West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications
Abstract
1. Introduction
2. Virus Biology
2.1. Genetic Organisation and Virus Replication
2.1.1. The Capsid (C) Protein
2.1.2. The Envelope (E) Protein
2.1.3. The prM/M Protein
2.1.4. NS1 Protein
2.1.5. NS2A Protein
2.1.6. NS2B Protein
2.1.7. NS3 Protein
2.1.8. NS4A Protein
2.1.9. NS4B Protein
2.1.10. NS5 Protein
| Viral Protein | Position in the Genome (Nucleotides) | Main Role | References |
|---|---|---|---|
| C | 97-465 |
| [62,81,82,85,132] |
| prM/M | 466-741-742-966 |
| [62,94,133] |
| E | 967-2469 |
| [48,62,86] |
| NS1 | 2470-3525 |
| [62,98,101,102,103] |
| NS2A | 3526-4218 |
| [62,113,134] |
| NS2B | 4219-4611 |
| [48,62,67] |
| NS3 | 4612-6468 |
| [48,62,118,119,120] |
| NS4A | 6469-6915 |
| [62] |
| NS4B | 6916-7680 |
| [62,125,135] |
| NS5 | 768-10395 |
| [62,67,128,129,130,131] |
2.2. The Life Cycle of WNV
3. Genetic Diversification within the WNV Species
4. WNV Ecology
4.1. Virus Transmission
4.2. Biological Vectors of WNV
4.3. WNV Reservoirs
5. Pathogenesis of WNV
5.1. Pathogenesis of Neuroinvasive Form
5.1.1. Hematogenous Route
5.1.2. Virus Passive Migration
5.1.3. The “Trojan Horse” Mechanism
5.1.4. Transneuronal Mechanism
5.2. Pathogenesis of the Cutaneous Form
5.3. Pathogenesis of the Gastrointestinal Form
5.4. Renal Form
6. Clinical Presentation, Epidemiology, Infection Sequalae, and Persistence
6.1. Clinical Presentation and Epidemiology
6.2. Sequalae of WNV Infection
6.3. Persistence of WNV Infection
6.4. WNV Surper-Infection
6.5. Coinfections of WNV with Other Pathogens
7. West Nile Virus in Reptiles
8. Diagnostic Approaches of WNV
9. Biomarkers of WNV Infection
10. WNV and One Health
11. Control of WNV Infection and Disease
11.1. Vaccination and Vaccine Development
11.2. Other Control Strategies
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Milošević, B.; Urošević, A.; Poluga, J.; Lavadinović, L.; Nedelijković, J.; Jevtović, D.; Dulović, O. Outbreak of West Nile virus infection among humans in Serbia, August to October 2012. Euro. Surveill. 2013, 18, 20613. [Google Scholar] [CrossRef]
- Brandler, S.; Tangy, F. Vaccines in development against West Nile virus. Viruses 2013, 5, 2384–2409. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Marfin, A.A.; Gubler, D.J. West nile virus. JAMA 2003, 290, 524–528. [Google Scholar] [CrossRef]
- Phalen, D.N.; Dahlhausen, B. West Nile virus. Semin. Avian Exot. Pet. Med. 2004, 13, 67–78. [Google Scholar] [CrossRef]
- van der Meulen, K.M.; Pensaert, M.B.; Nauwynck, H.J. West Nile virus in the vertebrate world. Arch. Virol 2005, 150, 637–657. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr. Top. Microbiol. Immunol. 2002, 267, 309–322. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Godsey, M.S.; King, R.J.; Guptill, S.C. Avian diversity and West Nile virus: Testing associations between biodiversity and infectious disease risk. Proc. Biol. Sci. 2006, 273, 109–117. [Google Scholar] [CrossRef]
- Kinney, R.M.; Huang, C.Y.; Whiteman, M.C.; Bowen, R.A.; Langevin, S.A.; Miller, B.R.; Brault, A.C. Avian virulence and thermostable replication of the North American strain of West Nile virus. J. Gen. Virol 2006, 87, 3611–3622. [Google Scholar] [CrossRef]
- McLean, R.; Ubico, S. Arboviruses in Birds. In Infectious Diseases of Wild Birds; Thomas, N., Hunter, D., Atkinson, C., Eds.; Blacwell Publishing: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Höfle, U.; Blanco, J.M.; Crespo, E.; Naranjo, V.; Jimenez-Clavero, M.A.; Sanchez, A.; la Fuente, J.; Gortazar, C. West Nile virus in the endangered Spanish imperial eagle. Vet. Microbiol. 2008, 129, 171–178. [Google Scholar] [CrossRef]
- Tag-El-Din-Hassan, H.T.; Sasaki, N.; Moritoh, K.; Torigoe, D.; Maeda, A.; Agui, T. The chicken 2′-5′ oligoadenylate synthetase A inhibits the replication of West Nile virus. Jpn. J. Vet. Res. 2012, 60, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Vo, N.P.; Boros, Á.; Pankovics, P.; Reuter, G.; Li, O.T.; Wang, C.; Deng, X.; Poon, L.L.; Delwart, E. The viruses of wild pigeon droppings. PLoS ONE 2013, 8, 72787. [Google Scholar] [CrossRef]
- Nyamwaya, D.; Wang’ondu, V.; Amimo, J.; Michuki, G.; Ogugo, M.; Ontiri, E.; Sang, R.; Lindahl, J.; Grace, D.; Bett, B. Detection of West Nile virus in wild birds in Tana River and Garissa Counties, Kenya. BMC Infect. Dis. 2016, 16, 696. [Google Scholar] [CrossRef]
- Jiménez-Clavero, M.A.; Sotelo, E.; Fernandez-Pinero, J.; Llorente, F.; Blanco, J.M.; Rodriguez-Ramos, J.; Perez-Ramirez, E.; Höfle, U. West Nile virus in golden eagles, Spain, 2007. Emerg. Infect. Dis. 2008, 14, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, E.; Schuffenecker, I.; Korimbocus, J.; Reynard, S.; Murri, S.; Kayser, Y.; Gauthier-Clerc, M.; Sabatier, P.; Zeller, H.G. West Nile virus in wild resident birds, Southern France, 2004. Vector Borne Zoonotic Dis. 2007, 7, 448–452. [Google Scholar] [CrossRef]
- Stockman, J.; Hawkins, M.G.; Burns, R.E.; Fang, Y.; Brault, A.C.; Lowenstine, L.J. West Nile virus infection in a green-winged macaw (Ara chloropterus). Avian Dis. 2010, 54, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T. West Nile Virus and Equine Encephalitis Viruses. Vet. Clin. N. Am. Equine Pract. 2014, 30, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, E.; Bernard, C.; Lecollinet, S.; Rakotoharinome, V.M.; Ravaomanana, J.; Roger, M.; Olive, M.-M.; Meenowa, D.; Jaumally, M.R.; Melanie, J. West Nile virus infection in horses, Indian ocean. Comp. Immunol. Microbiol. Infect. Dis. 2017, 53, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, G.; Mete, A.; Adaska, J.; Anderson, M.; Symmes, K.; Diab, S. West Nile Virus Infection in Sheep. Vet. Pathol. 2017, 54, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Ariel, E. Viruses in reptiles. Vet. Res. 2011, 42, 100. [Google Scholar] [CrossRef] [PubMed]
- Machain-Williams, C.; Padilla-Paz, S.E.; Weber, M.; Cetina-Trejo, R.; Juarez-Ordaz, J.A.; Loroño-Pino, M.A.; Ulloa, A.; Wang, C.; Garcia-Rejon, J.; Blitvich, B.J. Antibodies to West Nile virus in wild and farmed crocodiles in southeastern Mexico. J. Wildl. Dis. 2013, 49, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.R.; Hughes, D.F.; Meshaka, W.E.; Coleman, C.; Henning, J.D. Wild snakes harbor West Nile virus. One Health 2016, 2, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Egberink, H.; Addie, D.D.; Boucraut-Baralon, C.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Horzinek, M.C.; Hosie, M.J.; Marsilio, F.; Lloret, A. West Nile virus infection in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2015, 17, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Root, J.J. West Nile virus associations in wild mammals: A synthesis. Arch. Virol. 2013, 158, 735–752. [Google Scholar] [CrossRef]
- Rossi, S.L.; Ross, T.M.; Evans, J.D. West Nile virus. Clin. Lab. Med. 2010, 30, 47–65. [Google Scholar] [CrossRef]
- Steinman, A.; Banet-Noach, C.; Tal, S.; Levi, O.; Simanov, L.; Perk, S.; Malkinson, M.; Shpigel, N. West Nile virus infection in crocodiles. Emerg. Infect. Dis. 2003, 9, 887–889. [Google Scholar] [CrossRef]
- Suthar, M.S.; Diamond, M.S.; Gale, M., Jr. West Nile virus infection and immunity. Nat. Rev. Microbiol. 2013, 11, 115–128. [Google Scholar] [CrossRef]
- Marschang, R.E. Viruses infecting reptiles. Viruses 2011, 3, 2087–2126. [Google Scholar] [CrossRef]
- Muñoz, J.; Ruiz, S.; Soriguer, R.; Alcaide, M.; Viana, D.S.; Roiz, D.; Vázquez, A.; Figuerola, J. Feeding patterns of potential West Nile virus vectors in south-west Spain. PLoS ONE 2012, 7, 39549. [Google Scholar] [CrossRef]
- Dauphin, G.; Zientara, S.; Zeller, H.; Murgue, B. West Nile: Worldwide current situation in animals and humans. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 343–355. [Google Scholar] [CrossRef]
- Garcia, M.N.; Hasbun, R.; Murray, K.O. Persistence of West Nile virus. Microb. Infect. 2015, 17, 163–168. [Google Scholar] [CrossRef]
- Sbrana, E.; Tonry, J.H.; Xiao, S.-Y.; da Rosa, A.P.T.; Higgs, S.; Tesh, R.B. Oral transmission of West Nile virus in a hamster model. Am. J. Trop. Med. Hyg. 2005, 72, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Nir, Y.; Beemer, A.; Goldwasser, R. West Nile Virus infection in mice following exposure to a viral aerosol. Br. J. Exp. Pathol. 1965, 46, 443. [Google Scholar] [PubMed]
- Hinckley, A.F.; O′Leary, D.R.; Hayes, E.B. Transmission of West Nile virus through human breast milk seems to be rare. Pediatrics 2007, 119, 666–671. [Google Scholar] [CrossRef] [PubMed]
- CDC. Intrauterine West Nile virus infection–New York. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 1135–1136. [Google Scholar]
- CDC. Possible West Nile virus transmission to an infant through breast-feeding–Michigan. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 877. [Google Scholar]
- Jacobson, E.R.; Ginn, P.E.; Troutman, J.M.; Farina, L.; Stark, L.; Klenk, K.; Burkhalter, K.L.; Komar, N. West Nile virus infection in farmed American alligators (Alligator mississippiensis) in Florida. J. Wildl. Dis. 2005, 41, 96–106. [Google Scholar] [CrossRef]
- Klenk, K.; Snow, J.; Morgan, K.; Bowen, R.; Stephens, M.; Foster, F.; Gordy, P.; Beckett, S.; Komar, N.; Gubler, D. Alligators as West Nile virus amplifiers. Emerg. Infect. Dis. 2004, 10, 2150–2155. [Google Scholar] [CrossRef]
- Nevarez, J.G.; Mitchell, M.A.; Kim, D.Y.; Poston, R.; Lampinen, H.M. West Nile virus in alligator, Alligator mississippiensis, ranches from Louisiana. J. Herpetol. Med. Surg. 2005, 15, 4–9. [Google Scholar] [CrossRef]
- Habarugira, G.; Moran, J.; Colmant, A.M.; Davis, S.S.; O’Brien, C.A.; Hall-Mendelin, S.; McMahon, J.; Hewitson, G.; Nair, N.; Barcelon, J. Mosquito-Independent Transmission of West Nile virus in Farmed Saltwater Crocodiles (Crocodylus porosus). Viruses 2020, 12, 198. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: Biology, Transmission, and Human Infection. Clin. Microbiol. Rev. 2012, 25, 635. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O′Leary, D.R.; Campbell, G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Eaton, E.; Concepcion, W.; Blackburn, B. West Nile virus encephalitis acquired via liver transplantation and clinical response to intravenous immunoglobulin: Case report and review of the literature. Transpl. Infect. Dis. 2011, 13, 312–317. [Google Scholar] [CrossRef]
- Cervantes, D.T.; Chen, S.; Sutor, L.J.; Stonecipher, S.; Janoski, N.; Wright, D.J.; Busch, M.P. West Nile virus infection incidence based on donated blood samples and neuroinvasive disease reports, Northern Texas, USA, 2012. Emerg. Infect. Dis. 2015, 21, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Pacenti, M.; Franchin, E.; Pagni, S.; Martello, T.; Cattai, M.; Cusinato, R.; Palù, G. Excretion of West Nile virus in urine during acute infection. J. Infect. Dis. 2013, 208, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.; Umeyama, Y.; Hama, N.; Kobayashi, T.; Han, N.; Wada, H.; Seino, K.I. Interleukin-34, a comprehensive review. J. Leukoc. Biol. 2018, 104, 931–951. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.; Colpitts, T.M. A Brief Review of West Nile Virus Biology. Methods Mol. Biol. 2016, 1435, 1–13. [Google Scholar] [CrossRef]
- Martin, M.-F.; Nisole, S. West Nile Virus Restriction in Mosquito and Human Cells: A Virus under Confinement. Vaccines 2020, 8, 256. [Google Scholar] [CrossRef]
- Byas, A.D.; Ebel, G.D. Comparative Pathology of West Nile Virus in Humans and Non-Human Animals. Pathogens 2020, 9, 48. [Google Scholar] [CrossRef]
- Esser, H.J.; Mögling, R.; Cleton, N.B.; van der Jeugd, H.; Sprong, H.; Stroo, A.; Koopmans, M.P.G.; de Boer, W.F.; Reusken, C.B.E.M. Risk factors associated with sustained circulation of six zoonotic arboviruses: A systematic review for selection of surveillance sites in non-endemic areas. Parasites Vectors 2019, 12, 265. [Google Scholar] [CrossRef]
- Gray, T.J.; Webb, C.E. A review of the epidemiological and clinical aspects of West Nile virus. Int. J. Gen. Med. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Mbanzulu, K.M.; Mboera, L.E.G.; Luzolo, F.K.; Wumba, R.; Misinzo, G.; Kimera, S.I. Mosquito-borne viral diseases in the Democratic Republic of the Congo: A review. Parasites Vectors 2020, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Ferrarini, I.; Rigo, A.; Gandini, A.; Vinante, F. West Nile Virus Encephalitis in Haematological Setting: Report of Two Cases and a Brief Review of the Literature. Mediterr. J. Hematol. Infect. Dis. 2019, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Yamanaka, A.; Konishi, E. A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine 2014, 32, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Kousoulas, K. A review of vaccine approaches for West Nile virus. Int. J. Environ. Res. Public Health 2013, 10, 4200–4223. [Google Scholar] [CrossRef] [PubMed]
- Jiménez de Oya, N.; Escribano-Romero, E.; Blázquez, A.-B.; Martín-Acebes, M.A.; Saiz, J.-C. Current Progress of Avian Vaccines Against West Nile Virus. Vaccines 2019, 7, 126. [Google Scholar] [CrossRef]
- Beasley, D.; Davis, C.; Whiteman, M.; Granwehr, B.; Kinney, R.; Barrett, A.D. Molecular determinants of virulence of West Nile virus in North America. In Emergence and Control of Zoonotic Viral Encephalitides; Springer: Berlin/Heidelberg, Germany, 2004; pp. 35–41. [Google Scholar] [CrossRef]
- Hayes, E.B.; Sejvar, J.J.; Zaki, S.R.; Lanciotti, R.S.; Bode, A.V.; Campbell, G.L. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1174–1179. [Google Scholar] [CrossRef]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef]
- Dubrau, D.; Tortorici, M.A.; Rey, F.A.; Tautz, N. A positive-strand RNA virus uses alternative protein-protein interactions within a viral protease/cofactor complex to switch between RNA replication and virion morphogenesis. PLoS Pathog. 2017, 13, 1006134. [Google Scholar] [CrossRef]
- Brinton, M.A. The molecular biology of West Nile Virus: A new invader of the western hemisphere. Annu Rev. Microbiol 2002, 56, 371–402. [Google Scholar] [CrossRef]
- Bressanelli, S.; Stiasny, K.; Allison, S.L.; Stura, E.A.; Duquerroy, S.; Lescar, J.; Heinz, F.X.; Rey, F.A. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004, 23, 728–738. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Wang, M.; Cheng, A. Innate immune evasion mediated by flaviviridae non-structural proteins. Viruses 2017, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.T.; Mackenzie, J.S.; Bingham, J. Flaviviruses. Dis. Swine 2019. [Google Scholar] [CrossRef]
- Ryu, W.-S. Flaviviruses. In Molecular Virology of Human Pathogenic Viruses; Ryu, W.-S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 165–175. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, H. Flavivirus NS2B/NS3 Protease: Structure, Function, and Inhibition. In Viral Proteases and Their Inhibitors; Gupta, S.P., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 163–188. [Google Scholar] [CrossRef]
- Best, S.M. Flaviviruses. Curr. Biol. 2016, 26, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus entry receptors: An update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Tuplin, A.; Evans, D.; Buckley, A.; Jones, I.; Gould, E.; Gritsun, T. Replication enhancer elements within the open reading frame of tick-borne encephalitis virus and their evolution within the Flavivirus genus. Nucleic Acids Res. 2011, 39, 7034–7048. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.E.; Atkins, J.F. A conserved predicted pseudoknot in the NS2A-encoding sequence of West Nile and Japanese encephalitis flaviviruses suggests NS1’may derive from ribosomal frameshifting. Virol. J. 2009, 6, 14. [Google Scholar] [CrossRef]
- Yun, T.; Ye, W.; Ni, Z.; Zhang, D.; Zhang, C. Identification and molecular characterization of a novel flavivirus isolated from Pekin ducklings in China. Vet. Microbiol. 2012, 157, 311–319. [Google Scholar] [CrossRef]
