Concurrent Infection of Skunk Adenovirus-1, Listeria monocytogenes, and a Regionally Specific Clade of Canine Distemper Virus in One Gray Fox (Urocyon cinereoargenteus) and Concurrent Listeriosis and Canine Distemper in a Second Gray Fox
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pathology
2.2. Rabies Virus Testing
2.3. Canine Distemper Virus Characterization
2.4. Specific Canine Adenovirus 2 and Generic Adenovirus PCRs
2.5. Bacterial Culture, Speciation, and Whole Genome Sequencing
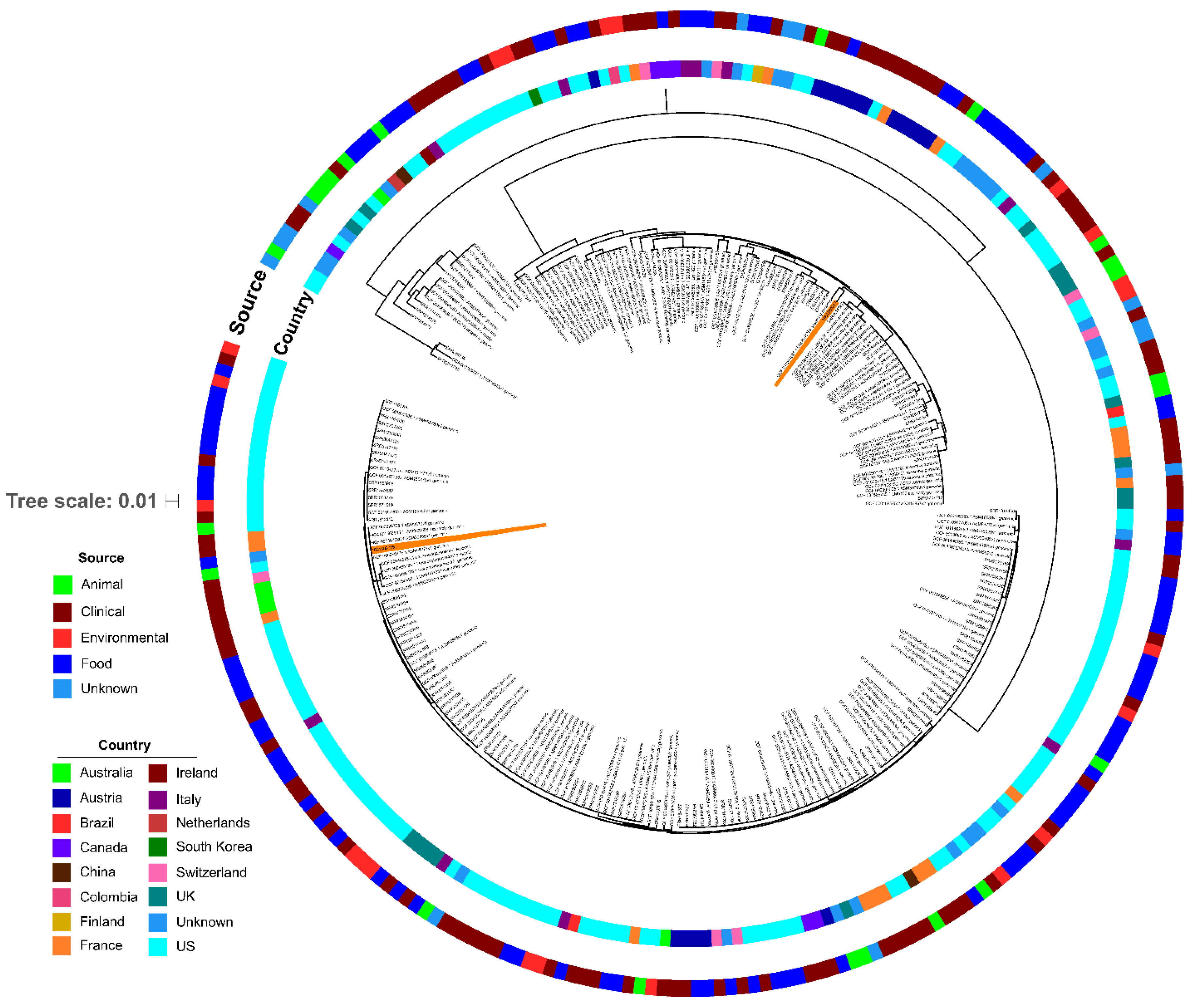
2.6. Genomic and Phylogenetic Analyses of L. monocytogenes
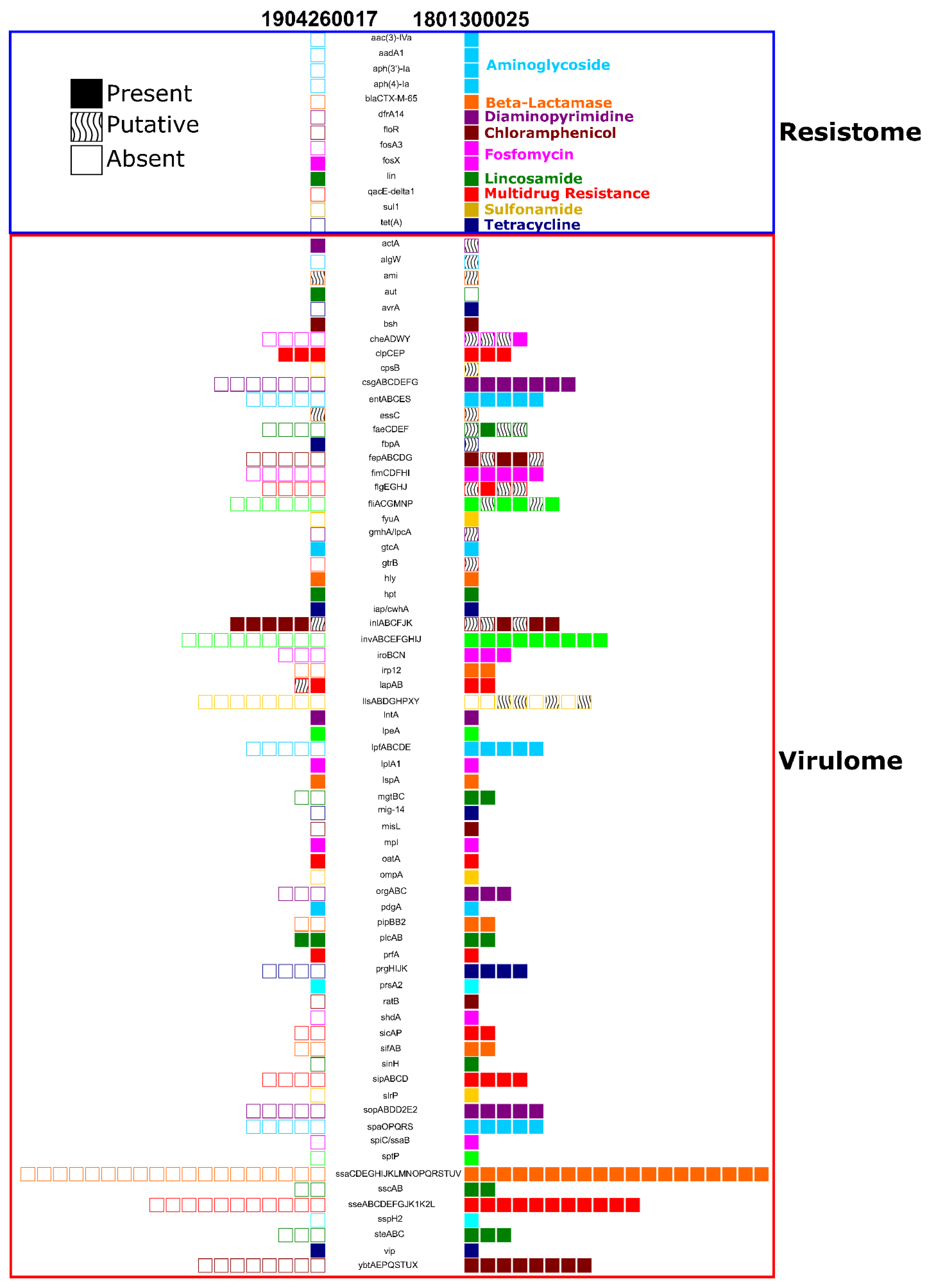
2.7. In Silico Identification of Resistome and Virulome Profiles
3. Results
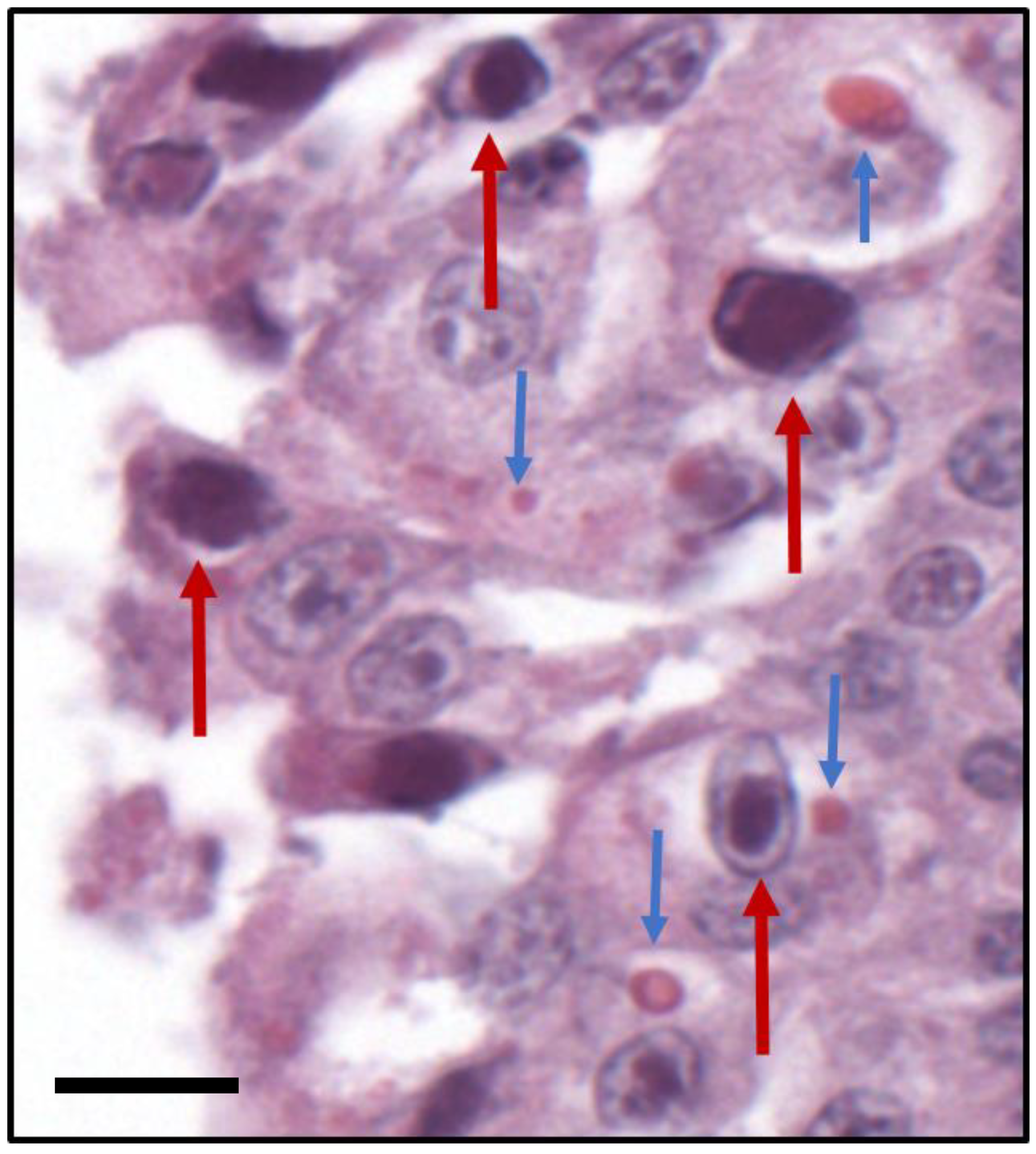
3.1. Pathology
3.2. Rabies Virus Testing
3.3. CDV Characterization
3.4. Skunk Adenovirus-1 Identification
3.5. Bacterial Culture, Speciation, and Whole Genome Sequencing
4. Discussion
5. Conclusions
6. Disclaimers
Author Contributions
Funding
Conflicts of Interest
References
- Madhusudana, S.N.; Subha, S.; Thankappan, U.; Ashwin, Y.B. Evaluation of a direct rapid immunohistochemical test (dRIT) for rapid diagnosis of rabies in animals and humans. Virol. Sin. 2012, 27, 299–302. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23055005 (accessed on 17 February 2020). [CrossRef] [PubMed]
