. Acarol. 2009, 12, 101. [Google Scholar] [CrossRef]
- Gaínza, Y.A.; Martínez-Marrero, S.; Corona-González, B. Ticks of veterinary interest in Cuba, and its importance in the changing climatic conditions. Rev. Electrónica Vet. 2014, 15, 1–22. [Google Scholar]
- ONEI. Agricultura, Ganadería, Silvicultura y Pesca. In Anuario Estadístico de Cuba 2018; Oficina Nacional de Estadística e Información: Havana, Cuba, 2019. [Google Scholar]
- Alonso, M.; Arellano-Sota, C.; Cereser, V.H.; Cordoves, C.O.; Guglielmone, A.A.; Kessler, R.; Mangold, A.J.; Nari, A.; Patarroyo, J.H.; Solari, M.A.; et al. Epidemiology of bovine anaplasmosis and babesiosis in Latin America and the Caribbean. Int. J. Epidemiol. 1992, 11, 713–733. [Google Scholar]
- Camus, E.; Barre, N. Vector situation of tick-borne diseases in the Caribbean islands. Vet. Parasitol. 1995, 57, 167–176. [Google Scholar] [CrossRef]
- Obregón, D.; Cabezas-cruz, A.; Armas, Y.; Silva, J.B.; Fonseca, A.H.; André, M.R.; Alfonso, P.; Oliveira, M.C.S.; Machado, R.Z.; Corona-González, B. High co-infection rates of Babesia bovis, Babesia bigemina, and Anaplasma marginale in water buffalo in Western Cuba. Parasitol. Res. 2019, 118, 955–967. [Google Scholar] [CrossRef]
- de La Cruz, J.; Černý, V. Dinámica anual del desarrollo de las larvas de garrapatas común del ganado bovino en Cuba, Boophilus microplus (Canesrini, 1887). Poeyana 1971, 91, 1–6. [Google Scholar]
- de la Vega, R.; Camejo, A.; Dias, G. Dynamic of Boophilus microplus (Acari: Ixodidae) female feeding on bovinse. Rev. Salud Anim. 2003, 25, 192–195. [Google Scholar]
- Díaz-Sánchez, A.A.; Pires, M.S.; Estrada, C.Y.; Cañizares, E.V.; del Castillo Domínguez, S.L.; Cabezas-Cruz, A.; Rivero, E.L.; da Fonseca, A.H.; Massard, C.L.; Corona-González, B. First molecular evidence of Babesia caballi and Theileria equi infections in horses in Cuba. Parasitol. Res. 2018, 117, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.G.; Cordeiro, M.D.; Silva, C.B.; Massard, C.L.; López, E.R.; Rodríguez, J.C.A.; Ribeiro, C.C.D.U.; Rodríguez, O.F.; Fonseca, A.H. Serological and molecular diagnosis of Ehrlichia canis and associated risk factors in dogs domiciled in western Cuba. Vet. Parasitol. Reg. Stud. Reports 2018, 14, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Burri, C.; Noda, A.A.; Douet, V.; Gern, L. Multiplex PCR for molecular screening of Borrelia burgdorferi sensu lato, Anaplasma spp. and Babesia spp. Ann. Agric. Environ. Med. 2015, 22, 642–646. [Google Scholar] [CrossRef] [PubMed]
- González, I.R.; Alberto, I.Á.; Ramos, N.; Echevarria, I.E.; Elena, I.M.; Barrera, R.; Iglesias, I.T.M. About Lyme disease in Cuba and its knowledge by medical personnel. Rev. Cuba. Salud Pública 2018, 44, 3–13. [Google Scholar]
- Rodríguez, I.; Fernández, C.; Sánchez, L.; Martínez, B.; Siegrist, H.H.; Lienhard, R. Serological evidences suggest Borrelia burgdorferi sensu lato infection in Cuba. Brazilian J. Infect. Dis. 2012, 16, 405–406. [Google Scholar] [CrossRef]
- Nava, S.; Venzal, J.M.; González-Acuña, D.; Martins, T.F.; Guglielmone, A.A. Genera and species of Ixodidae. In Ticks of the Southern Cone of America; Elsevier Academic Press: London, UK, 2016; pp. 25–267. [Google Scholar]
- Horak, I.G.; Camicas, J.L.; Keirans, J.E. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida): A world list of valid tick names. Exp. Appl. Acarol. 2002, 28, 27–54. [Google Scholar] [CrossRef]
- Peñalver, E.; Arillo, A.; Delclòs, X.; Peris, D.; Grimaldi, D.A.; Anderson, S.R.; Nascimbene, P.C.; Pérez-De La Fuente, R. Parasitised feathered dinosaurs as revealed by Cretaceous amber assemblages. Nat. Commun. 2017, 8, 1924. [Google Scholar] [CrossRef]
- de la Vega, R.; Díaz, G.; Fonseca, A. A multivariate analysis of Boophilus microplus (acari: Ixodidae): Non-parasitic phase. Rev. Salud Anim. 2010, 32, 89–96. [Google Scholar]
- León, L.F.C. Dr. Ildefonso Pérez Vigueras: Un Cazador de Parasitos; Consejo Nacional de Sociedades Científicas, Ministerio de Salud Pública: Havana, Cuba, 1981; Volume 62.