- Wengler, G.; Wengler, G. Terminal sequences of the genome and replicatioe-form RNA of the flavivirus West Nile virus: Absence of poly (A) and possible role in RNA replication. Virology 1981, 113, 544–555. [Google Scholar] [CrossRef]
- De Filette, M.; Ulbert, S.; Diamond, M.; Sanders, N. Recent progress in West Nile virus diagnosis and vaccination. Vet. Res. 2012, 43, 16. [Google Scholar] [CrossRef]
- Martins, I.C.; Gomes-Neto, F.; Faustino, A.F.; Carvalho, F.A.; Carneiro, F.A.; Bozza, P.T.; Mohana-Borges, R.; Castanho, M.A.; Almeida, F.C.; Santos, N.C. The disordered N-terminal region of dengue virus capsid protein contains a lipid-droplet-binding motif. Biochem. J. 2012, 444, 405–415. [Google Scholar] [CrossRef]
- Botha, E.M.; Markotter, W.; Wolfaardt, M.; Paweska, J.T.; Swanepoel, R.; Palacios, G.; Nel, L.H.; Venter, M. Genetic determinants of virulence in pathogenic lineage 2 West Nile virus strains. Emerg. Infect. Dis. 2008, 14, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Khan, S.A.; Dutta, P.; Topno, R.; Mahanta, J. Characterization of West Nile virus (WNV) isolates from Assam, India: Insights into the circulating WNV in northeastern India. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 39–47. [Google Scholar] [CrossRef] [PubMed]
- van Marle, G.; Antony, J.; Ostermann, H.; Dunham, C.; Hunt, T.; Halliday, W.; Maingat, F.; Urbanowski, M.D.; Hobman, T.; Peeling, J. West Nile virus-induced neuroinflammation: Glial infection and capsid protein-mediated neurovirulence. J. Virol. 2007, 81, 10933–10949. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.T.; Ma, L.; Burgner, J.W.; Groesch, T.D.; Post, C.B.; Kuhn, R.J. Flavivirus capsid is a dimeric alpha-helical protein. J. Virol. 2003, 77, 7143–7149. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanakantham, R.; Ng, M.-L. West Nile virus and dengue virus capsid protein negates the antiviral activity of human Sec3 protein through the proteasome pathway. Cell. Microbiol. 2013, 15, 1688–1706. [Google Scholar] [CrossRef]
- Byk, L.A.; Iglesias, N.G.; De Maio, F.A.; Gebhard, L.G.; Rossi, M.; Gamarnik, A.V. Dengue virus genome uncoating requires ubiquitination. MBio 2016, 7, e00804–e00816. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Zu, X.; Liu, Y.; Chen, L.; Zhu, X.; Zhang, L.; Zhou, Z.; Xiao, G.; Wang, W. The ubiquitin-proteasome system is essential for the productive entry of Japanese encephalitis virus. Virology 2016, 498, 116–127. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yoshii, K.; Phongphaew, W.; Muto, M.; Hirano, M.; Orba, Y.; Sawa, H.; Kariwa, H. West Nile virus capsid protein inhibits autophagy by AMP-activated protein kinase degradation in neurological disease development. PLoS Pathog. 2020, 16, 1008238. [Google Scholar] [CrossRef]
- Oh, W.; Yang, M.-R.; Lee, E.-W.; Park, K.-M.; Pyo, S.; Yang, J.-S.; Lee, H.-W.; Song, J. Jab1 mediates cytoplasmic localization and degradation of West Nile virus capsid protein. J. Biol. Chem. 2006, 281, 30166–30174. [Google Scholar] [CrossRef]
- Yang, J.-S.; Ramanathan, M.P.; Muthumani, K.; Choo, A.Y.; Jin, S.-H.; Yu, Q.-C.; Hwang, D.S.; Choo, D.K.; Lee, M.D.; Dang, K. Induction of inflammation by West Nile virus capsid through the caspase-9 apoptotic pathway. Emerg. Infect. Dis. 2002, 8, 1379. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.; Stiasny, K. Flaviviruses and their antigenic structure. J. Clin. Virol. 2012, 55, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Mandl, C.W.; Guirakhoo, F.; Holzmann, H.; Heinz, F.X.; Kunz, C. Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J. Virol. 1989, 63, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.A.; Heinz, F.X.; Mandl, C.; Kunz, C.; Harrison, S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 1995, 375, 291–298. [Google Scholar] [CrossRef]
- Slon Campos, J.L.; Mongkolsapaya, J.; Screaton, G.R. The immune response against flaviviruses. Nat. Immunol. 2018, 19, 1189–1198. [Google Scholar] [CrossRef]
- Adams, S.; Broom, A.; Sammels, L.; Hartnett, A.; Howard, M.; Coelen, R.; Mackenzie, J.; Hall, R. Glycosylation and antigenic variation among Kunjin virus isolates. Virology 1995, 206, 49–56. [Google Scholar] [CrossRef]
- Ebel, G.D.; Carricaburu, J.; Young, D.; Bernard, K.A.; Kramer, L.D. Genetic and phenotypic variation of West Nile virus in New York. Am. J. Trop. Med. Hyg. 2004, 71, 493–500. [Google Scholar] [CrossRef]
- Hanna, S.L.; Pierson, T.C.; Sanchez, M.D.; Ahmed, A.A.; Murtadha, M.M.; Doms, R.W. N-linked glycosylation of west nile virus envelope proteins influences particle assembly and infectivity. J. Virol. 2005, 79, 13262–13274. [Google Scholar] [CrossRef]
- Wengler, G.; Castle, E.; Leidner, U.; Nowak, T.; Wengler, G. Sequence analysis of the membrane protein V3 of the flavivirus West Nile virus and of its gene. Virology 1985, 147, 264–274. [Google Scholar] [CrossRef]
- Beasley, D.W.; Whiteman, M.C.; Zhang, S.; Huang, C.Y.-H.; Schneider, B.S.; Smith, D.R.; Gromowski, G.D.; Higgs, S.; Kinney, R.M.; Barrett, A.D. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J. Virol. 2005, 79, 8339–8347. [Google Scholar] [CrossRef]
- Scherret, J.; Mackenzie, J.; Khromykh, A.; Hall, R. Biological significance of glycosylation of the envelope protein of Kunjin virus. Ann. N. Y. Acad. Sci. 2001, 951, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Edmonds, J.H.; Williams, D.T.; Setoh, Y.X.; Bielefeldt-Ohmann, H.; Suen, W.W.; Hobson-Peters, J.; van den Hurk, A.F.; Pyke, A.T.; Hall-Mendelin, S. Virulence and evolution of West Nile virus, Australia, 1960–2012. Emerg. Infect. Dis. 2016, 22, 1353. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, P.D.; Langevin, S.A.; Bolling, B.G.; Andrade, C.C.; Engle, X.A.; Ramey, W.N.; Bosco-Lauth, A.; Bowen, R.A.; Sanders, T.A.; Huang, C.Y.H.; et al. N-linked glycosylation of the West Nile virus envelope protein is not a requisite for avian virulence or vector competence. PLoS Negl. Trop. Dis. 2019, 13, 0007473. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Young, P.R. The flavivirus NS1 protein: Molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef]
- Rastogi, M.; Sharma, N.; Singh, S.K. Flavivirus NS1: A multifaceted enigmatic viral protein. Virol. J. 2016, 13, 131. [Google Scholar] [CrossRef]
- Hall, R.A.; Khromykh, A.A.; Mackenzie, J.M.; Scherret, J.H.; Khromykh, T.I.; Mackenzie, J.S. Loss of dimerisation of the nonstructural protein NS1 of Kunjin virus delays viral replication and reduces virulence in mice, but still allows secretion of NS1. Virology 1999, 264, 66–75. [Google Scholar] [CrossRef]
- Gutsche, I.; Coulibaly, F.; Voss, J.E.; Salmon, J.; d′Alayer, J.; Ermonval, M.; Larquet, E.; Charneau, P.; Krey, T.; Megret, F.; et al. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc. Natl. Acad. Sci. USA 2011, 108, 8003–8008. [Google Scholar] [CrossRef]
- Mackenzie, J.M.; Jones, M.K.; Young, P.R. Immunolocalization of the Dengue Virus Nonstructural Glycoprotein NS1 Suggests a Role in Viral RNA Replication. Virology 1996, 220, 232–240. [Google Scholar] [CrossRef]
- Chung, K.M.; Liszewski, M.K.; Nybakken, G.; Davis, A.E.; Townsend, R.R.; Fremont, D.H.; Atkinson, J.P.; Diamond, M.S. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. USA 2006, 103, 19111–19116. [Google Scholar] [CrossRef]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef]
- Macdonald, J.; Tonry, J.; Hall, R.A.; Williams, B.; Palacios, G.; Ashok, M.S.; Jabado, O.; Clark, D.; Tesh, R.B.; Briese, T.; et al. NS1 protein secretion during the acute phase of West Nile virus infection. J. Virol. 2005, 79, 13924–13933. [Google Scholar] [CrossRef]
- Zainah, S.; Wahab, A.H.; Mariam, M.; Fauziah, M.K.; Khairul, A.H.; Roslina, I.; Sairulakhma, A.; Kadimon, S.S.; Jais, M.S.; Chua, K.B. Performance of a commercial rapid dengue NS1 antigen immunochromatography test with reference to dengue NS1 antigen-capture ELISA. J. Virol. Methods 2009, 155, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Setoh, Y.X.; Biron, R.M.; Sester, D.P.; Kim, K.S.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. The West Nile virus-like flavivirus Koutango is highly virulent in mice due to delayed viral clearance and the induction of a poor neutralizing antibody response. J. Virol. 2014, 88, 9947–9962. [Google Scholar] [CrossRef] [PubMed]
- Suen, W.W.; Prow, N.A.; Setoh, Y.X.; Hall, R.A.; Bielefeldt-Ohmann, H. End-point disease investigation for virus strains of intermediate virulence as illustrated by flavivirus infections. J. Gen. Virol. 2016, 97, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Castro-Jorge, L.A.; Machado, P.R.; Fávero, C.A.; Borges, M.C.; Passos, L.M.; de Oliveira, R.M.; Fonseca, B.A. Clinical evaluation of the NS1 antigen-capture ELISA for early diagnosis of dengue virus infection in Brazil. J. Med. Virol. 2010, 82, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Suen, W.; Uddin, M.; Wang, W.; Brown, V.; Adney, D.; Broad, N.; Prow, N.; Bowen, R.; Hall, R.; Bielefeldt-Ohmann, H. Experimental West Nile virus infection in rabbits: An alternative model for studying induction of disease and virus control. Pathogens 2015, 4, 529. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Scanlon, D.; Shiell, B.J.; Mackenzie, J.S.; Hall, R.A. Identification and analysis of truncated and elongated species of the flavivirus NS1 protein. Virus Res. 1999, 60, 67–79. [Google Scholar] [CrossRef]
- Melian, E.B.; Hinzman, E.; Nagasaki, T.; Firth, A.E.; Wills, N.M.; Nouwens, A.S.; Blitvich, B.J.; Leung, J.; Funk, A.; Atkins, J.F. NS1′ of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frameshifting and plays a role in viral neuroinvasiveness. J. Virol. 2010, 84, 1641–1647. [Google Scholar] [CrossRef]
- Leung, J.Y.; Pijlman, G.P.; Kondratieva, N.; Hyde, J.; Mackenzie, J.M.; Khromykh, A.A. Role of Nonstructural Protein NS2A in Flavivirus Assembly. J. Virol. 2008, 82, 4731. [Google Scholar] [CrossRef]
- Chappell, K.; Stoermer, M.; Fairlie, D.; Young, P. West Nile Virus NS2B/NS3 protease as an antiviral target. Curr. Med. Chem. 2008, 15, 2771–2784. [Google Scholar] [CrossRef]
- Chappell, K.J.; Stoermer, M.J.; Fairlie, D.P.; Young, P.R. Mutagenesis of the West Nile virus NS2B cofactor domain reveals two regions essential for protease activity. J. Gen. Virol. 2008, 89, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Donchenko, A.P.; Koonin, E.V.; Blinov, V.M. N-terminal domains of putative helicases of flavi-and pestiviruses may be serine proteases. Nucleic Acids Res. 1989, 17, 3889–3897. [Google Scholar] [CrossRef]
- Wengler, G.; Czaya, G.; Färber, P.M.; Hegemann, J.H. In vitro synthesis of West Nile virus proteins indicates that the amino-terminal segment of the NS3 protein contains the active centre of the protease which cleaves the viral polyprotein after multiple basic amino acids. J. Gen. Virol. 1991, 72, 851–858. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Koonin, E.V.; Donchenko, A.P.; Blinov, V.M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989, 17, 4713–4730. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, S.A.; Chernov, A.V.; Aleshin, A.E.; Shiryaeva, T.N.; Strongin, A.Y. NS4A regulates the ATPase activity of the NS3 helicase: A novel cofactor role of the non-structural protein NS4A from West Nile virus. J. Gen. Virol. 2009, 90, 2081–2085. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, E.; Milani, M.; Bollati, M.; Selisko, B.; Peyrane, F.; Pandini, V.; Sorrentino, G.; Canard, B.; Konarev, P.V.; Svergun, D.I. Crystal structure and activity of Kunjin virus NS3 helicase; protease and helicase domain assembly in the full length NS3 protein. J. Mol. Biol. 2007, 372, 444–455. [Google Scholar] [CrossRef]
- Li, K.; Phoo, W.W.; Luo, D. Functional interplay among the flavivirus NS3 protease, helicase, and cofactors. Virol. Sin. 2014, 29, 74–85. [Google Scholar] [CrossRef][Green Version]
- White, G.; Ottendorfer, C.; Graham, S.; Unnasch, T.R. Competency of reptiles and amphibians for eastern equine encephalitis virus. Am. Soc. Trop. Med. Hyg. 2011, 85, 421–425. [Google Scholar] [CrossRef]
- Ambrose, R.; Mackenzie, J. A conserved peptide in West Nile virus NS4A protein contributes to proteolytic processing and is essential for replication. J. Virol. 2011, 85, 11274–11282. [Google Scholar] [CrossRef]
- Wicker, J.A.; Whiteman, M.C.; Beasley, D.W.; Davis, C.T.; McGee, C.E.; Lee, J.C.; Higgs, S.; Kinney, R.M.; Huang, C.Y.-H.; Barrett, A.D. Mutational analysis of the West Nile virus NS4B protein. Virology 2012, 426, 22–33. [Google Scholar] [CrossRef]
- Youn, S.; Li, T.; McCune, B.T.; Edeling, M.A.; Fremont, D.H.; Cristea, I.M.; Diamond, M.S. Evidence for a genetic and physical interaction between nonstructural proteins NS1 and NS4B that modulates replication of West Nile virus. J. Virol. 2012, 86, 7360–7371. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Shan, C.; Deng, C.-L.; Ye, H.-Q.; Shi, P.-Y.; Yuan, Z.-M.; Gong, P.; Zhang, B. The Interface between Methyltransferase and Polymerase of NS5 Is Essential for Flavivirus Replication. PLoS Negl. Trop. Dis. 2014, 8, 2891. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A.; Tan, S.E.; Selisko, B.; Slade, R.; Hobson-Peters, J.; Canard, B.; Hughes, M.; Leung, J.Y.; Balmori-Melian, E.; Hall-Mendelin, S.; et al. Monoclonal antibodies to the West Nile virus NS5 protein map to linear and conformational epitopes in the methyltransferase and polymerase domains. J. Gen. Virol 2009, 90, 2912–2922. [Google Scholar] [CrossRef] [PubMed]
- Baleotti, F.G.; Moreli, M.L.; Figueiredo, L.T.M. Brazilian Flavivirus phylogeny based on NS5. Mem. Inst. Oswaldo Cruz 2003, 98, 379–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Best, S.M. The Many Faces of the Flavivirus NS5 Protein in Antagonism of Type I Interferon Signaling. J. Virol. 2017, 91, 01970-16. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Thurmond, S.; Hai, R.; Song, J. Structure and function of Zika virus NS5 protein: Perspectives for drug design. Cell. Mol. Life Sci. 2018, 75, 1723–1736. [Google Scholar] [CrossRef]
- Shah, P.S.; Link, N.; Jang, G.M.; Sharp, P.P.; Zhu, T.; Swaney, D.L.; Johnson, J.R.; Von Dollen, J.; Ramage, H.R.; Satkamp, L.; et al. Comparative Flavivirus-Host Protein Interaction Mapping Reveals Mechanisms of Dengue and Zika Virus Pathogenesis. Cell 2018, 175, 1931–1945. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, C.; Wu, J.; Nerurkar, V.R.; Yanagihara, R.; Lu, Y. Inhibition of west nile virus replication by retrovirus-delivered small interfering RNA in human neuroblastoma cells. J. Med. Virol. 2008, 80, 930–936. [Google Scholar] [CrossRef]
- Mukherjee, S.; Lin, T.-Y.; Dowd, K.A.; Manhart, C.J.; Pierson, T.C. The Infectivity of prM-Containing Partially Mature West Nile Virus Does Not Require the Activity of Cellular Furin-Like Proteases. J. Virol. 2011, 85, 12067. [Google Scholar] [CrossRef]
- Melian, E.B.; Edmonds, J.H.; Nagasaki, T.K.; Hinzman, E.; Floden, N.; Khromykh, A.A. West Nile virus NS2A protein facilitates virus-induced apoptosis independently of interferon response. J. Gen. Virol. 2013, 94, 308–313. [Google Scholar] [CrossRef]
- Welte, T.; Xie, G.; Wicker, J.A.; Whiteman, M.C.; Li, L.; Rachamallu, A.; Barrett, A.; Wang, T. Immune responses to an attenuated West Nile virus NS4B-P38G mutant strain. Vaccine 2011, 29, 4853–4861. [Google Scholar] [CrossRef]
- Girard, Y.A.; Popov, V.; Wen, J.; Han, V.; Higgs, S. Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2005, 42, 429–444. [Google Scholar] [CrossRef]