- Wilkes, R.P.; Sanchez, E.; Riley, M.C.; Kennedy, M.A. Real-Time reverse transcription polymerase chain reaction method for detection of Canine distemper virus modified live vaccine shedding for differentiation from infection with wild-type strains. J. Vet. Diagn. Investig. 2014, 26, 27–34. Available online: http://journals.sagepub.com/doi/full/10.1177/1040638713517232?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 25 March 2019). [CrossRef] [PubMed] [Green Version]
- Needle, D.B.; Burnell, V.C.; Forzán, M.J.; Dubovi, E.J.; Schuler, K.L.; Bernier, C.; Hollingshead, N.A.; Ellis, J.C.; Stevens, B.A.; Tate, P.; et al. Infection of eight mesocarnivores in New Hampshire and Vermont with a distinct clade of canine distemper virus in 2016–2017. J. Vet. Diagn. Investig. 2019, 31, 562–567. Available online: http://journals.sagepub.com/doi/10.1177/1040638719847510 (accessed on 7 June 2019). [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software Version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23329690 (accessed on 1 April 2019). [CrossRef] [Green Version]
- Balboni, A.; Dondi, F.; Prosperi, S.; Battilani, M. Development of a SYBR Green real-time PCR assay with melting curve analysis for simultaneous detection and differentiation of canine adenovirus type 1 and type 2. J. Virol. Methods. 2015, 222, 34–40. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0166-0934 (15)00198-6 (accessed on 5 April 2019). [CrossRef]
- Wellehan, J.F.X.; Johnson, A.J.; Harrach, B.; Benkö, M.; Pessier, A.P.; Johnson, C.M.; Garner, M.M.; Childress, A.; Jacobson, E.R. Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the atadenoviruses. J. Virol. 2004, 78, 13366–13369. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15542689 (accessed on 1 February 2017). [CrossRef] [Green Version]
- Needle, D.B.; Selig, M.K.; Jackson, K.A.; Delwart, E.; Tighe, E.; Leib, S.L.; Seuberlich, T.; Pesavento, P.A. Fatal bronchopneumonia caused by skunk adenovirus 1 in an African pygmy hedgehog. J. Vet. Diagn. Investig. 2019, 31, 103–106. Available online: http://journals.sagepub.com/doi/10.1177/1040638718812123 (accessed on 5 December 2018). [CrossRef] [Green Version]