- Pérez-Vigueras, I. Los Ixodidos y Culicidos de Cuba. Su Historia Natural y Medica, 1st ed.; Imprenta Universidad de la Habana: La Habana, Cuba, 1956. [Google Scholar]
- Pérez-Vigueras, I. Lista de los Ixódidos de Cuba. Circ. Mus. Bibl. Zool. La Habana 1954, 9, 1389–1390. [Google Scholar]
- Pérez-Vigueras, I. On the ticks of Cuba, with description of a new species, Amblyomma torrei, from Cyclura macleayi. Psyche (Stuttg) 1934, 41, 13–18. [Google Scholar] [CrossRef]
- Černý, V. Two new species of argasid ticks (Ixodidea, Argasidae) from Cuba. Folia Parasitol. 1967, 14, 148–149. [Google Scholar]
- Černý, V. The tick fauna of Cuba. Folia Parasitol. 1969, 16, 279–284. [Google Scholar]
- Woods, C.A.; Kilpatrick, C. Infraorder Hystricognathi. In Mammal Species of the World; Wilson, D.E., Reeder, D., Eds.; The Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 1538–1600. [Google Scholar]
- de la Cruz, J.; Estrada-Peña, A. Four new species of Antricola ticks (Argasidae: Antricolinae) from bat guano in Cuba and Curaçao. Acarologia 1995, 36, 277–286. [Google Scholar]
- de La Cruz, J. Composición Zoogeográfica de la fauna de garrapata (Acarina: Ixodidae) en Cuba. Poeyana 1978, 185, 1–5. [Google Scholar]
- de la Cruz, J. Notas adicionales a la fauna de las garrapatas (Ixodoidea) de Cuba. VI. Cuatro nuevas espécies del género Antricola Cooley e Kohls, 1942 (Argasidae: Ornithodorinae). Poeyana 1978, 184, 1–17. [Google Scholar]
- de la Cruz, J. Notas adicionales a la fauna de las garrapatas (Ixodoidea) de Cuba. V. Una nueva especie del género Antricola Cooley e Kohls, 1942 (Argasidae). Poeyana 1976, 151, 1–8. [Google Scholar]
- de la Cruz, J. Notas adicionales a la fauna de las garrapatas (Ixodoidea) de Cuba. IV. Presencia de Argas (Persicargas) persicus (Oken, 1818). Misc. Zologica 1976, 2, 3. [Google Scholar]
- de la Cruz, J. Notas adicionales a la fauna de las garrapatas (Ixodoidea) de Cuba. II. Nuevo status para Parantricola Cerny. Poeyana 1974, 138, 1–5. [Google Scholar]
- de la Cruz, J. Notas adicionales a la fauna de las garrapatas (Ixodoidea) de Cuba. I. Argasidae de las aves. Poeyana 1974, 129, 1–3. [Google Scholar]
- de la Cruz, J. Notas sobre las garrapatas del género Antricola (Cooley e Kohls, 1942) (Ixodiformes, Argasidae) con la descripción de una nueva especie. Acad. Cienc. Cuba Ser. Espeleol. y Carsólica 1973, 44, 1–13. [Google Scholar]
- Černý, V. Nueva espécie de garrapata Del género Ixodes Latreille (Ixodoidea, Ixodidae) em la jutía conga de la Isla de Pinos. Poeyana 1966, 24, 1–9. [Google Scholar]
- Beati, L.; Nava, S.; Burkman, E.J.; Barros-battesti, D.M.; Labruna, M.B.; Guglielmone, A.A.; Cáceres, A.G.; Guzmán-cornejo, C.M.; León, R. (Acari: Ixodidae), the Cayenne tick: Phylogeography and evidence for allopatric speciation. BMC Evol. Biol. 2013, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Beati, L.; Labruna, M.B.; Cáceres, A.G.; Mangold, A.J.; Guglielmone, A. A Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum, and Amblyomma sculptum (Ixodida: Ixodidae). Ticks Tick-Borne Dis. 2014, 5, 252–276. [Google Scholar] [PubMed]
- Noda, A.A.; Rodríguez, I.; Miranda, J.; Contreras, V.; Mattar, S. First molecular evidence of Coxiella burnetii infecting ticks in Cuba. Ticks Tick-Borne Dis. 2016, 7, 68–70. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, R. Estudio de la biología de Boophilus Microplus; Universidad de La Habana. Imp. Universitaria: La Habana, Cuba, 1975. [Google Scholar]
- Comité Editorial CENSA. OBITUARIO Rafael de la Vega Ruibal. Rev. Salud Anim. 2012, 34, 68. [Google Scholar]
- de la Vega, R.; Farradá, F.; Díaz, G. Aplicación de las constantes térmicas en el control de la garrapata del ganado vacuno (Boophilus microplus). VI. Cuarentena. Rev. Salud Anim. 1988, 10, 71–75. [Google Scholar]
- de la Vega, R.; Días, G. Aplicación de las constantes térmicas en el control de la garrapata del ganado vacuno (Boophilus microplus). V. Supervivencia larvaria en el laboratorio. Rev. Salud Anim. 1987, 9, 259–265. [Google Scholar]
- de la Vega, R.; Días, G. Aplicación de las constantes térmicas en el control de la garrapata del ganado vacuno (Boophilus microplus). VIII. Validación del programa de cuarentena. Rev. Salud Anim. 1992, 14, 133–136. [Google Scholar]
- de la Vega, R.; Días, G. Aplicación de las constantes térmicas en el control de la garrapata del ganado vacuno (Boophilus microplus). IV. Pronóstico del inicio de la eclosión en condiciones de intemperie. Rev. Salud Anim. 1986, 8, 337–345. [Google Scholar]
- de la Vega, R.; Días, G. Aplicación de las constantes térmicas en el control de la garrapata del ganado vacuno (Boophilus microplus). II. Estimación del inicio de la eclosión en condiciones naturales simuladas. Rev. Salud Anim. 1985, 7, 307–316. [Google Scholar]
- de la Vega, R.; Días, G. Aplicación de las constantes térmicas en el control de la garrapata del ganado vacuno (Boophilus microplus) I: Cálculo de las constantes térmicas. Rev. Salud Anim. 1985, 7, 141–148. [Google Scholar]
- de la Vega, R.; Días, G. Aplicación de las constantes térmicas en el control de la garrapata del ganado vacuno (Boophilus microplus). III. Simplificaciones del método de estimación de los períodos de la fase no parasitaria. Rev. Salud Anim. 1985, 7, 441–446. [Google Scholar]
- LNP (National Parasitology Laboratory). Serie Histórica de la Anaplasmosis y Babesiosis en Cuba; Sistema de Información Estadística del IMV. Annual Balance Report: 2014; Ministry of Agriculture of the Republic of Cuba: Habana, Cuba, 2014. [Google Scholar]
- INSMET. Instituto de Meteorología de la República de CUBA. Centro del Clima. El clima de Cuba. Características Generales. Available online: http://www.met.inf.cu/asp/genesis.asp?TB0=PLANTILLAS&TB1=CLIMAC&TB2=/clima/ClimaCuba.htm (accessed on 1 January 2016).