- Girard, Y.A.; Klingler, K.A.; Higgs, S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoonotic Dis. 2004, 4, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Mueller, C.R.; Fuchs, J.F.; McElroy, K.; Wessely, V.; Higgs, S.; Christensen, B.M. Evaluation of the function of a type I peritrophic matrix as a physical barrier for midgut epithelium invasion by mosquito-borne pathogens in Aedes aegypti. Vector Borne Zoonotic Dis. 2008, 8, 701–712. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; McLean, B.J.; Bielefeldt-Ohmann, H.; Hobson-Peters, J.; Hall, R.A.; van den Hurk, A.F. The insect-specific Palm Creek virus modulates West Nile virus infection in and transmission by Australian mosquitoes. Parasit Vectors 2016, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Colmant, A.M.G.; Hall-Mendelin, S.; Ritchie, S.A.; Bielefeldt-Ohmann, H.; Harrison, J.J.; Newton, N.D.; O’Brien, C.A.; Cazier, C.; Johansen, C.A.; Hobson-Peters, J.; et al. The recently identified flavivirus Bamaga virus is transmitted horizontally by Culex mosquitoes and interferes with West Nile virus replication in vitro and transmission in vivo. PLoS Negl. Trop. Dis. 2018, 12, 0006886. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.S.; Soong, L.; Coffey, L.L.; Stevenson, H.L.; McGee, C.E.; Higgs, S. Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile virus infection. PLoS ONE 2010, 5, 11704. [Google Scholar] [CrossRef]
- Davis, C.W.; Nguyen, H.-Y.; Hanna, S.L.; Sánchez, M.D.; Doms, R.W.; Pierson, T.C. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 2006, 80, 1290–1301. [Google Scholar] [CrossRef]
- Lee, E.; Hall, R.A.; Lobigs, M. Common E protein determinants for attenuation of glycosaminoglycan-binding variants of Japanese encephalitis and West Nile viruses. J. Virol. 2004, 78, 8271–8280. [Google Scholar] [CrossRef] [PubMed]
- Tassaneetrithep, B.; Burgess, T.H.; Granelli-Piperno, A.; Trumpfheller, C.; Finke, J.; Sun, W.; Eller, M.A.; Pattanapanyasat, K.; Sarasombath, S.; Birx, D.L. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003, 197, 823–829. [Google Scholar] [CrossRef]
- Kimura, T.; Ohyama, A. Association between the pH-dependent conformational change of West Nile flavivirus E protein and virus-mediated membrane fusion. J. Gen. Virol. 1988, 69, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.A.; Fegan, M.; O′Riley, K.; Motha, J.; Warner, S. Molecular characterization and phylogenetic analysis of Murray Valley encephalitis virus and West Nile virus (Kunjin subtype) from an arbovirus disease outbreak in horses in Victoria, Australia, in 2011. J. Vet. Diagn. Invest. 2013, 25, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Jimenez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Euro. Surveill. 2015, 20. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A.; Scherret, J.H.; Mackenzie, J.S. Kunjin virus: An Australian variant of West Nile. Ann. N. Y. Acad. Sci. 2001, 951, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Papa, A. West Nile virus infections in Greece: An update. Expert Rev. Anti-Infect. Ther. 2012, 10, 743–750. [Google Scholar] [CrossRef]
- Papa, A.; Bakonyi, T.; Xanthopoulou, K.; Vázquez, A.; Tenorio, A.; Nowotny, N. Genetic characterization of West Nile virus lineage 2, Greece, 2010. Emerg. Infect. Dis. 2011, 17, 920. [Google Scholar] [CrossRef]
- Pachler, K.; Lebl, K.; Berer, D.; Rudolf, I.; Hubalek, Z.; Nowotny, N. Putative new West Nile virus lineage in Uranotaenia unguiculata mosquitoes, Austria, 2013. Emerg. Infect. Dis. 2014, 20, 2119. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, E.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef]
- Bakonyi, T.; Hubálek, Z.; Rudolf, I.; Nowotny, N. Novel flavivirus or new lineage of West Nile virus, central Europe. Emerg. Infect. Dis. 2005, 11, 225–231. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Ebel, G.D.; Deubel, V.; Kerst, A.J.; Murri, S.; Meyer, R.; Bowen, M.; McKinney, N.; Morrill, W.E.; Crabtree, M.B.; et al. Complete Genome Sequences and Phylogenetic Analysis of West Nile Virus Strains Isolated from the United States, Europe, and the Middle East. Virology 2002, 298, 96–105. [Google Scholar] [CrossRef]
- Bagnarelli, P.; Marinelli, K.; Trotta, D.; Monachetti, A.; Tavio, M.; Del Gobbo, R.; Capobianchi, M.; Menzo, S.; Nicoletti, L.; Magurano, F. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Euro. Surveill. 2011, 16, 20002. [Google Scholar] [PubMed]
- Bowen, R.A.; Bosco-Lauth, A.; Syvrud, K.; Thomas, A.; Meinert, T.R.; Ludlow, D.R.; Cook, C.; Salt, J.; Ons, E. Protection of horses from West Nile virus Lineage 2 challenge following immunization with a whole, inactivated WNV lineage 1 vaccine. Vaccine 2014, 32, 5455–5459. [Google Scholar] [CrossRef]
- Ciccozzi, M.; Peletto, S.; Cella, E.; Giovanetti, M.; Lai, A.; Gabanelli, E.; Acutis, P.L.; Modesto, P.; Rezza, G.; Platonov, A.E. Epidemiological history and phylogeography of West Nile virus lineage 2. Infect. Genet. Evol. 2013, 17, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Sanchez-Seco, M.P.; Campelo, C.; de Ory, F.; Martin, O.; Herrero, L.; Salmeron Beliz, O.J.; Minguito, T.; Campos, M.C.; Molero, F.; et al. Imported Human West Nile Virus Lineage 2 Infection in Spain: Neurological and Gastrointestinal Complications. Viruses 2020, 12, 156. [Google Scholar] [CrossRef]
- Erdélyi, K.; Ursu, K.; Ferenczi, E.; Szeredi, L.; Rátz, F.; Skáre, J.; Bakonyi, T. Clinical and pathologic features of lineage 2 West Nile virus infections in birds of prey in Hungary. Vector Borne Zoonotic Dis. 2007, 7, 181–188. [Google Scholar] [CrossRef]
- Aliota, M.T.; Kramer, L.D. Replication of West Nile virus, Rabensburg lineage in mammalian cells is restricted by temperature. Parasites Vectors 2012, 5, 293. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Jones, S.A.; Dupuis, A.P., II; Ciota, A.T.; Hubalek, Z.; Kramer, L.D. Characterization of Rabensburg Virus, a Flavivirus Closely Related to West Nile Virus of the Japanese Encephalitis Antigenic Group. PLoS ONE 2012, 7, 39387. [Google Scholar] [CrossRef] [PubMed]
- ECDC. West Nile Virus Transmission Season Starting in Europe: First Case Reported, in Russia. Available online: https://www.ecdc.europa.eu/en/news-events/west-nile-virus-transmission-season-starting-europe-first-case-reported-russia (accessed on 29 April 2020).
- Lvov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Kovtunov, A.I.; Prilipov, A.G.; Kinney, R.; Aristova, V.A.; Dzharkenov, A.F.; Samokhvalov, E.I.; Savage, H.M.; et al. West Nile virus and other zoonotic viruses in Russia: Examples of emerging-reemerging situations. Arch. Virol. Suppl. 2004. [Google Scholar] [CrossRef]
- Vazquez, A.; Sanchez-Seco, M.P.; Ruiz, S.; Molero, F.; Hernandez, L.; Moreno, J.; Magallanes, A.; Tejedor, C.G.; Tenorio, A. Putative new lineage of west nile virus, Spain. Emerg. Infect. Dis. 2010, 16, 549–552. [Google Scholar] [CrossRef]
- Butenko, A.M.; Semashko, I.V.; Skvortsova, T.M.; Gromashevskii, V.L.; Kondrashina, N.G. Detection of the Koutango virus (Flavivirus, Togaviridae) in Somalia. Med. Parazitol. (Mosk) 1986, 3, 65–68. [Google Scholar]
- Coz, J.; Valade, M.; Cornet, M.; Robin, Y. Transovarian transmission of a Flavivirus, the Koutango virus, in Aedes aegypti L. C R Acad. Hebd. Seances Acad. Sci. D 1976, 283, 109–110. [Google Scholar]
- Charrel, R.; Brault, A.; Gallian, P.; Lemasson, J.-J.; Murgue, B.; Murri, S.; Pastorino, B.; Zeller, H.; De Chesse, R.; De Micco, P. Evolutionary relationship between Old World West Nile virus strains: Evidence for viral gene flow between Africa, the Middle East, and Europe. Virology 2003, 315, 381–388. [Google Scholar] [CrossRef]
- Fall, G.; Diallo, M.; Loucoubar, C.; Faye, O.; Sall, A.A. Vector Competence of Culex neavei and Culex quinquefasciatus (Diptera: Culicidae) from Senegal for Lineages 1, 2, Koutango and a Putative New Lineage of West Nile Virus. Am. J. Trop. Med. Hyg. 2014, 90, 747–754. [Google Scholar] [CrossRef]
- Barrera, R.; MacKay, A.; Amador, M.; Vasquez, J.; Smith, J.; Díaz, A.; Acevedo, V.; Cabán, B.; Hunsperger, E.; Muñoz-Jordán, J. Mosquito vectors of West Nile virus during an epizootic outbreak in Puerto Rico. J. Med. Entomol. 2014, 47, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Fall, A.G.; Diaïté, A.; Seck, M.T.; Bouyer, J.; Lefrançois, T.; Vachiéry, N.; Aprelon, R.; Faye, O.; Konaté, L.; Lancelot, R. West Nile virus transmission in sentinel chickens and potential mosquito vectors, Senegal River Delta, 2008–2009. Int. J. Environ. Res. Public Health 2013, 10, 4718–4727. [Google Scholar] [CrossRef] [PubMed]
- Bartlow, W.A.; Manore, C.; Xu, C.; Kaufeld, A.K.; Del Valle, S.; Ziemann, A.; Fairchild, G.; Fair, M.J. Forecasting Zoonotic Infectious Disease Response to Climate Change: Mosquito Vectors and a Changing Environment. Vet. Sci. 2019, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Jernigan, D.B.; Guasch, A.; Trepka, M.J.; Blackmore, C.G.; Hellinger, W.C.; Pham, S.M.; Zaki, S.; Lanciotti, R.S.; Lance-Parker, S.E. Transmission of West Nile virus from an organ donor to four transplant recipients. N. Engl. J. Med. 2003, 348, 2196–2203. [Google Scholar] [CrossRef]
- CDC. West Nile virus infection among turkey breeder farm workers–Wisconsin. MMWR. Morb. Mortal. Wkly. Rep. 2003, 52, 1017. [Google Scholar]
- CDC. Possible dialysis-related west nile virus transmission–Georgia. MMWR. Morb. Mortal. Wkly. Rep. 2004, 53, 738. [Google Scholar]
- CDC. Laboratory-acquired West Nile virus infections–United States. MMWR. Morb. Mortal. Wkly. Rep. 2002, 51, 1133. [Google Scholar]
- CDC. Update: West Nile virus screening of blood donations and transfusion-associated transmission–United States. MMWR. Morb. Mortal. Wkly. Rep. 2004, 53, 281. [Google Scholar]
- Pealer, L.N.; Marfin, A.A.; Petersen, L.R.; Lanciotti, R.S.; Page, P.L.; Stramer, S.L.; Stobierski, M.G.; Signs, K.; Newman, B.; Kapoor, H. Transmission of West Nile virus through blood transfusion in the United States in 2002. N. Engl. J. Med. 2003, 349, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Mauel, M.J.; Baldwin, C.; Burtle, G.; Ingram, D.; Hines, M.E. West Nile virus in farmed alligators. Emerg. Infect. Dis. 2003, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Banet-Noach, C.; Simanov, L.; Malkinson, M. Direct (non-vector) transmission of West Nile virus in geese. Avian Pathol. 2003, 32, 489–494. [Google Scholar] [CrossRef]
- Lindsey, N.P.; Staples, J.E.; Lehman, J.A.; Fischer, M. Medical Risk Factors for Severe West Nile Virus Disease, United States, 2008–2010. Am. J. Trop. Med. Hyg. 2012, 87, 179–184. [Google Scholar] [CrossRef]
- Yango, A.F.; Fischbach, B.V.; Levy, M.; Chandrakantan, A.; Tan, V.; Spak, C.; Melton, L.; Rice, K.; Barri, Y.; Rajagopal, A.; et al. West Nile virus infection in kidney and pancreas transplant recipients in the Dallas-Fort Worth Metroplex during the 2012 Texas epidemic. Transplantation 2014, 97, 953–957. [Google Scholar] [CrossRef]
- Pisani, G.; Cristiano, K.; Pupella, S.; Liumbruno, G.M. West Nile Virus in Europe and Safety of Blood Transfusion. Transfus. Med. Hemother. 2016, 43, 158–167. [Google Scholar] [CrossRef]
- Betsem, E.; Kaidarova, Z.; Stramer, S.L.; Shaz, B.; Sayers, M.; LeParc, G.; Custer, B.; Busch, M.P.; Murphy, E.L. Correlation of West Nile Virus Incidence in Donated Blood with West Nile Neuroinvasive Disease Rates, United States, 2010–2012. Emerg. Infect. Dis. 2017, 23, 212–219. [Google Scholar] [CrossRef]
- Kumar, D.; Drebot, M.A.; Wong, S.J.; Lim, G.; Artsob, H.; Buck, P.; Humar, A. A seroprevalence study of West Nile virus infection in solid organ transplant recipients. Am. J. Transplant. 2004, 4, 1883–1888. [Google Scholar] [CrossRef]
- Dong, E.; Morris, K.; Sodhi, G.; Chang, D.; Czer, L.; Chung, J.; Zabner, R.; Raastad, K.; Klapper, E.; Kobashigawa, J.; et al. Neuroinvasive West Nile Virus Post-Heart Transplantation: A Case Report. Transplant. Proc. 2018, 50, 4057–4061. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Meza, J.; Schweitzer, B.; Shafer, L.; Kalil, A.C.; Sambol, A.R. Seroprevalence of West Nile virus infection in solid organ transplant recipients. Transpl. Infect. Dis. 2010, 12, 120–126. [Google Scholar] [CrossRef]
- Gomez, A.J.; Waggoner, J.J.; Itoh, M.; Hollander, S.A.; Gutierrez, K.M.; Budvytiene, I.; Banaei, N.; Pinsky, B.A. Fatal West Nile virus encephalitis in a heart transplant recipient. J. Clin. Microbiol. 2015, 53, 2749–2752. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, K.; Embil, J.M.; McKibbin, L.R.; McEachern, J.; Drebot, M.A. West Nile Virus infection in a renal transplant recipient resulting in polioencephalomylelitis, quadriplegia, and global brain atrophy. IDCases 2019, 17, 00551. [Google Scholar] [CrossRef] [PubMed]
- Komar, N. West Nile virus: Epidemiology and ecology in North America. Adv. Virus Res. 2003, 61, 185–234. [Google Scholar] [CrossRef] [PubMed]
- Balenghien, T.; Vazeille, M.; Grandadam, M.; Schaffner, F.; Zeller, H.; Reiter, P.; Sabatier, P.; Fouque, F.; Bicout, D.J. Vector Competence of Some French Culex and Aedes Mosquitoes for West Nile Virus. Vector Borne Zoonotic Dis. 2008, 8, 589–596. [Google Scholar] [CrossRef]
- Golding, N.; Nunn, M.A.; Medlock, J.M.; Purse, B.V.; Vaux, A.G.; Schäfer, S.M. West Nile virus vector Culex modestus established in southern England. Parasites Vectors 2012, 5, 32. [Google Scholar] [CrossRef]
- Fros, J.J.; Miesen, P.; Vogels, C.B.; Gaibani, P.; Sambri, V.; Martina, B.E.; Koenraadt, C.J.; van Rij, R.P.; Vlak, J.M.; Takken, W.; et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health 2015, 1, 31–36. [Google Scholar] [CrossRef]
- Vogels, C.B.; Göertz, G.P.; Pijlman, G.P.; Koenraadt, C.J. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes Infect. 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Jupp, P.G. The Ecology of West Nile Virus in South Africa and the Occurrence of Outbreaks in Humans. Ann. N. Y. Acad. Sci. 2001, 951, 143–152. [Google Scholar] [CrossRef]
- Uejio, C.K.; Kemp, A.; Comrie, A.C. Climatic Controls on West Nile Virus and Sindbis Virus Transmission and Outbreaks in South Africa. Vector Borne Zoonotic Dis. 2011, 12, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Work, T.; Hurlbut, H.; Rizk, F. A Study of the Ecology of West Nile Virus in Egypt1. Am. J. Trop. Med. Hyg. 1956, 5, 579–620. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J. West Nile fever--a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999, 5, 643. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Saron, M.; Diop, O.; Mathiot, C.; Adeniji, J.; Olaleye, O. West Nile virus in mosquitoes and febrile patients in a semi-arid zone, Nigeria. J. Am. Sci. 2006, 2, 28–34. [Google Scholar]
- Miller, B.R.; Nasci, R.S.; Godsey, M.S.; Savage, H.M.; Lutwama, J.J.; Lanciotti, R.S.; Peters, C.J. First field evidence for natural vertical transmission of West Nile virus in Culex univittatus complex mosquitoes from Rift Valley Province, Kenya. Am. J. Trop. Med. Hyg. 2000, 62, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, A.; Ferguson, H.H.; Mendez-Sanchez, J.D.; Danis-Lozano, R.; Casas-Martinez, M.; Bond, J.G.; Garcia-Zebadua, J.C.; Orozco-Bonilla, A.; Juarez-Ordaz, J.A.; Farfan-Ale, J.A.; et al. West Nile virus activity in mosquitoes and domestic animals in Chiapas, Mexico. Vector Borne Zoonotic Dis. 2009, 9, 555–560. [Google Scholar] [CrossRef]
- Anderson, J.F.; Andreadis, T.G.; Vossbrinck, C.R.; Tirrell, S.; Wakem, E.M.; French, R.A.; Garmendia, A.E.; Van Kruiningen, H.J. Isolation of West Nile Virus from Mosquitoes, Crows, and a Cooper’s Hawk in Connecticut. Science 1999, 286, 2331. [Google Scholar] [CrossRef]
- Sardelis, M.R.; Turell, M.J. Ochlerotatus j. japonicus in Frederick County, Maryland: Discovery, distribution, and vector competence for West Nile virus. J. Am. Mosq. Control. Assoc. 2001, 17, 137–141. [Google Scholar]
- CDC. Mosquito Species in which West Nile Virus Has Been Detected–United States, 1999–2016. 2017. Available online: https://www.cdc.gov/westnile/resources/pdfs/MosquitoSpecies1999-2016.pdf (accessed on 29 April 2020).