- Needle, D.B.; Wise, A.G.; Gregory, C.R.; Maes, R.K.; Sidor, I.F.; Ritchie, B.W.; Agnew, D. Necrotizing ventriculitis in fledgling chimney swifts (Chaetura Pelagica) Associated with a novel adenovirus, chimney swift adenovirus-1 (CsAdV-1). Vet. Pathol. 2019, 56, 907–914. Available online: http://journals.sagepub.com/doi/10.1177/0300985819861717 (accessed on 23 July 2019). [CrossRef]
- Thouvenot, P.; Vales, G.; Bracq-Dieye, H.; Tessaud-Rita, N.; Maury, M.M.; Moura, A.; Lecuit, M.; Leclercq, A. MALDI-TOF mass spectrometry-based identification of Listeria species in surveillance: A prospective study. J. Microbiol. Methods. 2018, 144, 29–32. Available online: https://www.sciencedirect.com/science/article/pii/S0167701217302750 (accessed on 21 March 2019). [CrossRef] [Green Version]
- Barbuddhe, S.B.; Maier, T.; Schwarz, G.; Kostrzewa, M.; Hof, H.; Domann, E.; Chakraborty, T.; Hain, T. Rapid identification and typing of Listeria species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2008, 74, 5402–5407. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/18606788/ (accessed on 22 January 2020). [CrossRef] [Green Version]
- Chen, Y.; Gonzalez-Escalona, N.; Hammack, T.S.; Allard, M.W.; Strain, E.A.; Brown, E.W. Core genome multilocus sequence typing for identification of globally distributed clonal groups and differentiation of outbreak strains of listeria monocytogenes. Appl. Environ. Microbiol. 2016, 82, 6258–6272. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27520821 (accessed on 15 February 2020). [CrossRef] [PubMed] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. Available online: https://www.liebertpub.com/doi/full/10.1089/cmb.2012.0021?casa_token=5Aymcam4sFEAAAAA%3AsAdorwx9tHhSgsXmEpvxT0f-kyhhfXcQAlYlfSRQ0TU-kcZ_Pu8bjJAH6gpmlp9ZVECtUYKb1LKZ& (accessed on 20 October 2019). [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. Available online: https://academic.oup.com/bioinformatics/article-lookup/doi/10.1093/bioinformatics/btu153 (accessed on 20 October 2019). [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/26198102/ (accessed on 20 October 2019). [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/24451623/ (accessed on 20 October 2019). [CrossRef] [PubMed]
- Tavare, S. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect. Math. Life Sci. 1986, 17, 57–86. [Google Scholar]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/27095192/ (accessed on 24 October 2019). [CrossRef]
- GitHub—Tseemann/Abricate: Mass screening of contigs for antimicrobial and virulence genes. Available online: https://github.com/tseemann/abricate (accessed on 27 October 2019).