- Días, G.; de la Vega, R. Influencia de la temperatura y de la humedad relativa en la fase no parasitaria de Anocentor nitens (Ixodoidea: Ixodidae). Rev. Salud Anim. 1991, 13, 124–132. [Google Scholar]
- Días, G.; de la Vega, R. Fase no parasitaria de Anocentor nitens (Ixodoidea. Ixodidae) en condiciones controladas. Rev. Salud Anim. 1987, 9, 29–35. [Google Scholar]
- Días, G.; de la Vega, R. Larval survival of anocentor nitens under simulated natural conditions. Ann. N. Y. Acad. Sci. 2000, 916, 309–314. [Google Scholar] [CrossRef]
- de la Vega, R.; Díaz, G. Thermal constant estimation in tropical horse tick, Anocentor nitens (Acari: Ixodidae). Ann. N. Y. Acad. Sci. 2000, 916, 298–302. [Google Scholar] [CrossRef]
- Días, G.; de la Vega, R. Variación de parámetros biológicos en anocentor nitens (acari: Ixodidae) en relación con el hospedero. Rev. Salud Anim. 2007, 29, 32–35. [Google Scholar]
- Hernández, K.; Arece, J.; Simón, L.; Hernández, L.; Valdés, O. Behavior of ticks in different genotypes of large ruminants under silvopastoral system conditions. Pastos Forrajes 2013, 36, 72–76. [Google Scholar]
- Obregón, D.; Rodríguez, J.D.; Roque, E.; Alemán, Y. Rhipicephalus (Boophilus) microplus (acari: Ixodidae) en búfalos (Bubalus bubalis), en Cuba. Rev. Salud Anim. 2010, 32, 132–134. [Google Scholar]
- Obregón, D.; Corona-González, B.; Díaz-Sánchez, A.A.; Armas, Y.; Roque, E.; Oliveira, M.C.S.; Cabezas-Cruz, A. Efficient transovarial transmission of Babesia spp. in Rhipicephalus microplus ticks fed on water buffalo (Bubalus bubalis). Pathogens 2020, 9, 280. [Google Scholar] [CrossRef]
- Encinosa Guzmán, P.E.; Bello Soto, Y.; Rodríguez-Mallon, A. Genetic and biological characterization of a Cuban tick strain from Rhipicephalus sanguineus complex and its sensitivity to different chemical acaricides. Int. J. Acarol. 2015, 42, 18–25. [Google Scholar] [CrossRef]
- Sanches, G.S.; Évora, P.M.; Mangold, A.J.; Jittapalapong, S.; Rodriguez-Mallon, A.; Guzmán, P.E.E.; Bechara, G.H.; Camargo-Mathias, M.I. Molecular, biological, and morphometric comparisons between different geographical populations of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). Vet. Parasitol. 2016, 215, 78–87. [Google Scholar] [CrossRef]
- Grandío, O.; Fernández, A.; Fernández, M.; Valera, R.; Fuentes, O.; Pelegrino, J. Informe preliminar sobre investigaciones realizadas en el poblado “Las Terrazas”, Sierra del Rosario, sobre la existencia de la Enfermedad de Lyme. Rev. Cuba. Pediatría 1988, 60, 773. [Google Scholar]
- Rodríguez, I.; Pedroso, R.; Fernández, C.; Cinco, M.; Fuentes, O. Enfermedad de Lyme en Cuba? Presentación de posibles casos. Rev. Cubana Med. Trop. 2003, 55, 41–43. [Google Scholar]
- Rodríguez, I.; Ortega, L.M.; Fernández, C.; Rodríguez, M.E.; Scheurer, C.; Lienhard, R. Lyme borreliosis in Cuba. Based on new cases. Rev. Panam Infect 2009, 11, 37–41. [Google Scholar]
- Rodríguez, I.; Fernández, C.; Sánchez, L.; Martínez, B.; Siegrist, H.H.; Lienhard, R. Prevalence of antibodies to Borrelia burgdorferi sensu stricto in humans from a Cuban village. Braz. J. Infect. Dis. 2012, 16, 82–85. [Google Scholar]
- Rodríguez, I.; Noda, A.A.; Fuentes, O.; Lienhard, R.; Gern, L. Evidences about human tick-borne infections in Cuba. Acta Biom. Scient. 2018, 3, 164–165. [Google Scholar] [CrossRef][Green Version]
- Suárez-Hernández, M.; Alonso-Castellano, M.; Peláes-Martinez, R.; Sanchez-Perez, B.; Bravo-Gonzalez, J.; Sanchez-Sibello, A. Pesquisaje de Babesia en trabajadores agropecuarios y donantes en la provincia de Ciego de Ávila. Rev. Cubana Med. Trop. 1997, 49, 130–135. [Google Scholar]
- Noda, A.A.; Rodríguez, I.; Miranda, J.; Mattar, S.; Cabezas-Cruz, A. First report of spotted fever group Rickettsia in Cuba. Ticks Tick-Borne Dis. 2016, 7, 1057–1058. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Schaper, S.; Mansfeld, P.; Gonschorrek, J.; Bröker, M.; Nava, S. Detection of Amblyomma mixtum (Acari: Ixodidae) in Germany on a human traveler returning from Cuba. J. Med. Entomol. 2020, 57, 962–964. [Google Scholar] [CrossRef]
- Suarez, C.E.; Noh, S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet. Parasitol. 2011, 180, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Corona, B.; Rodríguez, M.; Martínez, S. Bovine anaplasmosis. Rev. Electrónica Vet VI 2005, 1, 1–27. [Google Scholar]
- Nari, A. Strategies for the control of one-host ticks and relationship with tick-borne diseases in South America. Vet. Parasitol. 1995, 57, 153–165. [Google Scholar] [CrossRef]
- Guglielmone, A.A. Epidemiology of babesiosis and anaplasmosis in South and Central America. Vet. Parasitol. 1995, 57, 109–119. [Google Scholar] [CrossRef]
- Gondard, M.; Cabezas-Cruz, A.; Charles, R.A.; Vayssier-Taussat, M.; Albina, E.; Moutailler, S. Ticks and tick-borne pathogens of the Caribbean: Current understanding and future directions for more comprehensive surveillance. Front. Cell. Infect. Microbiol. 2017, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- Fadraga, M.; Cordovés, C.O.; Puentes, T. Circulation of antibodies to haemoparasites in cattle (Bos taurus) of high genetic value in Cuba. Rev. Cuba. Cienc. Vet. 1991, 22, 249–255. [Google Scholar]
- Rodríguez, O.N.; Espaine, L.; Rivas, A.; Rodríguez, P. Epizootiología de las enfermedades de los bovinos causados por hemoparásitos en la República de Cuba. Rev. Cuba. Cienc. Vet. 1989, 20, 37–56. [Google Scholar]
- Toledo, M.; Seoane, G.; Fregel, N.; Encinosa, A.; Serrano, E. Sistema de información y vigilancia epizootiológica (SIVE) en la República de Cuba. Rev Cub. Cienc Vet. 2000, 26, 7–11. [Google Scholar]
- Perez, R. Integration of livestock in the sugarcane industry in Cuba. In Livestock Feed Resources within Integrated Farming Systems, Proceedings of the Second FAO Electronic Conference on Tropical Feeds, 9 September 1997–18 February 1998; FAO: Rome, Italy; pp. 117–130.
- Pérez, R. Changes in Cuban livestock production. World Anim. Rev. 1999, 96, 62–70. [Google Scholar]
- Marima, J.K.; Nel, C.L.; Marufu, M.C.; Jonsson, N.N.; Dube, B.; Dzama, K. A genetic and immunological comparison of tick-resistance in beef cattle following artificial infestation with Rhipicephalus ticks. Exp. Appl. Acarol. 2020, 80, 569–590. [Google Scholar] [CrossRef]
- Constantinoiu, C.C.; Jackson, L.; Jorgensen, W.K.; Lew-Tabor, E.; Piper, E.K.; Mayer, D.G.; Venus, B.; Jonsson, N.N. Local immune response against larvae of Rhipicephalus (Boophilus) microplus in Bos taurus indicus and Bos taurus taurus cattle. Int. J. Parasitol. 2010, 40, 865–875. [Google Scholar] [CrossRef]
- Tabor, A.E.; Ali, A.; Rehman, G.; Garcia, G.R.; Zangirolamo, A.F.; Malardo, T.; Jonsson, N.N. Cattle Tick Rhipicephalus microplus-host interface: A review of resistant and susceptible host responses. Front. Cell. Infect. Microbiol. 2017, 7, 506. [Google Scholar] [CrossRef] [PubMed]
- Zintl, A.; Gray, J.S.; Skerrett, H.E.; Mulcahy, G. Possible mechanisms underlying age-related resistance to bovine babesiosis. Parasite Immunol. 2005, 27, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.; Jackson, L.; Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.C.; Oreau, E.M.; Onnet, S.B.; Lantard, O.P.; Alandrin, L.M.; Moreau, E.; Bonnet, S.; Plantard, O.; Malandrin, L. Babesia and its hosts: Adaptation to long-lasting interactions as a way to achieve efficient transmission. Vet. Res. 2009, 40, 37. [Google Scholar] [CrossRef]
- Mitat, A.O.B. Búfalos de agua en Cuba: Origen y evolución. Rev. ACPA 2009, 3, 45–48. [Google Scholar]
- CENCOP, N.L.R.C. Existencia de Bufalos al Cierre del Año; MINAGRI: La Habana, Cuba, 2019.