- DeGroote, J.P.; Sugumaran, R.; Brend, S.M.; Tucker, B.J.; Bartholomay, L.C. Landscape, demographic, entomological, and climatic associations with human disease incidence of West Nile virus in the state of Iowa, USA. Int. J. Health Geogr. 2008, 7, 19. [Google Scholar] [CrossRef]
- Goddard, L.B.; Roth, A.E.; Reisen, W.K.; Scott, T.W. Vector competence of California mosquitoes for West Nile virus. Emerg. Infect. Dis. 2002, 8, 1385–1391. [Google Scholar] [CrossRef]
- Sardelis, M.R.; Turell, M.J.; Dohm, D.J.; O′Guinn, M.L. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 2001, 7, 1018–1022. [Google Scholar] [CrossRef]
- Reiter, P. West Nile virus in Europe: Understanding the present to gauge the future. Eurosurveillance 2010, 15, 19508. [Google Scholar] [CrossRef]
- Bolisetty, S.; Wheaton, G.; Whelan, P.; Smith, D.; Brown, A. Reappearance of human cases due to Murray Valley encephalitis virus and Kunjin virus in Central Australia after an absence of 26 years. Commun. Dis. Intell. Q. Rep. 2002, 26, 39. [Google Scholar]
- Mackenzie, J.S.; Lindsay, M.D.; Coelen, R.J.; Broom, A.K.; Hall, R.A.; Smith, D.W. Arboviruses causing human disease in the Australasian zoogeographic region. Arch. Virol 1994, 136, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Russell, R. Arboviruses and their vectors in Australia: An update on the ecology and epidemiology of some mosquito-borne arboviruses. Med. Vet. Entomol. 1995, 83, 141–158. [Google Scholar]
- Orshan, L.; Bin, H.; Schnur, H.; Kaufman, A.; Valinsky, A.; Shulman, L.; Weiss, L.; Mendelson, E.; Pener, H. Mosquito vectors of West Nile fever in Israel. J. Med. Entomol. 2008, 45, 939–947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paramasivan, R.; Mishra, A.; Mourya, D. West Nile virus: The Indian scenario. Indian J. Med. Res. 2003, 118, 101–108. [Google Scholar] [PubMed]
- Mumcuoglu, K.Y.; Banet-Noach, C.; Malkinson, M.; Shalom, U.; Galun, R. Argasid ticks as possible vectors of West Nile virus in Israel. Vector Borne Zoonotic Dis. 2005, 5, 65–71. [Google Scholar] [CrossRef]
- Sixl, W.; Stünzner, D.; Withalm, H. Serological examinations for antibodies against West Nile virus, Semlikivirus and chikungunyavirus in laboratory mice, parasitized by nidicole fauna from swallow’s nests. Geogr. Med. Suppl. 1988, 1, 51–55. [Google Scholar]
- Murgue, B.; Zeller, H.; Deubel, V. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. In Japanese Encephalitis and West Nile Viruses; Springer: Berlin/Heidelberg, Germany, 2002; pp. 195–221. [Google Scholar] [CrossRef]
- McLean, R.; Ubico, S.; Bourne, D.; Komar, N. West Nile virus in livestock and wildlife. In Japanese Encephalitis and West Nile Viruses; Springer: Berlin/Heidelberg, Germany, 2002; pp. 271–308. [Google Scholar] [CrossRef]
- Ludwig, G.V.; Calle, P.P.; Mangiafico, J.A.; Raphael, B.L.; Danner, D.K.; Hile, J.A.; Clippinger, T.L.; Smith, J.F.; Cook, R.A.; McNamara, T. An outbreak of West Nile virus in a New York City captive wildlife population. Am. J. Trop. Med. Hyg. 2002, 67, 67–75. [Google Scholar] [CrossRef]
- Campbell, G.L.; Ceianu, C.S.; Savage, H.M. Epidemic West Nile encephalitis in Romania: Waiting for history to repeat itself. Ann. N. Y. Acad. Sci. 2001, 951, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg. Infect. Dis. 2002, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Work, T.H.; Hurlbut, H.S.; Taylor, R. Indigenous Wild Birds of the Nile Delta as Potential West Nile Virus Circulating Reservoirs1. Am. J. Trop. Med. Hyg. 1955, 4, 872–888. [Google Scholar] [CrossRef]
- Eckstrand, C.; Woods, L.; Diab, S.S.; Crossley, B.; Giannitti, F. Diagnostic exercise: High mortality in a flock of chukar partridge chicks (Alectoris chukar) in California. Vet. Pathol. 2015, 52, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Wünschmann, A.; Ziegler, A. West Nile virus–associated mortality events in domestic Chukar partridges (Alectoris chukar) and domestic Impeyan pheasants (Lophophorus impeyanus). Avian Dis. 2006, 50, 456–459. [Google Scholar] [CrossRef]
- Ward, M.R.; Stallknecht, D.E.; Willis, J.; Conroy, M.J.; Davidson, W.R. Wild bird mortality and West Nile virus surveillance: Biases associated with detection, reporting, and carcass persistence. J. Wildl. Dis. 2006, 42, 92–106. [Google Scholar] [CrossRef]
- Yaremych, S.A.; Warner, R.E.; Mankin, P.C.; Brawn, J.D.; Raim, A.; Novak, R. West Nile virus and high death rate in American crows. Emerg. Infect. Dis. 2004, 10, 709–711. [Google Scholar] [CrossRef]
- LaDeau, S.L.; Kilpatrick, A.M.; Marra, P.P. West Nile virus emergence and large-scale declines of North American bird populations. Nature 2007, 447. [Google Scholar] [CrossRef]
- Panella, N.A.; Kerst, A.J.; Lanciotti, R.S.; Bryant, P.; Wolf, B.; Komar, N. Comparative West Nile virus detection in organs of naturally infected American Crows (Corvus brachyrhynchos). Emerg. Infect. Dis. 2001, 7, 754–755. [Google Scholar] [CrossRef]
- Steele, K.E.; Linn, M.J.; Schoepp, R.J.; Komar, N.; Geisbert, T.W.; Manduca, R.M.; Calle, P.P.; Raphael, B.L.; Clippinger, T.L.; Larsen, T.; et al. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City. Vet. Pathol 2000, 37, 208–224. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; LaDeau, S.L.; Marra, P.P. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk 2007, 124, 1121–1136. [Google Scholar] [CrossRef]
- DeCarlo, C.; Omar, A.H.; Haroun, M.I.; Bigler, L.; Bin Rais, M.N.; Abu, J.; Omar, A.R.; Mohammed, H.O. Potential Reservoir and Associated Factors for West Nile Virus in Three Distinct Climatological Zones. Vector Borne Zoonotic Dis. 2017, 17, 709–713. [Google Scholar] [CrossRef]
- Root, J.J.; Bentler, K.T.; Nemeth, N.M.; Gidlewski, T.; Spraker, T.R.; Franklin, A.B. Experimental infection of raccoons (Procyon lotor) with West Nile virus. Am. J. Trop Med. Hyg. 2010, 83, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Bentler, K.T.; Hall, J.S.; Root, J.J.; Klenk, K.; Schmit, B.; Blackwell, B.F.; Ramey, P.C.; Clark, L. Serologic evidence of West Nile virus exposure in North American mesopredators. Am. J. Trop. Med. Hyg. 2007, 76, 173–179. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Juarez, L.I.; Tucker, B.J.; Rowley, W.A.; Platt, K.B. Antibodies to West Nile virus in raccoons and other wild peridomestic mammals in Iowa. J. Wildl. Dis. 2009, 45, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Platt, K.B.; Tucker, B.J.; Halbur, P.G.; Blitvich, B.J.; Fabiosa, F.G.; Mullin, K.; Parikh, G.R.; Kitikoon, P.; Bartholomay, L.C.; Rowley, W.A. Fox squirrels (Sciurus niger) develop West Nile virus viremias sufficient for infecting select mosquito species. Vector Borne Zoonotic Dis. 2008, 8, 225–233. [Google Scholar] [CrossRef]
- Lim, S.M.; Koraka, P.; Osterhaus, A.D.; Martina, B.E. West Nile virus: Immunity and pathogenesis. Viruses 2011, 3, 811–828. [Google Scholar] [CrossRef]
- Briant, L.; Desprès, P.; Choumet, V.; Missé, D. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology 2014, 464, 26–32. [Google Scholar] [CrossRef]
- Martín-Fontecha, A.; Lanzavecchia, A.; Sallusto, F. Dendritic Cell Migration to Peripheral Lymph Nodes. In Dendritic Cells; Lombardi, G., Riffo-Vasquez, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 31–49. [Google Scholar] [CrossRef]
- Ben-Nathan, D.; Huitinga, I.; Lustig, S.; Van Rooijen, N.; Kobiler, D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch. Virol. 1996, 141, 459–469. [Google Scholar] [CrossRef]
- Diamond, M.S.; Shrestha, B.; Marri, A.; Mahan, D.; Engle, M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 2003, 77, 2578–2586. [Google Scholar] [CrossRef]
- Byrne, S.N.; Halliday, G.M.; Johnston, L.J.; King, N.J. Interleukin-1β but not tumor necrosis factor is involved in West Nile virus-induced Langerhans cell migration from the skin in C57BL/6 mice. J. Investig. Dermatol. 2001, 117, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tapia, D.; Loiacono, C.M.; Kleiboeker, S.B. Replication of West Nile virus in equine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 2006, 110, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.N.; Kent, K.A.; Bennett, C.J.; Bernard, K.A. Tissue tropism and neuroinvasion of West Nile virus do not differ for two mouse strains with different survival rates. Virology 2007, 368, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Gamino, V.; Höfle, U. Pathology and tissue tropism of natural West Nile virus infection in birds: A review. Vet. Res. 2013, 44, 39. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Bernard, K.A. West Nile virus infection in birds and mammals. Ann. N. Y. Acad. Sci. 2001, 951, 84–93. [Google Scholar] [CrossRef]
- Bernard, K.A.; Maffei, J.G.; Jones, S.A.; Kauffman, E.B.; Ebel, G.; Dupuis, A., 2nd; Ngo, K.A.; Nicholas, D.C.; Young, D.M.; Shi, P.Y.; et al. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg. Infect. Dis. 2001, 7, 679–685. [Google Scholar] [CrossRef]
- Homer, M.J.; Aguilar-Delfin, I.; Telford, S.R., 3rd; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef]
- DeBiasi, R.L. West Nile Virus Neuroinvasive Disease. Curr. Infect. Dis. Rep. 2011, 13, 350–359. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Tyler, K.L. West Nile virus meningoencephalitis. Nat. Clin. Pract. Neurol. 2006, 2, 264–275. [Google Scholar] [CrossRef]
- Davis, L.E.; DeBiasi, R.; Goade, D.E.; Haaland, K.Y.; Harrington, J.A.; Harnar, J.B.; Pergam, S.A.; King, M.K.; DeMasters, B.; Tyler, K.L. West Nile virus neuroinvasive disease. Ann. Neurol. 2006, 60, 286–300. [Google Scholar] [CrossRef]
- Palmieri, C.; Franca, M.; Uzal, F.; Anderson, M.; Barr, B.; Woods, L.; Moore, J.; Woolcock, P.; Shivaprasad, H. Pathology and immunohistochemical findings of West Nile virus infection in psittaciformes. Vet. Pathol. 2011, 48, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.R.; Stone, W.B.; Ebel, G.D.; Young, D.S.; Galinski, D.S.; Pensabene, J.P.; Franke, M.A.; Eidson, M.; Kramer, L.D. Crow deaths caused by West Nile virus during winter. Emerg. Infect. Dis. 2007, 13, 1912. [Google Scholar] [CrossRef]
- Suen, W.; Prow, N.; Hall, R.; Bielefeldt-Ohmann, H. Mechanism of West Nile virus neuroinvasion: A critical appraisal. Viruses 2014, 6, 2796–2825. [Google Scholar] [CrossRef]
- Hasebe, R.; Suzuki, T.; Makino, Y.; Igarashi, M.; Yamanouchi, S.; Maeda, A.; Horiuchi, M.; Sawa, H.; Kimura, T. Transcellular transport of West Nile virus-like particles across human endothelial cells depends on residues 156 and 159 of envelope protein. BMC Microbiol. 2010, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Dahm, T.; Rudolph, H.; Schwerk, C.; Schroten, H.; Tenenbaum, T. Neuroinvasion and Inflammation in Viral Central Nervous System Infections. Mediat. Inflamm. 2016, 2016, 8562805. [Google Scholar] [CrossRef] [PubMed]
- Dropulić, B.; Masters, C.L. Entry of neurotropic arboviruses into the central nervous system: An in vitro study using mouse brain endothelium. J. Infect. Dis. 1990, 161, 685–691. [Google Scholar] [CrossRef]
- Cantile, C.; Del Piero, F.; Di Guardo, G.; Arispici, M. Pathologic and immunohistochemical findings in naturally occurring West Nile virus infection in horses. Vet. Pathol. 2001, 38, 414–431. [Google Scholar] [CrossRef]
- Samuel, M.A.; Diamond, M.S. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 2005, 79, 13350–13361. [Google Scholar] [CrossRef]
- Wang, T.; Town, T.; Alexopoulou, L.; Anderson, J.F.; Fikrig, E.; Flavell, R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004, 10, 1366. [Google Scholar] [CrossRef]
- Garcia-Tapia, D.; Hassett, D.E.; Mitchell, W.J.; Johnson, G.C.; Kleiboeker, S.B. West Nile virus encephalitis: Sequential histopathological and immunological events in a murine model of infection. J. Neurovirol. 2007, 13, 130–138. [Google Scholar] [CrossRef]