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22782487 (accessed on 28 October 2019). [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/20003500/ (accessed on 28 October 2019). [CrossRef] [PubMed] [Green Version]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. Available online: http://nar.oxfordjournals.org/lookup/doi/10.1093/nar/gkv1239 (accessed on 28 October 2019). [CrossRef]
- Gerhold, R.W.; Allison, A.B.; Temple, D.L.; Chamberlain, M.J.; Strait, K.R.; Keel, M.K. Infectious canine hepatitis in a gray fox (Urocyon cinereoargenteus). J. Wildl. Dis. 2007, 43, 734–736. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2231712 (accessed on 21 November 2017). [CrossRef]
- Kozak, R.A.; Ackford, J.G.; Slaine, P.; Li, A.; Carman, S.; Campbell, D.; Welch, M.K.; Kropinski, A.M.; Nagy, É. Characterization of a novel adenovirus isolated from a skunk. Virology 2015, 485, 16–24. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26189043 (accessed on 12 March 2016). [CrossRef] [Green Version]
- Balik, S.; Bunting, E.; Dubovi, E.; Renshaw, R.; Childs-Sanford, S. Detection of skunk adenovirus 1 in two North American porcupines (Erethizon dorsatum) with respiratory disease. J. Zoo Wildl. Med. 2020, 50, 1012. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31926539 (accessed on 18 February 2020). [CrossRef] [PubMed]
- Kohl, C.; Vidovszky, M.Z.; Mühldorfer, K.; Dabrowski, P.W.; Radonić, A.; Nitsche, A.; Wibbelt, G.; Kurth, A.; Harrach, B. Genome analysis of bat adenovirus 2: Indications of interspecies transmission. J. Virol. 2012, 86, 1888–1892. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22130531 (accessed on 20 December 2016). [CrossRef] [Green Version]
- Hoppe, E.; Pauly, M.; Gillespie, T.R.; Akoua-Koffi, C.; Hohmann, G.; Fruth, B.; Karhemere, S.; Madinda, N.F.; Mugisha, L.; Muyembe, J.J.; et al. Multiple cross-species transmission events of human adenoviruses (HAdV) during hominine evolution. Mol. Biol. Evol. 2015, 32, 2072–2084. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25862141 (accessed on 6 March 2017). [CrossRef] [PubMed] [Green Version]
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. Available online: http://download.springer.com/static/pdf/653/art%253A10.1186%252Fs12917-016-0702-z.pdf?originUrl=http%3A%2F%2Fbmcvetres.biomedcentral.com%2Farticle%2F10.1186%2Fs12917-016-0702z&token2=exp=1497353798~acl=%2Fstatic%2Fpdf%2F653%2Fart%25253A10.1186%25252Fs129 (accessed on 9 January 2018). [CrossRef] [PubMed] [Green Version]
- Davidson, W.R.; Nettles, V.F.; Hayes, L.E.; Howerth, E.W.; Couvillion, C.E. Diseases diagnosed in gray foxes (Urocyon cinereoargenteus) from the Southeaster United States. J. Wildl. Dis. 1992, 28, 28–33. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1548799 (accessed on 9 January 2018). [CrossRef] [Green Version]
- Laksono, B.M.; de Vries, R.D.; Verburgh, R.J.; Visser, E.G.; de Jong, A.; Fraaij, P.L.A.; Ruijs, W.L.M.; Nieuwenhuijse, D.F.; van den Ham, H.J.; Koopmans, M.P.G.; et al. Studies into the mechanism of measles-associated immune suppression during a measles outbreak in the Netherlands. Nat. Commun. 2018, 9, 4944. Available online: www.nature.com/naturecommunications (accessed on 18 June 2020). [CrossRef]
- Mina, M.J.; Kula, T.; Leng, Y.; Li, M.; de Vries, R.D.; Knip, M.; Siljander, H.; Rewers, M.; Choy, D.F.; Wilson, M.S.; et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 2019, 366, 599–606. Available online: http://science.sciencemag.org/ (accessed on 18 June 2020). [CrossRef] [PubMed] [Green Version]
- Riley, M.C.; Wilkes, R.P. Sequencing of emerging canine distemper virus strain reveals new distinct genetic lineage in the United States associated with disease in wildlife and domestic canine populations. Virol. J. 2015, 12, 219. Available online: http://www.virologyj.com/content/12/1/219 (accessed on 18 June 2020). [CrossRef] [PubMed] [Green Version]
- Kapil, S.; Yeary, T.J. Canine distemper spillover in domestic dogs from urban wildlife. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 1069–1086. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/22041204/ (accessed on 18 June 2020). [CrossRef] [PubMed]
- Orsi, R.H.; Bakker HC den Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1438-4221(10)00061-5 (accessed on 18 June 2020). [CrossRef] [PubMed]
- Dhama, K.; Verma, A.K.; Rajagunalan, S.; Kumar, A.; Tiwari, R.; Chakraborty, S.; Kumar, R. Listeria monocytogenes infection in poultry and its public health importance with special reference to food borne zoonoses. Pakistan J. Biol. Sci. PJBS 2013, 16, 301–308. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24498796 (accessed on 15 November 2019). [CrossRef] [PubMed] [Green Version]
- Low, J.C.; Donachie, W. A review of Listeria monocytogenes and listeriosis. Vet. J. 1997, 153, 9–29. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1090-0233(97)80005-6 (accessed on 15 November 2019). [CrossRef]
- Schlech, W.F. Foodborne listeriosis. Clin. Infect. Dis. 2000, 31, 770–775. Available online: https://academic.oup.com/cid/article-lookup/doi/10.1086/314008 (accessed on 15 November 2019). [CrossRef]
- Reportable disease in New Hampshire. Division of Animal Industry. 2002. Available online: https://www.agriculture.nh.gov/publications-forms/documents/reportable-diseases.pdf (accessed on 15 November 2019).