- Obregón, D.; Oliveira, M.C.S.; Tizioto, P.; Funnes, E.M.; Martínez, S.; Roque, E.; Fonseca, A.H.; Corona, B. Diagnóstico de Babesia bovis en búfalos de la región occidental de Cuba a través de un ensayo de nPCR. Rev. Salud Anim. 2012, 34, 101–108. [Google Scholar]
- Obregón, D.; Corona, B.G.; de la Fuente, J.; Cabezas-Cruz, A.; Gonçalves, L.R.; Matos, C.A.; Armas, Y.; Hinojosa, Y.; Alfonso, P.; Oliveira, M.C.S.; et al. Molecular evidence of the reservoir competence of water buffalo (Bubalus bubalis) for Anaplasma marginale in Cuba. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 180–187. [Google Scholar] [CrossRef]
- Obregón, D.; Rabelo, M.D.; Giglioti, R.; Bilhassi, T.B.; Néo, T.A.; Corona, B.; Alfonso, P.; Machado, R.Z.; Oliveira, M.C.S. Standardization of a SYBR green based real-time PCR system for detection and molecular quantification of Babesia bovis and B. bigemina in water buffaloes (Bubalus bubalis). J. Buffalo Sci. 2016, 5, 44–52. [Google Scholar] [CrossRef]
- Obregón, D.; Zamora, B.C.-G.P.A. El búfalo (Bubalus bubalis) como reservorio de Babesia bovis, B. bigemina y Anaplasma marginale en el Occidente de Cuba. An. Acad. Cienc. Cuba 2019, 9, 31–33. [Google Scholar]
- Onyiche, T.E.; Suganuma, K.; Igarashi, I.; Yokoyama, N.; Xuan, X.; Thekisoe, O. A review on equine piroplasmosis: Epidemiology, vector ecology, risk factors, host immunity, diagnosis and control. Int. J. Environ. Res. Public Health 2019, 16, 1736. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Ueti, M.W. Vector ecology of equine piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef]
- OIE, W.O. for A. H. Equine piroplasmosis. In Terrestrial Manual. Version adopted by the World Assembly of Delegates of the OIE; OIE: Paris, France, 2014. [Google Scholar]
- Wise, L.N.; Pelzel-McCluskey, A.; Mealey, R.H.; Knowles, D.P. Equine piroplasmosis. Vet. Clin. Equine N. Am. Pract. 2014, 30, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Hernandez, J.; Roman, E.I. Concomitant haemoparasitosis with Piroplasma caballi and Nuttallia equi in a horse from the province of Ciego de Avila. Cienc. Técnica Agric. Vet. 1985, 7, 79–82. [Google Scholar]
- Asgarali, Z.; Coombs, D.K.; Mohammed, F.; Campbell, M.D.; Caesar, E. A serological study of Babesia caballi and Theileria equi in Thoroughbreds in Trinidad. Vet. Parasitol. 2007, 144, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Salabarria, F.; Godshaev, A.; Ferrer, J.; Jimenez, T.; Villalba, G.; Jorge, J. Babesia (Nuttalia) equi y Babesia caballi en cebras (Equus zebra). Diagnostico morfologico y serologico. Rev. Cuba. Cienc. Vet. 1981, 12, 171–176. [Google Scholar]
- Salabarria, F.F.; Gonzalez, M.; Jimenez, T. Complement fixation in the serological diagnosis of Nuttalia (Babesia) equi. Rev. Cuba. Cienc. Vet. 1982, 13, 81–84. [Google Scholar]
- Hall, C.M.; Busch, J.D.; Scoles, G.A.; Palma-Cagle, K.A.; Ueti, M.W.; Kappmeyer, L.S.; Wagner, D.M. Genetic characterization of Theileria equi infecting horses in North America: Evidence for a limited source of U.S. introductions. Parasites Vectors 2013, 6, 35. [Google Scholar] [CrossRef]
- Sant, C.; Allicock, O.M.; d’Abadie, R.; Charles, R.A.; Georges, K. Phylogenetic analysis of Theileria equi and Babesia caballi sequences from thoroughbred mares and foals in Trinidad. Parasitol. Res. 2019, 118, 1171–1177. [Google Scholar] [CrossRef]
- Vitari, G.; Costa, R.L.; Abreu, A.; Peixoto, M.; Silva, C.; Paulino, P.; Pires, M.; Massard, C.; Santos, H. Genetic diversity of Theileria equi from horses In different regions of Brazil based on the 18S rRNA gene. J. Parasitol. 2019, 105, 186–194. [Google Scholar] [CrossRef]
- Pérez, B.; Valdés, R.; Vitorte, S. Epidemiología de las enfermedades transmitidas por garrapatas en Cuba. Rev. Cuba. Cienc. Vet. 2002, 20, 78–87. [Google Scholar]
- León, A.; Demedio, J.; Márquez, M.; Castillo, E.; Perera, A.; Zuaznaba, O.; Caníbal, J.; González, B.; Reynaldo, L.; Vega, N.; et al. Diagnóstico de ehrlichiosis en caninos en la ciudad de La Habana. Redvet 2008, 3, 1–22. [Google Scholar]
- Silva, C.B.; Santos, H.A.; Navarrete, M.G.; Ribeiro, C.C.D.U.; Gonzalez, B.C.; Zaldivar, M.F.; Pires, M.S.; Peckle, M.; Costa, R.L.; Vitari, G.L.V.; et al. Molecular detection and characterization of Anaplasma platys in dogs and ticks in Cuba. Ticks Tick-Borne Dis. 2016, 7, 938–944. [Google Scholar] [CrossRef] [PubMed]
- González-Navarrete, M.; Cordeiro, M.D.; da Silva, C.B.; Pires, M.S.; Uzedo Ribeiro, C.C.D.; Cabezas-Cruz, A.; Massard, C.L.; López, E.R.; da Fonseca, A.H. Molecular detection of Ehrlichia canis and Babesia canis vogeli in Rhipicephalus sanguineus sensu lato ticks from Cuba. Rev. Bras. Med. Vet. 2016, 38, 63–67. [Google Scholar]
- González-Navarrete, M.; Bezerra da Silva, C.; Cuello Portal, S.; Rodríguez Alonso, M.B.; Fonseca, A.H. Diagnosis of Ehrlichia canis in domestic dogs of Havana, Cuba. Rev. Salud Anim. 2019, 41, 1–6. [Google Scholar]
- Fernández, R.D. Caracterización de los sistemas de producción caprina en la provincia Ciego de Ávila. Pastos Forrajes 2016, 39, 64–71. [Google Scholar]
- Herrera, J.; Jordán, H.; Senra, A.F. Aspectos del manejo y alimentación de la reproductora ovina Pelibuey en Cuba. Rev. Cuba. Cienc. Agrícola 2010, 44, 211–219. [Google Scholar]