- Roe, K.; Kumar, M.; Lum, S.; Orillo, B.; Nerurkar, V.R.; Verma, S. West Nile virus-induced disruption of the blood–brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. J. Gen. Virol. 2012, 93, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kumar, M.; Gurjav, U.; Lum, S.; Nerurkar, V.R. Reversal of West Nile virus-induced blood–brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 2010, 397, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Arjona, A.; Foellmer, H.G.; Town, T.; Leng, L.; McDonald, C.; Wang, T.; Wong, S.J.; Montgomery, R.R.; Fikrig, E.; Bucala, R. Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. J. Clin. Investig. 2007, 117, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Yu, L.; Cao, S.; Wang, K.; Yuan, J.; Wang, C.; Wang, K.; Cui, M.; Fu, Z.F. Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J. Virol. 2015, 89, 5602–5614. [Google Scholar] [CrossRef]
- Bielefeldt-Ohmann, H.; Smirnova, N.P.; Tolnay, A.E.; Webb, B.T.; Antoniazzi, A.Q.; van Campen, H.; Hansen, T.R. Neuro-invasion by a ‘Trojan Horse’ strategy and vasculopathy during intrauterine flavivirus infection. Int. J. Exp. Pathol. 2012, 93, 24–33. [Google Scholar] [CrossRef]
- Samuel, M.A.; Wang, H.; Siddharthan, V.; Morrey, J.D.; Diamond, M.S. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl. Acad. Sci. USA 2007, 104, 17140–17145. [Google Scholar] [CrossRef]
- Wang, H.; Siddharthan, V.; Hall, J.O.; Morrey, J.D. West Nile virus preferentially transports along motor neuron axons after sciatic nerve injection of hamsters. J. Neurovirol. 2009, 15, 293–299. [Google Scholar] [CrossRef]
- Maximova, O.A.; Bernbaum, J.G.; Pletnev, A.G. West Nile Virus Spreads Transsynaptically within the Pathways of Motor Control: Anatomical and Ultrastructural Mapping of Neuronal Virus Infection in the Primate Central Nervous System. PLoS Negl. Trop. Dis. 2016, 10, 0004980. [Google Scholar] [CrossRef]
- Ferguson, D.D.; Gershman, K.; LeBailly, A.; Petersen, L.R. Characteristics of the rash associated with West Nile virus fever. Clin. Infect. Dis. 2005, 41, 1204–1207. [Google Scholar] [CrossRef]
- Anderson, R.C.; Horn, K.B.; Hoang, M.P.; Gottlieb, E.; Bennin, B. Punctate exanthem of West Nile virus infection: Report of 3 cases. J. Am. Acad. Dermatol. 2004, 51, 820–823. [Google Scholar] [CrossRef]
- Appler, K.K.; Brown, A.N.; Stewart, B.S.; Behr, M.J.; Demarest, V.L.; Wong, S.J.; Bernard, K.A. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS ONE 2010, 5, 10649. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.-Y.; Behr, M.J.; Chadwick, C.M.; Shi, P.-Y.; Bernard, K.A. Keratinocytes are cell targets of West Nile virus in vivo. J. Virol. 2011, 85, 5197–5201. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.J.; Osvath, S.R.; Hall, R.A.; King, N.J.; Sedger, L.M. Regulation of antigen processing and presentation molecules in West Nile virus-infected human skin fibroblasts. Virology 2004, 324, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; King, N.J.; Kesson, A.M. The role of tumor necrosis factor in modulating responses of murine embryo fibroblasts by flavivirus, West Nile. Virology 2004, 329, 361–370. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.; King, N.J.; Kesson, A.M. Major histocompatibility complex class I (MHC-I) induction by West Nile virus: Involvement of 2 signaling pathways in MHC-I up-regulation. J. Infect. Dis. 2004, 189, 658–668. [Google Scholar] [CrossRef]
- Fredericksen, B.L.; Smith, M.; Katze, M.G.; Shi, P.-Y.; Gale, M. The host response to West Nile Virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J. Virol. 2004, 78, 7737–7747. [Google Scholar] [CrossRef]
- Jarman, R.V.; Morgan, P.N.; Duffy, C.E. Persistence of West Nile virus in L-929 mouse fibroblasts. Proc. Soc. Exp. Biol. Med. 1968, 129, 633–637. [Google Scholar] [CrossRef]
- Kajaste-Rudnitski, A.; Mashimo, T.; Frenkiel, M.-P.; Guénet, J.-L.; Lucas, M.; Desprès, P. The 2′, 5′-oligoadenylate synthetase 1b is a potent inhibitor of West Nile virus replication inside infected cells. J. Biol. Chem. 2006, 281, 4624–4637. [Google Scholar] [CrossRef]
- Wünschmann, A.; Shivers, J.; Bender, J.; Carroll, L.; Fuller, S.; Saggese, M.; Van Wettere, A.; Redig, P. Pathologic and immunohistochemical findings in goshawks (Accipiter gentilis) and great horned owls (Bubo virginianus) naturally infected with West Nile virus. Avian Dis. 2005, 49, 252–259. [Google Scholar] [CrossRef]
- Nevarez, J.G.; Mitchell, M.A.; Morgan, T.; Roy, A.; Johnson, A. Association of West Nile virus with lymphohistiocytic proliferative cutaneous lesions in American alligators (Alligator mississippiensis) detected by RT-PCR. J. Zoo Wildl. Med. 2008, 39, 562–566. [Google Scholar] [CrossRef]
- Isberg, S.; Moran, J.; De Araujo, R.; Elliott, N.; Davis, S.; Melville, L. First evidence of Kunjin strain of West Nile virus associated with saltwater crocodile (Crocodylus porosus) skin lesions. Aust. Vet. J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vandergaast, R.; Fredericksen, B.L. West Nile virus (WNV) replication is independent of autophagy in mammalian cells. PLoS ONE 2012, 7, 45800. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Kimiagar, I.; Pollak, L.; Gandelman-Marton, R.; Itzhaki, A.; Milo, R.; Rabey, J. Neurological features of West Nile virus infection during the 2000 outbreak in a regional hospital in Israel. J. Neurol. Sci. 2002, 200, 63–66. [Google Scholar] [CrossRef]
- Racsa, L.; Gander, R.; Chung, W.; Southern, P.; Le, J.; Beal, S.; Lee, F.; Cavuoti, D.; Reisch, J.; Alatoom, A. Clinical features of West Nile virus epidemic in Dallas, Texas, 2012. Diagn. Microbiol. Infect. Dis. 2014, 78, 132–136. [Google Scholar] [CrossRef]
- Ceauşu, E.; Erşcoiu, S.; Calistru, P.; Ispas, D.; Dorobăţ, O.; Homoş, M.; Bărbulescu, C.; Cojocaru, I.; Simion, C.; Cristea, C. Clinical manifestations in the West Nile virus outbreak. Rom. J. Virol. 1997, 48, 3–11. [Google Scholar]
- Goldblum, N.; Sterk, V.; Paderski, B. West Nile fever. The clinical features of the disease and the isolation of West Nile virus from the blood of nine human cases. Am. J. Hyg. 1954, 59, 89–103. [Google Scholar]
- Marberg, K.; Goldbltjm, N.; Sterk, V.V.; Jasinska-Klingbehg, W.; Klingberg, M.A. The natural history of West Nile Fever. I. Clinical observations during an epidemic in Israel. Am. J. Hyg. 1956, 64, 259–269. [Google Scholar] [CrossRef]
- Chowers, M.Y.; Lang, R.; Nassar, F.; Ben-David, D.; Giladi, M.; Rubinshtein, E.; Itzhaki, A.; Mishal, J.; Siegman-Igra, Y.; Kitzes, R. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg. Infect. Dis. 2001, 7, 675–678. [Google Scholar] [CrossRef]
- Nevarez, J.G. Lymphohistiocytic Proliferative Syndrome of Alligators (Alligator Mississippiensis): A Cutaneous Manifestation of West. Nile Virus. Ph.D. Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 2007. [Google Scholar]
- Brener, Z.Z.; Harbord, N.B.; Zhuravenko, I.; Nicastri, A.D.; Bergman, M.; Dubrow, A.; Feinfeld, D.; Winchester, J. Acute renal failure in a patient with West Nile viral encephalitis. Nephrol. Dial. Transplant. 2007, 22, 662–663. [Google Scholar] [CrossRef]
- Paddock, C.D.; Nicholson, W.L.; Bhatnagar, J.; Goldsmith, C.S.; Greer, P.W.; Hayes, E.B.; Risko, J.A.; Henderson, C.; Blackmore, C.G.; Lanciotti, R.S. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin. Infect. Dis. 2006, 42, 1527–1535. [Google Scholar] [CrossRef]
- Huang, C.; Slater, B.; Rudd, R.; Parchuri, N.; Hull, R.; Dupuis, M.; Hindenburg, A. First isolation of West Nile virus from a patient with encephalitis in the United States. Emerg. Infect. Dis. 2002, 8, 1367. [Google Scholar] [CrossRef] [PubMed]
- Baty, S.A.; Gibney, K.B.; Staples, J.E.; Patterson, A.B.; Levy, C.; Lehman, J.; Wadleigh, T.; Feld, J.; Lanciotti, R.; Nugent, C.T. Evaluation for West Nile Virus (WNV) RNA in urine of patients within 5 months of WNV infection. J. Infect. Dis. 2012, 205, 1476–1477. [Google Scholar] [CrossRef] [PubMed]
- Tonry, J.H.; Xiao, S.-y.; Siirin, M.; Chen, H.; Da Rosa, A.P.T.; Tesh, R.B. Persistent shedding of West Nile virus in urine of experimentally infected hamsters. Am. J. Trop. Med. Hyg. 2005, 72, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Civen, R.; Villacorte, F.; Robles, D.T.; Dassey, D.E.; Croker, C.; Borenstein, L.; Harvey, S.M.; Mascola, L. West Nile virus infection in the pediatric population. Pediatr. Infect. Dis. J. 2006, 25, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.B.; Trikamji, B.V.; Mathisen, G.E.; Mishra, S.K. Southern California neuroinvasive West Nile virus case series. Neurol. Sci. 2018, 39, 251–257. [Google Scholar] [CrossRef] [PubMed]
- CDC. Acute flaccid paralysis syndrome associated with West Nile virus infection–Mississippi and Louisiana, July-August 2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 825–828. [Google Scholar]
- Yim, R.; Posfay-Barbe, K.M.; Nolt, D.; Fatula, G.; Wald, E.R. Spectrum of clinical manifestations of West Nile virus infection in children. Pediatrics 2004, 114, 1673–1675. [Google Scholar] [CrossRef]
- Roehrig, J.T. West Nile virus in the United States—a historical perspective. Viruses 2013, 5, 3088–3108. [Google Scholar] [CrossRef]
- Sejvar, J.J. Clinical manifestations and outcomes of West Nile virus infection. Viruses 2014, 6, 606–623. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Marfin, A.A. Manifestations of West Nile neuroinvasive disease. Rev. Med. Virol. 2006, 16, 209–224. [Google Scholar] [CrossRef]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef]
- Jiménez de Oya, N.; Camacho, M.-C.; Blázquez, A.-B.; Lima-Barbero, J.-F.; Saiz, J.-C.; Höfle, U.; Escribano-Romero, E. High susceptibility of magpie (Pica pica) to experimental infection with lineage 1 and 2 West Nile virus. PLoS Negl. Trop. Dis. 2018, 12, 0006394. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- D’Agostino, J.J.; Isaza, R. Clinical signs and results of specific diagnostic testing among captive birds housed at zoological institutions and infected with West Nile virus. J. Am. Vet. Med. Assoc. 2004, 224, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Steinman, A.; Banet-Noach, C.; Simanov, L.; Grinfeld, N.; Aizenberg, Z.; Levi, O.; Lahav, D.; Malkinson, M.; Perk, S.; Shpigel, N.Y. Experimental infection of common garter snakes (Thamnophis sirtalis) with West Nile virus. Vector Borne Zoonotic Dis. 2006, 6, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.P.; Florescu, S.A.; Cotar, A.I.; Badescu, D.; Ceianu, C.S.; Zaharia, M.; Tardei, G.; Codreanu, D.; Ceausu, E.; Ruta, S.M. Re-emergence of severe West Nile virus neuroinvasive disease in humans in Romania, 2012 to 2017-implications for travel medicine. Travel Med. Infect. Dis. 2018, 22, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.-F.; Delroux, K.; Wang, X.; Qian, F.; Arjona, A.; Malawista, S.E.; Fikrig, E.; Montgomery, R.R. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J. Virol. 2008, 82, 7613–7623. [Google Scholar] [CrossRef]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef]
- O’Leary, D.R.; Marfin, A.A.; Montgomery, S.P.; Kipp, A.M.; Lehman, J.A.; Biggerstaff, B.J.; Elko, V.L.; Collins, P.D.; Jones, J.E.; Campbell, G.L. The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis. 2004, 4, 61–70. [Google Scholar] [CrossRef]
- Nett, R.; Kuehnert, M.; Ison, M.; Orlowski, J.; Fischer, M.; Staples, J. Current practices and evaluation of screening solid organ donors for West Nile virus. Transpl. Infect. Dis. 2012, 14, 268–277. [Google Scholar] [CrossRef]
- Han, L.L.; Popovici, F.; Alexander, J.P., Jr.; Laurentia, V.; Tengelsen, L.A.; Cernescu, C.; Gary, H.E., Jr.; Ion-Nedelcu, N.; Campbell, G.L.; Tsai, T.F. Risk factors for West Nile virus infection and meningoencephalitis, Romania, 1996. J. Infect. Dis. 1999, 179, 230–233. [Google Scholar] [CrossRef]
- Miramontes, R., Jr.; Lafferty, W.E.; Lind, B.K.; Oberle, M.W. Is agricultural activity linked to the incidence of human West Nile virus. Am. J. Prev. Med. 2006, 30, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Paz, S. Climate change impacts on West Nile virus transmission in a global context. Philos. Trans. R. Soc. B 2015, 370, 20130561. [Google Scholar] [CrossRef] [PubMed]
- OSHA. Workplace Precautions against West Nile Virus. Available online: https://www.osha.gov/dts/shib/WestNileVirus_8-29-03.pdf (accessed on 11 July 2020).