- Black, S.S.; Austin, F.W.; McKinley, E. Isolation of yersinia pseudotuberculosis and listeria monocytogenes serotype 4 from a gray fox (Urocyon cinereoargenteus) with canine distemper. J. Wildl. Dis. 1996, 32, 362–366. [Google Scholar] [CrossRef]
- Jakowski, R.; Wyland, D. Listeriosis associated with canine distemper in a gray fox. J. Am. Vet. Med. Assoc. 1971, 159, 626–628. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Needle, D.B.; Marr, J.L.; Park, C.J.; Andam, C.P.; Wise, A.G.; Maes, R.K.; Wilkes, R.P.; Anis, E.A.; Sidor, I.F.; Agnew, D.; et al. Concurrent Infection of Skunk Adenovirus-1, Listeria monocytogenes, and a Regionally Specific Clade of Canine Distemper Virus in One Gray Fox (Urocyon cinereoargenteus) and Concurrent Listeriosis and Canine Distemper in a Second Gray Fox. Pathogens 2020, 9, 591. https://doi.org/10.3390/pathogens9070591
Needle DB, Marr JL, Park CJ, Andam CP, Wise AG, Maes RK, Wilkes RP, Anis EA, Sidor IF, Agnew D, et al. Concurrent Infection of Skunk Adenovirus-1, Listeria monocytogenes, and a Regionally Specific Clade of Canine Distemper Virus in One Gray Fox (Urocyon cinereoargenteus) and Concurrent Listeriosis and Canine Distemper in a Second Gray Fox. Pathogens. 2020; 9(7):591. https://doi.org/10.3390/pathogens9070591
Chicago/Turabian StyleNeedle, David B., Jacqueline L. Marr, Cooper J. Park, Cheryl P. Andam, Annabel G. Wise, Roger K. Maes, Rebecca P. Wilkes, Eman A. Anis, Inga F. Sidor, Dalen Agnew, and et al. 2020. "Concurrent Infection of Skunk Adenovirus-1, Listeria monocytogenes, and a Regionally Specific Clade of Canine Distemper Virus in One Gray Fox (Urocyon cinereoargenteus) and Concurrent Listeriosis and Canine Distemper in a Second Gray Fox" Pathogens 9, no. 7: 591. https://doi.org/10.3390/pathogens9070591
APA StyleNeedle, D. B., Marr, J. L., Park, C. J., Andam, C. P., Wise, A. G., Maes, R. K., Wilkes, R. P., Anis, E. A., Sidor, I. F., Agnew, D., Ellis, J. C., Tate, P., Mathewson, A., Benton, C., & Gibson, R. (2020). Concurrent Infection of Skunk Adenovirus-1, Listeria monocytogenes, and a Regionally Specific Clade of Canine Distemper Virus in One Gray Fox (Urocyon cinereoargenteus) and Concurrent Listeriosis and Canine Distemper in a Second Gray Fox. Pathogens, 9(7), 591. https://doi.org/10.3390/pathogens9070591