- Mahieu, M.; Archimède, H.; Fleury, J.; Mandonnet, N.; Alexandre, G. Intensive grazing system for small ruminants in the Tropics: The French West Indies experience and perspectives. Small Rumin. Res. 2008, 77, 195–207. [Google Scholar] [CrossRef]
- Peron, N.; Limas, T.; Fuentes, J.L. El ovino Pelibuey de Cuba. Revision bibliografica de algunas caracteristicas reproductivas. Rev. Mund. Zootec. 1991, 66, 32–39. [Google Scholar]
- Alessandra, T.; Santo, C. Tick-borne diseases in sheep and goats: Clinical and diagnostic aspects. Small Rumin. Res. 2012, 106, S6–S11. [Google Scholar] [CrossRef]
- Camus, E.; Barré, N. Amblyomma variegatum and associated diseases in the Caribbean: Strategies for control and eradication in Guadeloupe. Parassitologia 1990, 32, 185–193. [Google Scholar] [PubMed]
- Camus, E.B.N. The role of Amblyomma variegatum in the transmission of heartwater with special reference to guadeloupe. Ann. N Y Acad. Sci. 1992, 653, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Joa, R.; Merino, N.; Alonso, M.; Blandino, T. Eperythrozoonosis (E. ovis) in sheep and goats in Cuba. Short communication. Rev. Salud Anim. 1987, 9, 85–86. [Google Scholar]
- Messick, J.B. Hemotrophic mycoplasmas (hemoplasmas): A review and new insights into pathogenic potential. Vet. Clin. Pathol. 2004, 33, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Nikol’skii, S.N.; Slipchenko, S.N. Experiments on the transmission of Eperythrozoon ovis by the ticks H. plumbeum and Rh. bursa. Veterinariya 1969, 46, 46–56. [Google Scholar]
- Rodríguez, O.N.; Espaine, L.; Rivas, A.; Rodriguez, P. Epidemiology of cattle diseases caused by haemoparasites in Cuba. Rev. Cuba. Cienc. Vet. 1989, 20, 37–56. [Google Scholar]
- Zhang, J.; Kelly, P.; Li, J.; Xu, C.; Wang, C. Molecular Detection of Theileria spp. in Livestock on Five Caribbean Islands. BioMed Res. Int. 2015, 2015, 624728. [Google Scholar] [CrossRef]
- Cordovés, C.O.; Camacho, A.; Leyva, A.; Fregel, N. Surveillance and control system for hemoparasitoses in the Republic of Cuba. Rev. Cuba. Cienc. Vet. 1991, 22, 197–232. [Google Scholar]
- Pino, R.; Salabarría, F.F. Prevalencia de Babesia bigemina (Smith y Kilborne, 1983) en el período enero-mayo de 1987 en las provincias de Ciudad de la Habana y La Habana. Rev. Cuba. Cienc. Vet. 1989, 20, 227–232. [Google Scholar]
- Salabarría, F.F.; Jiménez, T. Results obtained in the morphological diagnosis of bovine babesiosis and anaplasmosis in Habana province. Rev. Cuba. Cienc. Vet. 1983, 22–32. [Google Scholar]
- Corona-González, B.; Obregón, D.A.; Alemán, Y.; Alfonso, P.; Vega, E.; Díaz, A.; Martínez, S.; Corona, B.G.; Obregón, D.A.; Alemán, Y.; et al. Tendencias en el diagnóstico de la anaplasmosis bovina. Rev. Salud Anim. 2014, 36, 73–79. [Google Scholar]
- Blandino, T.; Alonso, M.; Barrera, M.; Mendoza, E. Validation and use of an elisa kit for the diagnosis of Babesia bovis in Cuba. In Final Research Co-Ordination Meeting on Immunoassay Methods for the Diagnosis and Epidemiology of Animal Diseases in Latin America; International Atomic Energy Agency (IAEA): Guadeloupe, France, 1998; pp. 247–251. [Google Scholar]
- Alonso, M.; Blandino, T.; Larramendi, R.; Jimenez, T.; Mesa, J. Inmunofluorescencia indirecta en el diagnóstico de la babesiosis bovina en Cuba. Rev. Salud Anim. 1988, 10, 197. [Google Scholar]
- Blandino, T.; Barrera, M.; Alonso, M. Prueba de inmunoperoxidasa para el diagnóstico serologico de Babesia bovis. Prog. Med. Vet. 1992, 1, 11. [Google Scholar]
- Rodríguez, O.N.; Espaine, L.; Rodríguez, P. Anaplasmosis y babesiosis en bovinos. Pruebas de aglutinación fijación para respuestas seguras y eficientes. Panagfa 1980, 8, 66–69. [Google Scholar]
- Corona-González, B.; Marrero, S. Detection of Anaplasma marginale in bovine, using the msp5 gene amplification by PCR. Rev Salud Anim. 2011, 33, 24–31. [Google Scholar]
- Corona-González, B.; Obregón, D.; Martínez, S.; Espinosa, I.; Fonseca, A.H.; Roque, E. Detección por PCR de Anaplasma marginale en búfalos de la región occidental de Cuba. Rev. Salud Anim. 2012, 34, 11–18. [Google Scholar]
- Díaz-Sánchez, A.A.; Corona-González, B.; Meli, M.L.; Álvarez, D.O.; Cañizares, E.V.; Rodríguez, O.F.; Rivero, E.L.; Hofmann-Lehmann, R. First molecular evidence of bovine hemoplasma species (Mycoplasma spp.) in water buffalo and dairy cattle herds in Cuba. Parasites Vectors 2019, 12, 78. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Naranjo, V.; Acevedo-Whitehouse, K.; Mangold, A.J.; Kocan, K.M.; de la Fuente, J. Phylogeographic analysis reveals association of tick-borne pathogen, Anaplasma marginale, MSP1a sequences with ecological traits affecting tick vector performance. BMC Biol. 2009, 7, 57. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Passos, L.M.F.; Lis, K.; Kenneil, R.; Valdés, J.J.; Ferrolho, J.; Tonk, M.; Pohl, A.E.; Grubhoffer, L.; Zweygarth, E.; et al. Functional and immunological relevance of Anaplasma marginale major surface protein 1a sequence and structural analysis. PLoS ONE 2013, 8, e65243. [Google Scholar] [CrossRef]
- Ruybal, P.; Moretta, R.; Perez, A.; Petrigh, R.; Zimmer, P.; Alcaraz, E.; Echaide, I.; Torioni de Echaide, S.; Kocan, K.M.; de la Fuente, J.; et al. Genetic diversity of Anaplasma marginale in Argentina. Vet. Parasitol. 2009, 162, 176–180. [Google Scholar] [CrossRef]