- Hyams, K.C.; Hanson, K.; Stephen Wignall, F.; Escamilla, J.; Oldfield, E.C., III. The impact of infectious diseases on the health of US troops deployed to the Persian Gulf during operations Desert Shield and Desert Storm. Clin. Infect. Dis. 1995, 20, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.J.; Brundage, M.; Cannon, C.; Cox, K.; Clements, T.E.; Cooper, E.D.; Elbert, Y.; Ludwig, G.V.; Mangiafico, J.A.; Malakooti, M. Department of Defense West Nile virus surveillance in 2002. Mil. Med. 2004, 169, 421–428. [Google Scholar] [CrossRef]
- Vonesch, N.; Binazzi, A.; Bonafede, M.; Melis, P.; Ruggieri, A.; Iavicoli, S.; Tomao, P. Emerging zoonotic viral infections of occupational health importance. Pathog. Dis. 2019, 77. [Google Scholar] [CrossRef]
- Trock, S.C.; Meade, B.J.; Glaser, A.L.; Ostlund, E.N.; Lanciotti, R.S.; Cropp, B.C.; Kulasekera, V.; Kramer, L.D.; Komar, N. West Nile virus outbreak among horses in New York State, 1999 and 2000. Emerg. Infect. Dis. 2001, 7, 745–747. [Google Scholar] [CrossRef]
- Shieh, W.-J.; Guarner, J.; Layton, M.; Fine, A.; Miller, J.; Nash, D.; Campbell, G.L.; Roehrig, J.T.; Gubler, D.J.; Zaki, S.R. The role of pathology in an investigation of an outbreak of West Nile encephalitis in New York, 1999. Emerg. Infect. Dis. 2000, 6, 370–372. [Google Scholar] [CrossRef]
- Ellis, A.E.; Mead, D.G.; Allison, A.B.; Stallknecht, D.E.; Howerth, E.W. Pathology and epidemiology of natural West Nile viral infection of raptors in Georgia. J. Wildl. Dis. 2007, 43. [Google Scholar] [CrossRef]
- Gibbs, S.E.J.; Hoffman, D.M.; Stark, L.M.; Marlenee, N.L.; Blitvich, B.J.; Beaty, B.J.; Stallknecht, D.E. Persistence of antibodies to West Nile virus in naturally infected rock pigeons (Columba livia). Clin. Diagn. Lab. Immunol. 2005, 12, 665–667. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saito, E.K.; Sileo, L.; Green, D.E.; Meteyer, C.U.; McLaughlin, G.S.; Converse, K.A.; Docherty, D.E. Raptor mortality due to West Nile virus in the United States, 2002. J. Wildl. Dis. 2007, 43, 206–213. [Google Scholar] [CrossRef]
- Murray, K.O.; Mertens, E.; Desprès, P. West Nile virus and its emergence in the United States of America. Vet. Res. 2010, 41, 67. [Google Scholar] [CrossRef] [PubMed]
- Gancz, A.Y.; Smith, D.A.; Barker, I.K.; Lindsay, R.; Hunter, B. Pathology and tissue distribution of West Nile virus in North American owls (family: Strigidae). Avian Pathol 2006, 35. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Fang, Y.; Lothrop, H.D.; Martinez, V.M.; Wilson, J.; Oconnor, P.; Carney, R.; Cahoon-Young, B.; Shafii, M.; Brault, A.C. Overwintering of West Nile virus in Southern California. J. Med. Entomol 2006, 43. [Google Scholar] [CrossRef]
- Ernest, H.B.; Woods, L.W.; Hoar, B.R. Pathology associated with West Nile virus infections in the yellow-billed magpie (Pica nuttalli): A California endemic bird. J. Wildl. Dis. 2010, 46, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, N.P.; Lehman, J.A.; Staples, J.E.; Fischer, M. West Nile virus and other arboviral diseases—United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 521–526. [Google Scholar]
- Frost, M.J.; Zhang, J.; Edmonds, J.H.; Prow, N.A.; Gu, X.; Davis, R.; Hornitzky, C.; Arzey, K.E.; Finlaison, D.; Hick, P. Characterization of virulent west nile virus kunjin strain, australia, 2011. Emerg. Infect. Dis. 2012, 18, 792–800. [Google Scholar] [CrossRef]
- Sirbu, A.; Ceianu, C.; Panculescu-Gatej, R.; Vazquez, A.; Tenorio, A.; Rebreanu, R.; Niedrig, M.; Nicolescu, G.; Pistol, A. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro. Surveill. 2011, 16, 19762. [Google Scholar]
- Platonov, A.E.; Shipulin, G.A.; Shipulina, O.Y.; Tyutyunnik, E.N.; Frolochkina, T.I.; Lanciotti, R.S.; Yazyshina, S.; Platonova, O.V.; Obukhov, I.L.; Zhukov, A.N. Outbreak of West Nile virus infection, Volgograd Region, Russia, 1999. Emerg. Infect. Dis. 2001, 7, 128–132. [Google Scholar] [CrossRef]
- Papa, A.; Danis, K.; Baka, A.; Bakas, A.; Dougas, G.; Lytras, T.; Theocharopoulos, G.; Chrysagis, D.; Vassiliadou, E.; Kamaria, F.; et al. Ongoing outbreak of West Nile virus infections in humans in Greece, July–August 2010. Euro. Surveill. 2010, 15, 19644. [Google Scholar] [CrossRef] [PubMed]
- Kutasi, O.; Bakonyi, T.; Lecollinet, S.; Biksi, I.; Ferenczi, E.; Bahuon, C.; Sardi, S.; Zientara, S.; Szenci, O. Equine encephalomyelitis outbreak caused by a genetic lineage 2 West Nile virus in Hungary. J. Vet. Intern. Med. 2011, 25, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Magurano, F.; Remoli, M.; Baggieri, M.; Fortuna, C.; Marchi, A.; Fiorentini, C.; Bucci, P.; Benedetti, E.; Ciufolini, M.; Rizzo, C. Circulation of West Nile virus lineage 1 and 2 during an outbreak in Italy. Clin. Microbiol. Infect. 2012, 18, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.; Amitai, Z.; Lustig, Y.; Mor, Z.; Weiberger, M.; Chowers, M.; Maayan, S.; Zimhony, O.; Ben-Ami, R.; Chazan, B. Outbreak of West Nile Virus disease in Israel (2015): A retrospective analysis of notified cases. Travel Med. Infect. Dis. 2019, 28, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Triki, H.; Murri, S.; Le, B.G.; Bahri, O.; Hili, K.; Sidhom, M.; Dellagi, K. West Nile viral meningo-encephalitis in Tunisia. Med. Trop. (Mars) 2001, 61, 487–490. [Google Scholar]
- Nur, Y.A.; Groen, J.; Heuvelmans, H.; Tuynman, W.; Copra, C.; Osterhaus, A. An outbreak of West Nile fever among migrants in Kisangani, Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 1999, 61, 885–888. [Google Scholar] [CrossRef]
- Depoortere, E.; Kavle, J.; Keus, K.; Zeller, H.; Murri, S.; Legros, D. Outbreak of West Nile virus causing severe neurological involvement in children, Nuba Mountains, Sudan, 2002. Trop Med. Int Health 2004, 9, 730–736. [Google Scholar] [CrossRef]
- Hachfi, W.; Bougmiza, I.; Bellazreg, F.; Bahri, O.; Kaabia, N.; Bahri, F.; Letaief, A. Une deuxième épidémie de méningo-encéphalite à virus West Nile en Tunisie. Med. Mal. Infect. 2010, 40, 456–461. [Google Scholar] [CrossRef]
- Zaayman, D.; Venter, M. West Nile virus neurologic disease in humans, South Africa, September 2008–May 2009. Emerg. Infect. Dis. 2012, 18, 2051–2054. [Google Scholar] [CrossRef]
- Mandji Lawson, J.M.; Mounguengui, D.; Ondounda, M.; Nguema Edzang, B.; Vandji, J.; Tchoua, R. [A case of meningo-encephalitis due to West Nile virus in Libreville, Gabon]. Med. Trop. (Mars) 2009, 69, 501–502. [Google Scholar]
- Morvan, J.; Chin, L.; Fontenille, D.; Rakotoarivony, I.; Coulanges, P. The Prevalence of West Nile virus-antibodies among children (5 to 20 years old) in Madagascar. Bull. Soc. Pathol. Exot. 1991, 84, 225–234. [Google Scholar]
- Venter, M.; Human, S.; Van Niekerk, S.; Williams, J.; Van Eeden, C.; Freeman, F. Fatal neurologic disease and abortion in mare infected with lineage 1 West Nile virus, South Africa. Emerg. Infect. Dis. 2011, 17, 1534–1536. [Google Scholar] [CrossRef] [PubMed]
- Simulundu, E.; Ndashe, K.; Chambaro, H.M.; Squarre, D.; Reilly, P.M.; Chitanga, S.; Changula, K.; Mukubesa, A.N.; Ndebe, J.; Tembo, J. West Nile Virus in Farmed Crocodiles, Zambia, 2019. Emerg. Infect. Dis. 2020, 26, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Wilson, M.E.; Sejvar, J.J. The long-term outcomes of human West Nile virus infection. Clin. Infect. Dis. 2007, 44, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Weatherhead, J.E.; Miller, V.E.; Garcia, M.N.; Hasbun, R.; Salazar, L.; Dimachkie, M.M.; Murray, K.O. Long-term neurological outcomes in West Nile virus-infected patients: An observational study. Am. J. Trop. Med. Hyg. 2015, 92, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Hasbun, R.; Garcia, M.N.; Kellaway, J.; Baker, L.; Salazar, L.; Woods, S.P.; Murray, K.O. West Nile Virus Retinopathy and Associations with Long Term Neurological and Neurocognitive Sequelae. PLoS ONE 2016, 11, 0148898. [Google Scholar] [CrossRef]
- Klee, A.L.; Maidin, B.; Edwin, B.; Poshni, I.; Mostashari, F.; Fine, A.; Layton, M.; Nash, D. Long-term prognosis for clinical West Nile virus infection. Emerg. Infect. Dis. 2004, 10, 1405–1411. [Google Scholar] [CrossRef]
- Berg, P.J.; Smallfield, S.; Svien, L. An investigation of depression and fatigue post West Nile virus infection. S. D. Med. 2010, 63, 127–133. [Google Scholar]
- Murray, K.O.; Resnick, M.; Miller, V. Depression after infection with West Nile virus. Emerg. Infect. Dis. 2007, 13, 479–481. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Haddad, M.B.; Tierney, B.C.; Campbell, G.L.; Marfin, A.A.; Van Gerpen, J.A.; Fleischauer, A.; Leis, A.A.; Stokic, D.S.; Petersen, L.R. Neurologic manifestations and outcome of West Nile virus infection. JAMA 2003, 290, 511–515. [Google Scholar] [CrossRef]
- Ouhoumanne, N.; Lowe, A.M.; Fortin, A.; Kairy, D.; Vibien, A.; Lensch, J.K.; Tannenbaum, T.N.; Milord, F. Morbidity, mortality and long-term sequelae of West Nile virus disease in Québec. Epidemiol. Infect. 2018, 146, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Siddharthan, V.; Wang, H.; Motter, N.E.; Hall, J.O.; Skinner, R.D.; Skirpstunas, R.T.; Morrey, J.D. Persistent West Nile Virus Associated with a Neurological Sequela in Hamsters Identified by Motor Unit Number Estimation. J. Virol. 2009, 83, 4251. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.S.; Demarest, V.L.; Wong, S.J.; Green, S.; Bernard, K.A. Persistence of virus-specific immune responses in the central nervous system of mice after West Nile virus infection. BMC Immunol. 2011, 12, 6. [Google Scholar] [CrossRef]
- Pogodina, V.V.; Frolova, M.P.; Malenko, G.V.; Fokina, G.I.; Koreshkova, G.V.; Kiseleva, L.L.; Bochkova, N.G.; Ralph, N.M. Study on West Nile virus persistence in monkeys. Arch. Virol. 1983, 75, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.; Walker, C.; Herrington, E.; Lewis, J.A.; McCormick, J.; Beasley, D.W.; Tesh, R.B.; Fisher-Hoch, S. Persistent infection with West Nile virus years after initial infection. J. Infect. Dis. 2010, 201, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.S.; Langevin, S.A.; Brault, A.C.; Woods, L.; Carroll, B.D.; Reisen, W.K. Detection of persistent West Nile virus RNA in experimentally and naturally infected avian hosts. Am. J. Trop. Med. Hyg. 2012, 87, 559–564. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Vineyard, M.P.; Woods, L.W.; Reisen, W.K. Dynamics of West Nile virus persistence in house sparrows (Passer domesticus). PLoS Negl. Trop. Dis. 2012, 6, 1860. [Google Scholar] [CrossRef]
- Nemeth, N.; Young, G.; Ndaluka, C.; Bielefeldt-Ohmann, H.; Komar, N.; Bowen, R. Persistent West Nile virus infection in the house sparrow (Passer domesticus). Arch. Virol. 2009, 154, 783–789. [Google Scholar] [CrossRef]
- Petersen, L.R. Patient Education: West Nile Virus Infection (Beyond the Basics). Available online: https://www.uptodate.com/contents/west-nile-virus-infection-beyond-the-basics (accessed on 11 July 2020).
- Papa, A.; Anastasiadou, A.; Delianidou, M. West Nile virus IgM and IgG antibodies three years post- infection. Hippokratia 2015, 19, 34–36. [Google Scholar]
- Carson, P.J.; Prince, H.E.; Biggerstaff, B.J.; Lanciotti, R.; Tobler, L.H.; Busch, M. Characteristics of Antibody Responses in West Nile Virus-Seropositive Blood Donors. J. Clin. Microbiol. 2014, 52, 57–60. [Google Scholar] [CrossRef]
- Graham, A.L.; Lamb, T.J.; Read, A.F.; Allen, J.E. Malaria-filaria coinfection in mice makes malarial disease more severe unless filarial infection achieves patency. J. Infect. Dis. 2005, 191, 410–421. [Google Scholar] [CrossRef]
- Telfer, S.; Lambin, X.; Birtles, R.; Beldomenico, P.; Burthe, S.; Paterson, S.; Begon, M. Species interactions in a parasite community drive infection risk in a wildlife population. Science 2010, 330, 243–246. [Google Scholar] [CrossRef]
- Graham, A.L. Ecological rules governing helminth–microparasite coinfection. Proc. Natl. Acad. Sci. USA. 2008, 105, 566–570. [Google Scholar] [CrossRef]
- Graham, A.L.; Cattadori, I.M.; Lloyd-Smith, J.O.; Ferrari, M.J.; Bjørnstad, O.N. Transmission consequences of coinfection: Cytokines writ large. Trends Parasitol. 2007, 23, 284–291. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Etienne, R.S.; Luikart, G.; Beja-Pereira, A.; Jolles, A.E. Hidden consequences of living in a wormy world: Nematode-induced immune suppression facilitates tuberculosis invasion in African buffalo. Am. Nat. 2010, 176, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.H.; Karp, C.L.; Auwaerter, P.G.; Mayer, K.H. Coinfection with HIV and tropical infectious diseases. I. Protozoal pathogens. Clin. Infect. Dis. 2007, 45, 1208–1213. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Lease, J.K.; Dusek, R.J.; Samuel, M.D. Prevalence of pox-like lesions and malaria in forest bird communities on leeward Mauna Loa Volcano, Hawaii. Condor 2005, 107, 537–546. [Google Scholar] [CrossRef]
- Valiant, W.G.; Mattapallil, M.J.; Higgs, S.; Huang, Y.-J.S.; Vanlandingham, D.L.; Lewis, M.G.; Mattapallil, J.J. Simultaneous Coinfection of Macaques with Zika and Dengue Viruses Does not Enhance Acute Plasma Viremia but Leads to Activation of Monocyte Subsets and Biphasic Release of Pro-inflammatory Cytokines. Sci. Rep. 2019, 9, 7877. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, R.; Patriota, J.V.; de Souza, M.D.L.; Felix, A.C.; Mamede, N.; Sanabani, S.S. Investigation into an outbreak of dengue-like illness in Pernambuco, Brazil, revealed a cocirculation of Zika, Chikungunya, and dengue virus type 1. Medicine 2016, 95, 3201. [Google Scholar] [CrossRef]
- Kambris, Z.; Cook, P.E.; Phuc, H.K.; Sinkins, S.P. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 2009, 326, 134–136. [Google Scholar] [CrossRef]
- Griffiths, E.C.; Pedersen, A.B.; Fenton, A.; Petchey, O.L. The nature and consequences of coinfection in humans. J. Infect. 2011, 63, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.C.; Anderson, T.K.; Higashiguchi, J.M.; Kitron, U.D.; Walker, E.D.; Brawn, J.D.; Krebs, B.L.; Ruiz, M.O.; Goldberg, T.L.; Ricklefs, R.E. An inverse association between West Nile virus serostatus and avian malaria infection status. Parasites Vectors 2014, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, J.-B.; Paradkar, P.N. Iron availability affects West Nile virus infection in its mosquito vector. Virol. J. 2017, 14, 103. [Google Scholar] [CrossRef]
- Baba, M.; Logue, C.H.; Oderinde, B.; Abdulmaleek, H.; Williams, J.; Lewis, J.; Laws, T.R.; Hewson, R.; Marcello, A.; Agaro, P.D. Evidence of arbovirus co-infection in suspected febrile malaria and typhoid patients in Nigeria. J. Infect. Dev. Ctries 2013, 7, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Gokozan, H.N.; Ball, M.K.; Otero, J.; McGwire, B.S. Fatal human eosinophilic meningo-encephalitis caused by CNS co-infection with Halicephalobus gingivalis and West Nile virus. Parasitol. Int. 2015, 64, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Dutta, P.; Chowdhury, P.; Borah, J.; Topno, R.; Mahanta, J. Co-infection of arboviruses presenting as Acute Encephalitis Syndrome. J. Clin. Virol. 2011, 51, 5–7. [Google Scholar] [CrossRef]
- Erdem, H.; Ergunay, K.; Yilmaz, A.; Naz, H.; Akata, F.; Inan, A.S.; Ulcay, A.; Gunay, F.; Ozkul, A.; Alten, B.; et al. Emergence and co-infections of West Nile virus and Toscana virus in Eastern Thrace, Turkey. Clin. Microbiol. Infect. 2014, 20, 319–325. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Woods, L.W.; Boyce, W.M.; Eckstrand, C.D.; Langevin, S.A.; Reisen, W.K.; Townsend, A.K. West Nile virus and non-West Nile virus mortality and coinfection of American crows (Corvus brachyrhynchos) in California. Avian Dis. 2014, 58, 255–261. [Google Scholar] [CrossRef]
- Dorigatti, I.; McCormack, C.; Nedjati-Gilani, G.; Ferguson, N.M. Using Wolbachia for Dengue Control: Insights from Modelling. Trends Parasitol. 2018, 34, 102–113. [Google Scholar] [CrossRef]
- Okamoto, K.W.; Amarasekare, P. The biological control of disease vectors. J. Theor. Biol. 2012, 309, 47–57. [Google Scholar] [CrossRef]
- Thomas, M.B. Biological control of human disease vectors: A perspective on challenges and opportunities. Biocontrol (Dordr. Neth.) 2018, 63, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Niang, E.H.A.; Bassene, H.; Fenollar, F.; Mediannikov, O. Biological Control of Mosquito-Borne Diseases: The Potential of Wolbachia-Based Interventions in an IVM Framework. J. Trop. Med. 2018, 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Dodson, B.L.; Hughes, G.L.; Paul, O.; Matacchiero, A.C.; Kramer, L.D.; Rasgon, J.L. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl. Trop. Dis. 2014, 8, 2965. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.L.; Meola, M.A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 2010, 5, 11977. [Google Scholar] [CrossRef] [PubMed]
- Hobson-Peters, J.; Yam, A.W.Y.; Lu, J.W.F.; Setoh, Y.X.; May, F.J.; Kurucz, N.; Walsh, S.; Prow, N.A.; Davis, S.S.; Weir, R. A new insect-specific flavivirus from northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS ONE 2013, 8, 56534. [Google Scholar] [CrossRef]
- Goenaga, S.; Kenney, J.; Duggal, N.; Delorey, M.; Ebel, G.; Zhang, B.; Levis, S.; Enria, D.; Brault, A. Potential for co-infection of a mosquito-specific flavivirus, Nhumirim virus, to block West Nile virus transmission in mosquitoes. Viruses 2015, 7, 5801–5812. [Google Scholar] [CrossRef]
- Colmant, A.M.G.; Bielefeldt-Ohmann, H.; Vet, L.J.; O’Brien, C.A.; Bowen, R.A.; Hartwig, A.E.; Davis, S.; Piyasena, T.B.H.; Habarugira, G.; Harrison, J.J.; et al. NS4/5 mutations enhance flavivirus Bamaga virus infectivity and pathogenicity in vitro and in vivo. PLoS Negl. Trop. Dis. 2020, 14, 0008166. [Google Scholar] [CrossRef]
- Farfan-Ale, J.A.; Blitvich, B.J.; Marlenee, N.L.; Loroño-Pino, M.A.; Puerto-Manzano, F.; García-Rejón, J.E.; Rosado-Paredes, E.P.; Flores-Flores, L.F.; Ortega-Salazar, A.; Chávez-Medina, J. Antibodies to West Nile virus in asymptomatic mammals, birds, and reptiles in the Yucatan Peninsula of Mexico. Am. J. Trop. Med. Hyg. 2006, 74, 908–914. [Google Scholar] [CrossRef]
- Klenk, K.; Komar, N. Poor replication of West Nile virus (New York 1999 strain) in three reptilian and one amphibian species. Am. J. Trop. Med. Hyg. 2003, 69, 260–262. [Google Scholar] [CrossRef]
- Townsend, H.M. Integumentary Lesions Known as “Pix” in the American Alligator (Alligator Mississippiensis). Citeseer: 2008; Volume 70. Available online: https://ufdc.ufl.edu/UFE0022415/00001 (accessed on 11 July 2020).