- Mutshembele, A.M.; Cabezas-Cruz, A.; Mtshali, M.S.; Thekisoe, O.M.M.; Galindo, R.C.; de la Fuente, J. Epidemiology and evolution of the genetic variability of Anaplasma marginale in South Africa. Ticks Tick-Borne Dis. 2014, 5, 624–631. [Google Scholar] [CrossRef]
- de la Fuente, J.; Ruybal, P.; Mtshali, M.S.; Naranjo, V.; Shuqing, L.; Mangold, A.J.; Rodríguez, S.D.; Jiménez, R.; Vicente, J.; Moretta, R.; et al. Analysis of world strains of Anaplasma marginale using major surface protein 1a repeat sequences. Vet. Microbiol. 2007, 119, 382–390. [Google Scholar] [CrossRef]
- Catanese, H.N.; Brayton, K.A.; Gebremedhin, A.H. RepeatAnalyzer: A tool for analysing and managing short-sequence repeat data. BMC Genom. 2016, 17, 422. [Google Scholar] [CrossRef]
- Corona, G.; Díaz, S.; Hinojosa, L.; Obregón, Á.; Da Silva, C.B.; Peixoto, M.P.; Pires, M.S.; Santos, H.A.; Martínez, M.; da Fonseca, A.H.; et al. Analysis of major surface protein 1a sequence in Cuban isolates of Anaplasma marginale. Rev. Salud Anim. 2016, 38, 14–18. [Google Scholar]
- Ybañez, A.P.; Ybañez, R.H.D.; Claveria, F.G.; Cruz-Flores, M.J.; Xuenan, X.; Yokoyama, N.; Inokuma, H. High genetic diversity of Anaplasma marginale detected from Philippine cattle. J. Vet. Med. Sci. 2014, 76, 1009–1014. [Google Scholar] [CrossRef]
- Jonsson, N.N.; Bock, R.E.; Jorgensen, W.K. Productivity and health effects of anaplasmosis and babesiosis on Bos indicus cattle and their crosses, and the effects of differing intensity of tick control in Australia. Vet. Parasitol. 2008, 155, 1–9. [Google Scholar] [CrossRef]
- Porto Neto, L.R.; Jonsson, N.N.; D’Occhio, M.J.; Barendse, W. Molecular genetic approaches for identifying the basis of variation in resistance to tick infestation in cattle. Vet. Parasitol. 2011, 180, 165–172. [Google Scholar] [CrossRef]
- Robbertse, L.; Richards, S.A.; Maritz-Olivier, C. Bovine immune factors underlying tick resistance: Integration and future directions. Front. Cell. Infect. Microbiol. 2017, 7, 522. [Google Scholar] [CrossRef]
- Hilleman, M.R. Vaccines in historic evolution and perspective: A narrative of vaccine discoveries. J. Hum. Virol. 2000, 3, 63–76. [Google Scholar] [CrossRef]
- Barker, S.C.; Murrell, A. Systematic and evolution of ticks with the list of valid genus and species names. In Ticks: Biology, Disease and Control; Bowman, A.S., Nuttall, P.A., Eds.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Cuba’s biotech boom. Nature 2009, 457, 130. [CrossRef][Green Version]
- Baracca, A.; Franconi, R. Introduction. Cuba’s exceptional scientific development. In Subalternity vs. Hegemony, Cuba’s Outstanding Achievements in Science and Biotechnology, 1959–2014; Baracca, A., Franconi, R., Eds.; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 1–10. [Google Scholar]
- Rand, K.N.; Moore, T.; Sriskantha, A.; Spring, K.; Tellam, R.; Willadsen, P.; Cobon, G.S. Cloning and expression of a protective antigen from the cattle tick Boophilus microplus. Proc. Natl. Acad. Sci. USA 1989, 86, 9657–9661. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, P.; Riding, G.A.; McKenna, R.V.; Kemp, D.H.; Tellam, R.L.; Nielsen, J.N.; Lahnstein, J.; Cobon, G.S.; Gough, J.M. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J. Immunol. 1989, 143, 1346–1351. [Google Scholar]
- Willadsen, P.; Kemp, D.H. Vaccination with “concealed” antigens for tick control. Parasitol. Today 1988, 4, 196–198. [Google Scholar] [CrossRef]
- Willadsen, P. The molecular revolution in the development of vaccines against ectoparasites. Vet. Parasitol. 2001, 101, 353–368. [Google Scholar] [CrossRef]
- Canales, M.; Enríquez, A.; Ramos, E.; Cabrera, D.; Dandie, H.; Soto, A.; Falcón, V.; Rodríguez, M.; de la Fuente, J. Large-scale production in Pichia pastoris of the recombinant vaccine Gavac against cattle tick. Vaccine 1997, 15, 414–422. [Google Scholar] [CrossRef]
- Rodríguez, M.; Rubiera, R.; Penichet, M.; Montesinos, R.; Cremata, J.; Falcón, V.; Sánchez, G.; Bringas, R.; Cordovés, C.; Valdés, M. High level expression of the B. microplus Bm86 antigen in the yeast Pichia pastoris forming highly immunogenic particles for cattle. J. Biotechnol. 1994, 33, 135–146. [Google Scholar] [CrossRef]
- Rodriguez, M.; Penichet, M.L.; Mouris, A.E.; Labarta, V.; Luaces, L.L.; Rubiera, R.; Cordoves, C.; Sanchez, P.A.; Ramos, E.; Soto, A. Control of Boophilus microplus populations in grazing cattle vaccinated with a recombinant Bm86 antigen preparation. Vet. Parasitol. 1995, 57, 339–349. [Google Scholar] [CrossRef]
- Rodríguez, M.; Massard, C.L.; da Fonseca, A.H.; Ramos, N.F.; Machado, H.; Labarta, V.; de la Fuente, J. Effect of vaccination with a recombinant Bm86 antigen preparation on natural infestations of Boophilus microplus in grazing dairy and beef pure and cross-bred cattle in Brazil. Vaccine 1995, 13, 1804–1808. [Google Scholar] [CrossRef]
- Redondo, M.; Fragoso, H.; Montero, C.; Lona, J.; Medellín, J.A.; Fría, R.; Hernández, V.; Franco, R.; Machado, H.; Rodríguez, M.; et al. Integrated control of acaricide-resistant Boophilus microplus populations on grazing cattle in Mexico using vaccination with Gavac and amidine treatments. Exp. Appl. Acarol. 1999, 23, 841–849. [Google Scholar] [CrossRef]
- Vanegas, L.F.; Parra, S.A.; Vanegas, C.G.; de la Fuente, J. Commercialization of the recombinant vaccine GavacTM against Boophilus microplus in Colombia. In Recombinant Vaccines for the Control of Cattle Tick; Elfos Scientiae: La Habana, Cuba, 1995; pp. 196–199. [Google Scholar]