- Rios, F.M.; Zimmerman, L.M. Immunology of Reptiles. In eLS; John Wiley & Sons, Ltd: Chichester, UK, 2015. [Google Scholar] [CrossRef]
- Mostashari, F.; Bunning, M.L.; Kitsutani, P.T.; Singer, D.A.; Nash, D.; Cooper, M.J.; Katz, N.; Liljebjelke, K.A.; Biggerstaff, B.J.; Fine, A.D. Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet 2001, 358, 261–264. [Google Scholar] [CrossRef]
- Pepperell, C.; Rau, N.; Krajden, S.; Kern, R.; Humar, A.; Mederski, B.; Simor, A.; Low, D.E.; McGeer, A.; Mazzulli, T. West Nile virus infection in 2002: Morbidity and mortality among patients admitted to hospital in southcentral Ontario. CMAJ 2003, 168, 1399–1405. [Google Scholar]
- Abroug, F.; Ouanes-Besbes, L.; Letaief, M.; Romdhane, F.B.; Khairallah, M.; Triki, H.; Bouzouiaia, N. A cluster study of predictors of severe West Nile virus infection. Mayo Clin. Proc. 2006, 81, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Tardei, G.; Ruta, S.; Chitu, V.; Rossi, C.; Tsai, T.; Cernescu, C. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection. J. Clin. Microbiol. 2000, 38, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T.; Nash, D.; Maldin, B.; Labowitz, A.; Martin, D.A.; Lanciotti, R.S.; Campbell, G.L. Persistence of virus-reactive serum immunoglobulin m antibody in confirmed west nile virus encephalitis cases. Emerg. Infect. Dis. 2003, 9, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Broom, A.; Hartnett, A.; Howard, M.; Mackenzie, J. Immunodominant epitopes on the NS1 protein of MVE and KUN viruses serve as targets for a blocking ELISA to detect virus-specific antibodies in sentinel animal serum. J. Virol. Methods 1995, 51, 201–210. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Bowen, R.A.; Marlenee, N.L.; Hall, R.A.; Bunning, M.L.; Beaty, B.J. Epitope-Blocking Enzyme-Linked Immunosorbent Assays for Detection of West Nile Virus Antibodies in Domestic Mammals. J. Clin. Microbiol. 2003, 41, 2676. [Google Scholar] [CrossRef]
- Hukkanen, R.R.; Liggitt, D.H.; Kelley, S.T.; Grant, R.; Anderson, D.; Beaty, B.J.; Marlenee, N.L.; Hall, R.A.; Bielefeldt-Ohmann, H. Comparison of commercially available and novel West Nile virus immunoassays for detection of seroconversion in pig-tailed macaques (Macaca nemestrina). Comp. Med. 2006, 56, 46–54. [Google Scholar] [PubMed]
- Prow, N.; Tan, C.; Wang, W.; Hobson-Peters, J.; Kidd, L.; Barton, A.; Wright, J.; Hall, R.; Bielefeldt-Ohmann, H. Natural exposure of horses to mosquito-borne flaviviruses in south-east Queensland, Australia. Int. J. Environ. Res. Public Health 2013, 10, 4432–4443. [Google Scholar] [CrossRef] [PubMed]
- Kitai, Y.; Kondo, T.; Konishi, E. Non-structural protein 1 (NS1) antibody-based assays to differentiate West Nile (WN) virus from Japanese encephalitis virus infections in horses: Effects of WN virus NS1 antibodies induced by inactivated WN vaccine. J. Virol. Methods 2011, 171, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Higbie, C.T.; Nevarez, J.G.; Roy, A.F.; Piero, F.D. Presence of West Nile Virus RNA in Tissues of American Alligators (Alligator mississippiensis) Vaccinated with a Killed West Nile Virus Vaccine. J. Herpetol. Med. Surg. 2017, 27, 18–21. [Google Scholar] [CrossRef]
- Domingo, C.; Patel, P.; Linke, S.; Achazi, K.; Niedrig, M. Molecular diagnosis of flaviviruses. Future Virol. 2011, 6, 1059–1074. [Google Scholar] [CrossRef]
- Pusterla, N.; Madigan, J.E.; Leutenegger, C. Real-time polymerase chain reaction: A novel molecular diagnostic tool for equine infectious diseases. J. Vet. Intern. Med. 2006, 20, 3–12. [Google Scholar] [CrossRef]
- Beasley, D.W. Recent advances in the molecular biology of West Nile virus. Curr. Mol. Med. 2005, 5, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.-Y.; Kramer, L.D. Molecular detection of West Nile virus RNA. Expert Rev. Mol. Diagn. 2003, 3, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Patrinos, G.P.; Danielson, P.B.; Ansorge, W.J. Molecular Diagnostics: Past, Present, and Future. In Molecular Diagnostics, 3rd ed.; Patrinos, G.P., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 1–11. [Google Scholar] [CrossRef]
- Sun, W. Nucleic extraction and amplification. In Molecular Diagnostics; Elsevier: Amsterdam, The Nederland, 2010; pp. 35–47. [Google Scholar]
- Moureau, G.; Temmam, S.; Gonzalez, J.; Charrel, R.; Grard, G.; De Lamballerie, X. A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector Borne Zoonotic Dis. 2007, 7, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Berthet, F.; Zeller, H.; Drouet, M.; Rauzier, J.; Digoutte, J.; Deubel, V. Extensive nucleotide changes and deletions within the envelope glycoprotein gene of Euro-African West Nile viruses. J. Gen. Virol. 1997, 78, 2293–2297. [Google Scholar] [CrossRef]
- Sampson, B.A.; Ambrosi, C.; Charlot, A.; Reiber, K.; Veress, J.F.; Armbrustmacher, V. The pathology of human West Nile virus infection. Hum. Pathol. 2000, 31, 527–531. [Google Scholar] [CrossRef]
- Bielefeldt-Ohmann, H.; Bosco-Lauth, A.; Hartwig, A.-E.; Uddin, M.J.; Barcelon, J.; Suen, W.W.; Wang, W.; Hall, R.A.; Bowen, R.A. Characterization of non-lethal West Nile Virus (WNV) infection in horses: Subclinical pathology and innate immune response. Microb. Pathog. 2017, 103, 71–79. [Google Scholar] [CrossRef]
- Suen, W. Investigation of Neuropathogenesis and Control of an Equine-pathogenic Australian West Nile Virus Isolate in an Established CD1 Swiss White Mouse and a Novel Rabbit Model. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2017. [Google Scholar]
- Shaikh, N.A.; Ge, J.; Zhao, Y.-X.; Walker, P.; Drebot, M. Development of a Novel, Rapid, and Sensitive Immunochromatographic Strip Assay Specific for West Nile Virus (WNV) IgM and Testing of Its Diagnostic Accuracy in Patients Suspected of WNV Infection. Clin. Chem. 2007, 53, 2031. [Google Scholar] [CrossRef]
- Qian, F.; Thakar, J.; Yuan, X.; Nolan, M.; Murray, K.O.; Lee, W.T.; Wong, S.J.; Meng, H.; Fikrig, E.; Kleinstein, S.H. Immune markers associated with host susceptibility to infection with West Nile virus. Viral Immunol. 2014, 27, 39–47. [Google Scholar] [CrossRef]
- Qian, F.; Goel, G.; Meng, H.; Wang, X.; You, F.; Devine, L.; Raddassi, K.; Garcia, M.N.; Murray, K.O.; Bolen, C.R. Systems immunology reveals markers of susceptibility to West Nile virus infection. Clin. Vaccine Immunol. 2015, 22, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Suen, W.W.; Uddin, M.J.; Prow, N.A.; Bowen, R.A.; Hall, R.A.; Bielefeldt-Ohmann, H. Tissue-specific transcription profile of cytokine and chemokine genes associated with flavivirus control and non-lethal neuropathogenesis in rabbits. Virology 2016, 494, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Town, T.; Qian, F.; Wang, P.; Kamanaka, M.; Connolly, T.M.; Gate, D.; Montgomery, R.R.; Flavell, R.A.; Fikrig, E. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009, 5, 1000610. [Google Scholar] [CrossRef] [PubMed]
- de Waal Malefyt, R.; Haanen, J.; Spits, H.; Roncarolo, M.-G.; te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; De Vries, J. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 1991, 174, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Fraisier, C.; Camoin, L.; Lim, S.; Bakli, M.; Belghazi, M.; Fourquet, P.; Granjeaud, S.; Osterhaus, A.D.; Koraka, P.; Martina, B. Altered protein networks and cellular pathways in severe West Nile disease in mice. PLoS ONE 2013, 8, 68318. [Google Scholar] [CrossRef]
- Baba, M.; Saron, M.; Adeniji, J. The seasonal effect of West Nile Virus in semi-arid zone, Nigeria. Acad. J. 2006, 1, 31–38. [Google Scholar]
- Parkash, V.; Woods, K.; Kafetzopoulou, L.; Osborne, J.; Aarons, E.; Cartwright, K. West Nile Virus Infection in Travelers Returning to United Kingdom from South Africa. Emerg. Infect. Dis. 2019, 25, 367–369. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Daszak, P.; Goodman, S.J.; Rogg, H.; Kramer, L.D.; Cedeno, V.; Cunningham, A.A. Predicting pathogen introduction: West Nile virus spread to Galapagos. Conserv. Biol. 2006, 20, 1224–1231. [Google Scholar] [CrossRef]
- Banet-Noach, C.; Malkinson, M.; Brill, A.; Samina, I.; Yadin, H.; Weisman, Y.; Pokamunski, S.; King, R.; Deubel, V.; Stram, Y. Phylogenetic Relationships of West Nile Viruses Isolated from Birds and Horses in Israel from 1997 to 2001. Virus Genes 2003, 26, 135–141. [Google Scholar] [CrossRef]
- Kilpatrick, A.M. Globalization, land use, and the invasion of West Nile virus. Science 2011, 334, 323–327. [Google Scholar] [CrossRef]
- Bowden, S.E.; Magori, K.; Drake, J.M. Regional differences in the association between land cover and West Nile virus disease incidence in humans in the United States. Am. J. Trop. Med. Hyg. 2011, 84, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, M.; Rizzoli, A.; Metz, M.; Rosà, R.; Marini, G.; Chadwick, E.; Neteler, M. Identifying the environmental conditions favouring West Nile virus outbreaks in Europe. PLoS ONE 2015, 10, e0121158. [Google Scholar] [CrossRef]
- Pradier, S.; Leblond, A.; Durand, B. Land cover, landscape structure, and West Nile virus circulation in southern France. Vector Borne Zoonotic Dis. 2008, 8, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Leblond, A.; Sandoz, A.; Lefebvre, G.; Zeller, H.; Bicout, D. Remote sensing based identification of environmental risk factors associated with West Nile disease in horses in Camargue, France. Prev. Vet. Med. 2007, 79, 20–31. [Google Scholar] [CrossRef]
- Hartley, D.M.; Barker, C.M.; Le Menach, A.; Niu, T.; Gaff, H.D.; Reisen, W.K. Effects of temperature on emergence and seasonality of West Nile virus in California. Am. J. Trop. Med. Hyg. 2012, 86, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Laperriere, V.; Brugger, K.; Rubel, F. Simulation of the seasonal cycles of bird, equine and human West Nile virus cases. Prev. Vet. Med. 2011, 98, 99–110. [Google Scholar] [CrossRef]
- Cotar, A.I.; Falcuta, E.; Prioteasa, L.F.; Dinu, S.; Ceianu, C.S.; Paz, S. Transmission dynamics of the West Nile virus in mosquito vector populations under the influence of weather factors in the Danube Delta, Romania. EcoHealth 2016, 13, 796–807. [Google Scholar] [CrossRef]