- de la Fuente, J.; Rodrıguez, M.; Montero, C.; Redondo, M.; Garcıa-Garcıa, J.C.; Méndez, L.; Serrano, E.; Valdés, M.; Enrıquez, A.; Canales, M. Vaccination against ticks (Boophilus spp.): The experience with the Bm86-based vaccine GavacTM. Genet. Anal. Biomol. Eng. 1999, 15, 143–148. [Google Scholar] [CrossRef]
- Valle, M.R.; Mèndez, L.; Valdez, M.; Redondo, M.; Espinosa, C.M.; Vargas, M.; Cruz, R.L.; Barrios, H.P.; Seoane, G.; Ramirez, E.S.; et al. Integrated control of Boophilus microplus ticks in Cuba based on vaccination with the anti-tick vaccine GavacTM. Exp. Appl. Acarol. 2004, 34, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.; Rubi, J.; Pérez, D.; Cordova, V.; Salazar, Y.; Vielma, A.; Barrios, F.; Gil, C.A.; Segura, N.; Carrillo, Y. High impact and effectiveness of GavacTM vaccine in the national program for control of bovine ticks Rhipicephalus microplus in Venezuela. Livest. Sci. 2016, 187, 48–52. [Google Scholar] [CrossRef]
- Eiden, A.L.; Kaufman, P.E.; Oi, F.M.; Allan, S.A.; Miller, R.J. Detection of permethrin resistance and fipronil tolerance in Rhipicephalus sanguineus (Acari: Ixodidae) in the United States. J. Med. Entomol. 2015, 52, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vivas, R.I.; Ojeda-Chi, M.M.; Trinidad-Martinez, I.; De León, A.A.P. First documentation of ivermectin resistance in Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). Vet. Parasitol. 2017, 233, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vivas, R.I.; Ojeda-Chi, M.M.; Trinidad-Martinez, I.; Bolio-Gonzalez, M.E. First report of amitraz and cypermethrin resistance in Rhipicephalus sanguineus sensu lato infesting dogs in M exico. Med. Vet. Entomol. 2017, 31, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; George, J.E.; Guerrero, F.; Carpenter, L.; Welch, J.B. Characterization of acaricide resistance in Rhipicephalus sanguineus (Latreille)(Acari: Ixodidae) collected from the Corozal army veterinary quarantine center, Panama. J. Med. Entomol. 2001, 38, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mallon, A.; Encinosa Guzmán, P.E.; Bello Soto, Y.; Rosales Perdomo, K.; Montero Espinosa, C.; Vargas, M.; Estrada García, M.P. A chemical conjugate of the tick P0 peptide is efficacious against Amblyomma mixtum. Transbound. Emerg. Dis. 2020, 67, 1–3. [Google Scholar] [CrossRef]
- Rodríguez-Mallon, A.; Encinosa, P.E.; Méndez-Pérez, L.; Bello, Y.; Fernández, R.R.; Garay, H.; Cabrales, A.; Méndez, L.; Borroto, C.; Estrada, M.P. High efficacy of a 20 amino acid peptide of the acidic ribosomal protein P0 against the cattle tick, Rhipicephalus microplus. Ticks Tick-Borne Dis. 2015, 6, 530–537. [Google Scholar] [CrossRef]
- Rodríguez-Mallon, A.; Fernández, E.; Encinosa, P.E.; Bello, Y.; Méndez-Pérez, L.; Ruiz, L.C.; Pérez, D.; González, M.; Garay, H.; Reyes, O. A novel tick antigen shows high vaccine efficacy against the dog tick, Rhipicephalus sanguineus. Vaccine 2012, 30, 1782–1789. [Google Scholar] [CrossRef]
- Mallón, A.R.; González, L.J.; Enrique, P.; Guzmán, E.; Bechara, G.H.; Sanches, G.S.; Pousa, S.; Cabrera, G. Functional and mass spectrometric evaluation of an anti-tick antigen based on the P0 peptide conjugated to Bm86 protein. Pathogens 2020, 9, 513. [Google Scholar] [CrossRef]
- Nari, A.; Hansen, J.W. Resistance of ecto and endo-parasites: Current and future soluctions. In Proceedings of the OIE International Committee. 67th General Sesion, París, France, 17–21 May 1999; pp. 13–22. [Google Scholar]
- Rodríguez-Vivas, I.; Rosado-Aguilar, J.A.; Ojeda-Chi, M.M.; Pérez-Cogollo, C.L.; Trinidad-martínez, I.; Bolio-González, M.E. Integrated control of ticks in bovine livestock Rhipicephalus microplus. Ecosistemas Recur. Agropecu. 2014, 1, 295–308. [Google Scholar]
- Rodriguez-vivas, R.I. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Int. 2017, 117, 3–29. [Google Scholar] [CrossRef]
- López, D. Cruzamientos en Cuba: Experiencias y perspectivas. Arch. Latinoam. Prod. Anim. 2005, 5, 29–36. [Google Scholar]
- Suárez, M.A.; Zubizarreta, I.; Pérez, T. Interacción genotipo ambiente en ganado bovino Siboney de Cuba. Livest. Res. Rural Dev. 2009, 21, 31. [Google Scholar]
- Uffo, O.; Acosta, A.; Ribot, A.; Ruiz, K.; Ronda, R.; Martinez, S. Molecular characterization of the Cuban Siboney cattle. Biotecnol. Apl. 2013, 30, 232–233. [Google Scholar]
- Allsopp, B.A. Natural History of Ehrlichia ruminantium. Vet. Parasitol. 2010, 167, 123–135. [Google Scholar] [CrossRef]
| Species | Main Hosts | Geographic Area (Provinces) |
|---|---|---|
| Argas persicus | Fowls | Artemisa, Mayabeque, Camagüey |
| Argas miniatus | Fowls | Pinar del Río, Camagüey |
| Argas radiatus | Fowls | Isla de la Juventud, Camagüey |
| Antricola silvai | Bat guano/Bats | Sancti Spiritus |
| Antricola granasi | Bat guano/Bats | Sancti Spiritus |
| Antricola habanensis | Bat guano/Bats | Mayabeque |
| Antricola cernyi | Bat guano/Bats | Cienfuegos |
| Antricola occidentalis | Bat guano/Bats | Pinar del Río |
| Antricola martelorum | Bat guano/Bats | Mayabeque |
| Antricola naomiae | Bat guano/Bats | Matanzas |
| Antricola armasi | Bat guano/Bats | Pinar del Río |
| Antricola centralis | Bat guano/Bats | Villa Clara |
| Antricola siboneyi | Bat guano/Bats | Santiago de Cuba |
| Antricola marginatus | Bat guano/Bats | Widespread in Cuba |
| Ornithodoros azteci | Bats | Mayabeque, Isla de la Juventud |
| Ornithodoros brodyi | Bats | Pinar del Río, Artemisa, Cienfuegos |
| Ornithodoros cyclurae | Reptiles | Granma |
| Ornithodoros denmarki | Birds | Matanzas |
| Ornithodoros dusbabeki | Bats | Isla de la Juventud |
| Ornithodoros kelleyi | Bats | Sancti Spiritus |
| Ornithodoros natalinus | Bats | Isla de la Juventud |
| Ornithodoros capensis 1 | Birds | Pinar del Río |
| Ornithodoros tadaridae | Bats | Camagüey |
| Ornithodoros viguerasi | Bats | Pinar del Río, Artemisa, Mayabeque, Matanzas, Villa Clara, Sancti Spiritus, Santiago de Cuba, Guantánamo |
| Otobius megnini | Equine | Artemisa |
| Species | Main hosts | Geographic Area (Provinces) |
|---|---|---|
| Amblyomma albopictum | Reptiles, rodents | Artemisa, Mayabeque, Isla de La Juventud, Camagüey |
| Amblyomma cajennense 1 | Domestic animals, humans | Pinar del Río, Artemisa, Mayabeque, Isla de la Juventud, Camagüey, Santiago de Cuba |
| Amblyomma dissimile | Reptiles, amphibians | Pinar del Río, Artemisa, Mayabeque, Isla de la Juventud, Santiago de Cuba |
| Amblyomma quadricavum 1 | Reptiles, amphibians | Pinar del Río, Artemisa, Mayabeque, Cienfuegos |
| Amblyomma torrei | Reptiles, amphibians | Pinar del Río, La Habana, Camagüey |
| Dermacentor nitens | Equines | Widespread in Cuba |
| Ixodes capromydis 2 | Cuban hutia | Isla de la Juventud |
| Rhipicephalus microplus | Bovines, equines | Widespread in Cuba |
| Rhipicephalus sanguineus 3 | Domestic dogs | Widespread in Cuba |
| Pathogens | Reported Host | Detection Method | References |
|---|---|---|---|
| A. marginale, B. bovis, B. bigemina | cattle | CFT, CAT | Rodríguez et al., 1980 [138] |
| A. marginale, B. bovis, B. bigemina | cattle | Morphological diagnosis | Salabarría and Jiménez, 1983 [133] |
| M. ovis | sheep, goat | Morphological diagnosis | Joa et al., 1987 [126] |
| A. ovis, B. ovis, M. ovis, B. motasi | sheep, goat | Morphological diagnosis | Rodriguez et al., 1989 [129] |
| Babesia spp. | cattle | IFA | Alonso et al., 1988 [136] |
| B. bovis, B. bigemina | cattle | IPT | Blandino et al., 1992 [137] |
| A. marginale, B. bovis, B. bigemina | cattle | CFT, CAT, IFA | Fadraga et al., 1991 [86] |
| B. bovis | cattle | ELISA | Blandino et al., 1998 [136] |
| A. marginale | cattle | PCR | Corona et al., 2011 [139] |
| B. bovis | buffalo | nPCR | Obregón et al., 2012 [99] |
| A. marginale | buffalo | nPCR | Corona et al., 2012 [140] |
| B. burgdorferi s.l. Anaplasma-Ehrlichia, Babesia-Theileria | D. nitens, A. cajennense s.l., R. microplus | Multiplex PCR | Rodríguez et al., 2015 [26] |
| B. bovis, B. bigemina | buffalo | SYBR Green qPCR | Obregón et al., 2016 [101] |
| R. amblyommii | A. mixtum | PCR | Noda et al., 2016 [79] |
| E. canis, B. canis vogeli | R. sanguineus s.l. | PCR, nPCR | Gonzalez et al., 2016 [118] |
| C. burnetii | A. mixtum | PCR | Noda et al., 2016 [51] |
| A. platys | R. sanguineus s.l., dog | nPCR | Silva et al., 2016 [116] |
| B. caballi, T. equi | horse | nPCR | Díaz-Sánchez et al., 2018 [24] |
| E. canis | dog | iELISA, nPCR | Gonzalez et al., 2018 [25] |
| A. marginale | buffalo | TaqMan qPCR | Obregón et al., 2018 [100] |
| A. marginale, T. annulata | cattle, buffalo | Multiplex TaqMan qPCR | Díaz-Sánchez et al., 2019 [14] |
| B. bovis, B. bigemina, A. marginale | buffalo | iELISA, nPCR | Obregón et al., 2019 [21] |
| M. wenyonii, Candidatus M. haemobus | cattle, buffalo | TaqMan qPCR | Díaz-Sánchez et al., 2019 [141] |
| B. bovis, B. bigemina | cattle, buffalo, R. microplus | SYBR Green qPCR | Obregón et al., 2020 [70] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obregón Alvarez, D.; Corona-González, B.; Rodríguez-Mallón, A.; Rodríguez Gonzalez, I.; Alfonso, P.; Noda Ramos, A.A.; Díaz-Sánchez, A.A.; González Navarrete, M.; Rodríguez Fernández, R.; Méndez Mellor, L.; et al. Ticks and Tick-Borne Diseases in Cuba, Half a Century of Scientific Research. Pathogens 2020, 9, 616. https://doi.org/10.3390/pathogens9080616
Obregón Alvarez D, Corona-González B, Rodríguez-Mallón A, Rodríguez Gonzalez I, Alfonso P, Noda Ramos AA, Díaz-Sánchez AA, González Navarrete M, Rodríguez Fernández R, Méndez Mellor L, et al. Ticks and Tick-Borne Diseases in Cuba, Half a Century of Scientific Research. Pathogens. 2020; 9(8):616. https://doi.org/10.3390/pathogens9080616
Chicago/Turabian StyleObregón Alvarez, Dasiel, Belkis Corona-González, Alina Rodríguez-Mallón, Islay Rodríguez Gonzalez, Pastor Alfonso, Angel A. Noda Ramos, Adrian A. Díaz-Sánchez, Maylin González Navarrete, Rafmary Rodríguez Fernández, Luis Méndez Mellor, and et al. 2020. "Ticks and Tick-Borne Diseases in Cuba, Half a Century of Scientific Research" Pathogens 9, no. 8: 616. https://doi.org/10.3390/pathogens9080616
APA StyleObregón Alvarez, D., Corona-González, B., Rodríguez-Mallón, A., Rodríguez Gonzalez, I., Alfonso, P., Noda Ramos, A. A., Díaz-Sánchez, A. A., González Navarrete, M., Rodríguez Fernández, R., Méndez Mellor, L., Catanese, H. N., Peláez, M., Alemán Gainza, Y., Marrero-Perera, R., Roblejo-Arias, L., Lobo-Rivero, E., Silva, C. B., Fonseca, A. H., Roque López, E., & Cabezas-Cruz, A. (2020). Ticks and Tick-Borne Diseases in Cuba, Half a Century of Scientific Research. Pathogens, 9(8), 616. https://doi.org/10.3390/pathogens9080616