- Chase, J.M.; Knight, T.M. Drought-induced mosquito outbreaks in wetlands. Ecol. Lett. 2003, 6, 1017–1024. [Google Scholar] [CrossRef]
- Ziyaeyan, M.; Behzadi, M.A.; Leyva-Grado, V.H.; Azizi, K.; Pouladfar, G.; Dorzaban, H.; Ziyaeyan, A.; Salek, S.; Jaber Hashemi, A.; Jamalidoust, M. Widespread circulation of West Nile virus, but not Zika virus in southern Iran. PLoS Negl. Trop. Dis. 2018, 12, 0007022. [Google Scholar] [CrossRef]
- Dayan, G.; Pugachev, K.; Bevilacqua, J.; Lang, J.; Monath, T. Preclinical and clinical development of a YFV 17 D-based chimeric vaccine against West Nile virus. Viruses 2013, 5, 3048–3070. [Google Scholar] [CrossRef]
- Coller, B.-A.; Pai, V.; Weeks-Levy, C.L.; Ogata, S.A. Recombinant subunit west nile virus vaccine for protection of human subjects. U.S. Patent 20,170,165,349A1, 15 June 2017. [Google Scholar]
- Coller, B.-A.; Pai, V.; Weeks-Levy, C.L.; Ogata, S.A. West nile virus vaccine comprising WN-80E recombinant subunit protein. U.S. Patent No. 10,039,820, 7 August 2018. [Google Scholar]
- Yamshchikov, V.; Manuvakhova, M.; Rodriguez, E.; Hébert, C. Development of a human live attenuated West Nile infectious DNA vaccine: Identification of a minimal mutation set conferring the attenuation level acceptable for a human vaccine. Virology 2017, 500, 122–129. [Google Scholar] [CrossRef]
- Yamshchikov, V.; Manuvakhova, M.; Rodriguez, E. Development of a human live attenuated West Nile infectious DNA vaccine: Suitability of attenuating mutations found in SA14-14-2 for WN vaccine design. Virology 2016, 487, 198–206. [Google Scholar] [CrossRef]
- Pletnev, A.G.; Putnak, J.R.; Chanock, R.M.; Murphy, B.R.; Whitehead, S.S.; Blaney, J.E. Construction of West Nile Virus and Dengue Virus Chimeras for Use in a Live Virus Vaccine to Prevent Disease Caused by West Nile Virus. U.S. Patent No. 10,058,602, 28 August 2018. [Google Scholar]
- Volz, A.; Lim, S.; Kaserer, M.; Lülf, A.; Marr, L.; Jany, S.; Deeg, C.A.; Pijlman, G.P.; Koraka, P.; Osterhaus, A.D. Immunogenicity and protective efficacy of recombinant Modified Vaccinia virus Ankara candidate vaccines delivering West Nile virus envelope antigens. Vaccine 2016, 34, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Prospects for development of a vaccine against the West Nile virus. Ann. N. Y. Acad. Sci. 2001, 951, 1–12. [Google Scholar] [CrossRef]
- Minke, J.; Siger, L.; Karaca, K.; Austgen, L.; Gordy, P.; Bowen, R.; Renshaw, R.; Loosmore, S.; Audonnet, J.; Nordgren, B. Recombinant canarypoxvirus vaccine carrying the prM/E genes of West Nile virus protects horses against a West Nile virus-mosquito challenge. In Emergence and Control of Zoonotic Viral Encephalitides; Springer: Berlin/Heidelberg, Germany, 2004; pp. 221–230. [Google Scholar]
- Roby, J.A.; Bielefeldt-Ohmann, H.; Prow, N.A.; Chang, D.C.; Hall, R.A.; Khromykh, A.A. Increased expression of capsid protein in trans enhances production of single-round infectious particles by West Nile virus DNA vaccine candidate. J. Gen. Virol. 2014, 95, 2176–2191. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.C.; Liu, W.J.; Anraku, I.; Clark, D.C.; Pollitt, C.C.; Suhrbier, A.; Hall, R.A.; Khromykh, A.A. Single-round infectious particles enhance immunogenicity of a DNA vaccine against West Nile virus. Nat. Biotechnol. 2008, 26, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Guirakhoo, F.; Barban, V.; Higgs, S.; Monath, T.P.; Lang, J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 2010, 28, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Hobson-Peters, J.; Harrison, J.J.; Watterson, D.; Hazlewood, J.E.; Vet, L.J.; Newton, N.D.; Warrilow, D.; Colmant, A.M.G.; Taylor, C.; Huang, B.; et al. A recombinant platform for flavivirus vaccines and diagnostics using chimeras of a new insect-specific virus. Sci. Transl. Med. 2019, 11, 7888. [Google Scholar] [CrossRef]
- Vet, L.J.; Setoh, Y.X.; Amarilla, A.A.; Habarugira, G.; Suen, W.W.; Newton, N.D.; Harrison, J.J.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. Protective Efficacy of a Chimeric Insect-Specific Flavivirus Vaccine against West Nile Virus. Vaccines 2020, 8, 258. [Google Scholar] [CrossRef]
- Ng, T.; Hathaway, D.; Jennings, N.; Champ, D.; Chiang, Y.; Chu, H. Equine vaccine for West Nile virus. Dev. Biol. (Basel) 2003, 114, 221–227. [Google Scholar]
- Davidson, A.H.; Traub-Dargatz, J.L.; Rodeheaver, R.M.; Ostlund, E.N.; Pedersen, D.D.; Moorhead, R.G.; Stricklin, J.B.; Dewell, R.D.; Roach, S.D.; Long, R.E. Immunologic responses to West Nile virus in vaccinated and clinically affected horses. J. Am. Vet. Med. Assoc. 2005, 226, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Siger, L.; Bowen, R.A.; Karaca, K.; Murray, M.J.; Gordy, P.W.; Loosmore, S.M.; Audonnet, J.-C.F.; Nordgren, R.M.; Minke, J.M. Assessment of the efficacy of a single dose of a recombinant vaccine against West Nile virus in response to natural challenge with West Nile virus-infected mosquitoes in horses. Am. J. Vet. Res. 2004, 65, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Karaca, K.; Bowen, R.; Austgen, L.; Teehee, M.; Siger, L.; Grosenbaugh, D.; Loosemore, L.; Audonnet, J.-C.; Nordgren, R.; Minke, J. Recombinant canarypox vectored West Nile virus (WNV) vaccine protects dogs and cats against a mosquito WNV challenge. Vaccine 2005, 23, 3808–3813. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Paul, A.M.; Sun, H.; He, J.; Yang, M.; Bai, F.; Chen, Q. A plant-produced vaccine protects mice against lethal West Nile virus infection without enhancing Zika or dengue virus infectivity. Vaccine 2018, 36, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Freeing vaccine adjuvants from dangerous immunological dogma. Expert Rev. Vaccines 2008, 7, 7–10. [Google Scholar] [CrossRef] [PubMed]
- El Garch, H.; Minke, J.M.; Rehder, J.; Richard, S.; Edlund Toulemonde, C.; Dinic, S.; Andreoni, C.; Audonnet, J.C.; Nordgren, R.; Juillard, V. A West Nile virus (WNV) recombinant canarypox virus vaccine elicits WNV-specific neutralizing antibodies and cell-mediated immune responses in the horse. Vet. Immunol. Immunopathol. 2008, 123, 230–239. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- May, F.J.; Lobigs, M.; Lee, E.; Gendle, D.J.; Mackenzie, J.S.; Broom, A.K.; Conlan, J.V.; Hall, R.A. Biological, antigenic and phylogenetic characterization of the flavivirus Alfuy. J. Gen. Virol. 2006, 87, 329–337. [Google Scholar] [CrossRef]
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989, 70, 37–43. [Google Scholar] [CrossRef]
- Goncalvez, A.P.; Purcell, R.H.; Lai, C.-J. Epitope determinants of a chimpanzee Fab antibody that efficiently cross-neutralizes dengue type 1 and type 2 viruses map to inside and in close proximity to fusion loop of the dengue type 2 virus envelope glycoprotein. J. Virol. 2004, 78, 12919–12928. [Google Scholar] [CrossRef]
- Crill, W.D.; Chang, G.-J.J. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J. Virol. 2004, 78, 13975–13986. [Google Scholar] [CrossRef]
- Chang, G.-J.J.; Crill, W.D. Localization and Characterization of Flavivirus Envelope Glycoprotein Cross-reactive Epitopes and Methods for their use. Patent No. 9,000,141, 7 April 2015. [Google Scholar]
- Tesh, R.B.; da Rosa, A.P.T.; Guzman, H.; Araujo, T.P.; Xiao, S.-Y. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg. Infect. Dis. 2002, 8, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Quiroga, D.; Elizondo-Quiroga, A. West Nile virus and its theories, a big puzzle in Mexico and Latin America. J. Glob. Infect. Dis. 2013, 5, 168–175. [Google Scholar] [CrossRef]
- Saron, W.A.; Rathore, A.P.; Ting, L.; Ooi, E.E.; Low, J.; Abraham, S.N.; John, A.L.S. Flavivirus serocomplex cross-reactive immunity is protective by activating heterologous memory CD4 T cells. Sci. Adv. 2018, 4, 4297. [Google Scholar] [CrossRef]
- Li, J.; Gao, N.; Fan, D.; Chen, H.; Sheng, Z.; Fu, S.; Liang, G.; An, J. Cross-protection induced by Japanese encephalitis vaccines against different genotypes of Dengue viruses in mice. Sci. Rep. 2016, 6, 19953. [Google Scholar] [CrossRef]
- Fox, A.; Whitehead, S.; Anders, K.L.; Thai, P.Q.; Yen, N.T.; Duong, T.N.; Thoang, D.D.; Farrar, J.; Wertheim, H.; Simmons, C. Investigation of dengue and Japanese encephalitis virus transmission in Hanam, Viet Nam. Am. J. Trop. Med. Hyg. 2014, 90, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. Anthropology of Infectious Disease; Routledge: London, UK, 2016. [Google Scholar]
- Capobianchi, M.; Sambri, V.; Castilletti, C.; Pierro, A.; Rossini, G.; Gaibani, P.; Cavrini, F.; Selleri, M.; Meschi, S.; Lapa, D. Retrospective screening of solid organ donors in Italy, 2009, reveals unpredicted circulation of West Nile virus. Euro. Surveill. 2010, 15, 19648. [Google Scholar] [CrossRef]
- Dambach, P.; Louis, V.R.; Kaiser, A.; Ouedraogo, S.; Sié, A.; Sauerborn, R.; Becker, N. Efficacy of Bacillus thuringiensis var. israelensis against malaria mosquitoes in northwestern Burkina Faso. Parasites Vectors 2014, 7, 371. [Google Scholar] [CrossRef]
- Ben-Dov, E. Bacillus thuringiensis subsp. israelensis and its dipteran-specific toxins. Toxins (Basel) 2014, 6, 1222–1243. [Google Scholar] [CrossRef] [PubMed]
- Weston, D.P.; Amweg, E.L.; Mekebri, A.; Ogle, R.S.; Lydy, M.J. Aquatic effects of aerial spraying for mosquito control over an urban area. Environ. Sci. Technol. 2006, 40, 5817–5822. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.M.; Husted, S.; Jean, C.; Glaser, C.; Kramer, V. Efficacy of aerial spraying of mosquito adulticide in reducing incidence of West Nile virus, California, 2005. Emerg. Infect. Dis. 2008, 14, 747–754. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, N.B.; Schneider, Z.D.; Little, E.; Barker, C.; Caillouet, K.A.; Campbell, S.R.; Damian, D.; Irwin, P.; Jones, H.M.P.; Townsend, J.; et al. Use of temperature to improve West Nile virus forecasts. PLoS Comput. Biol. 2018, 14, 1006047. [Google Scholar] [CrossRef] [PubMed]
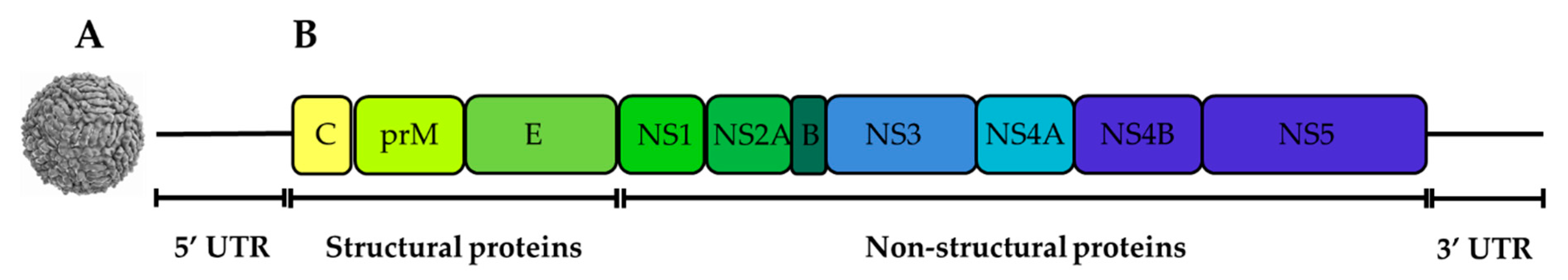
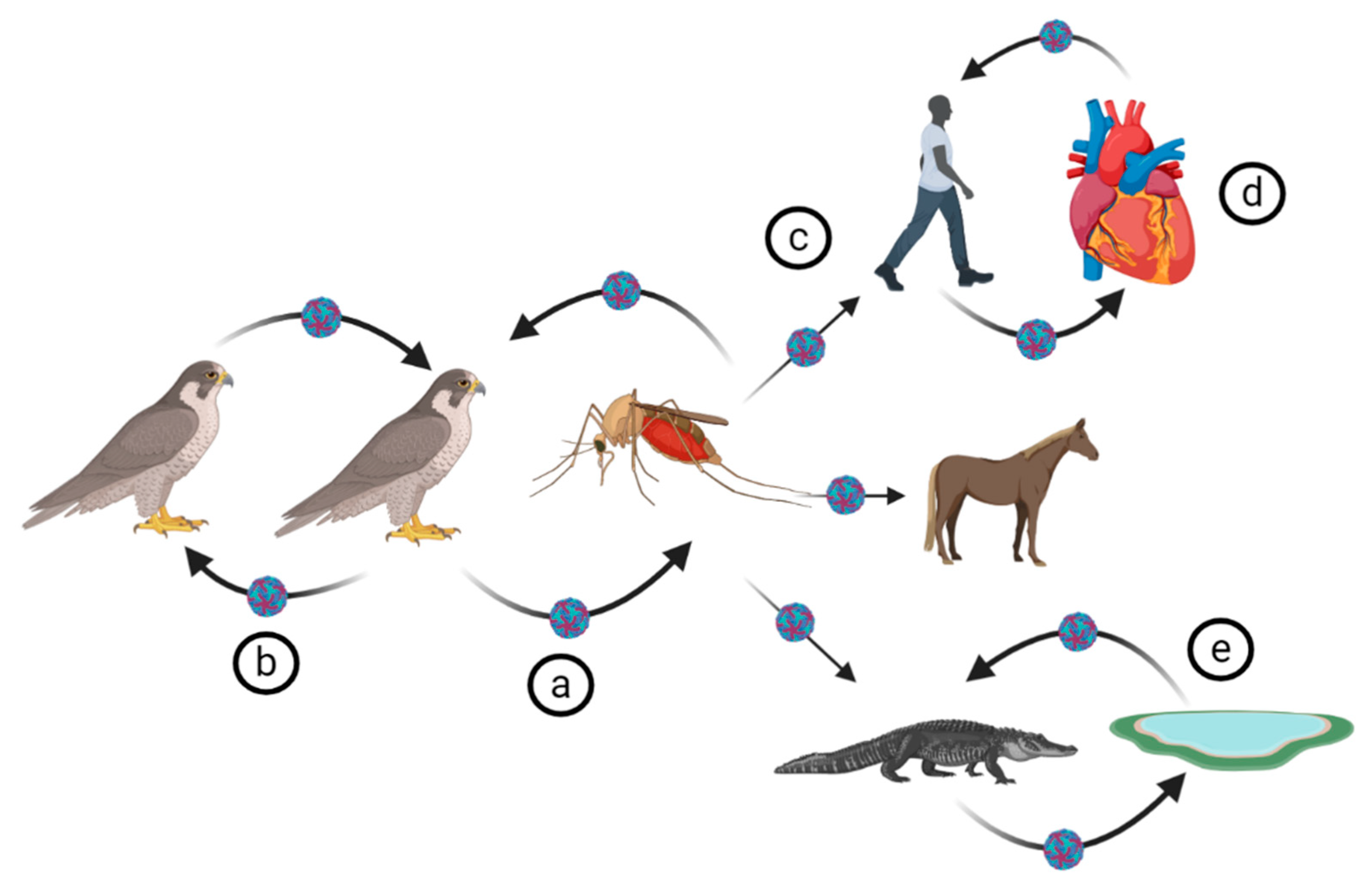
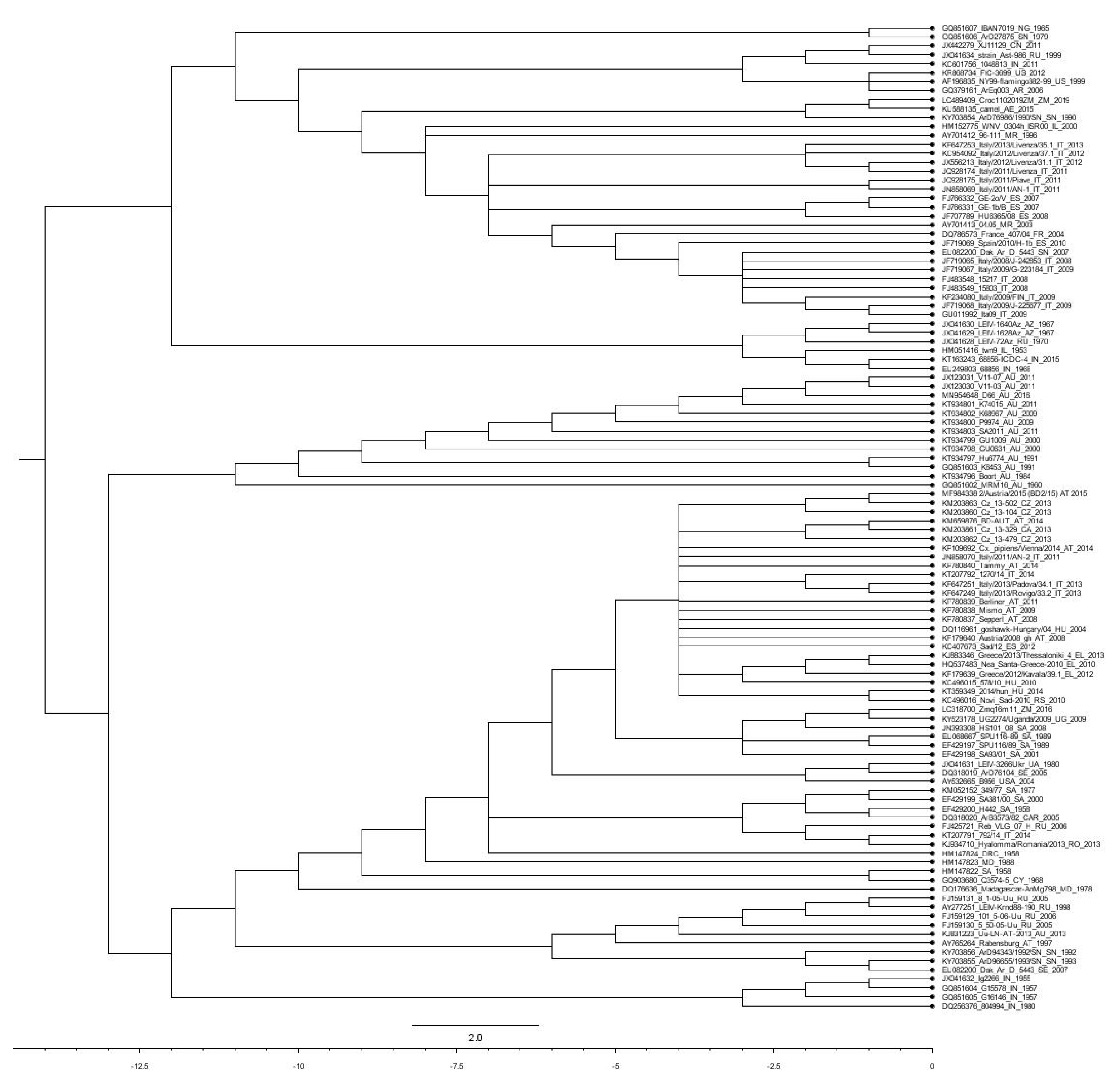
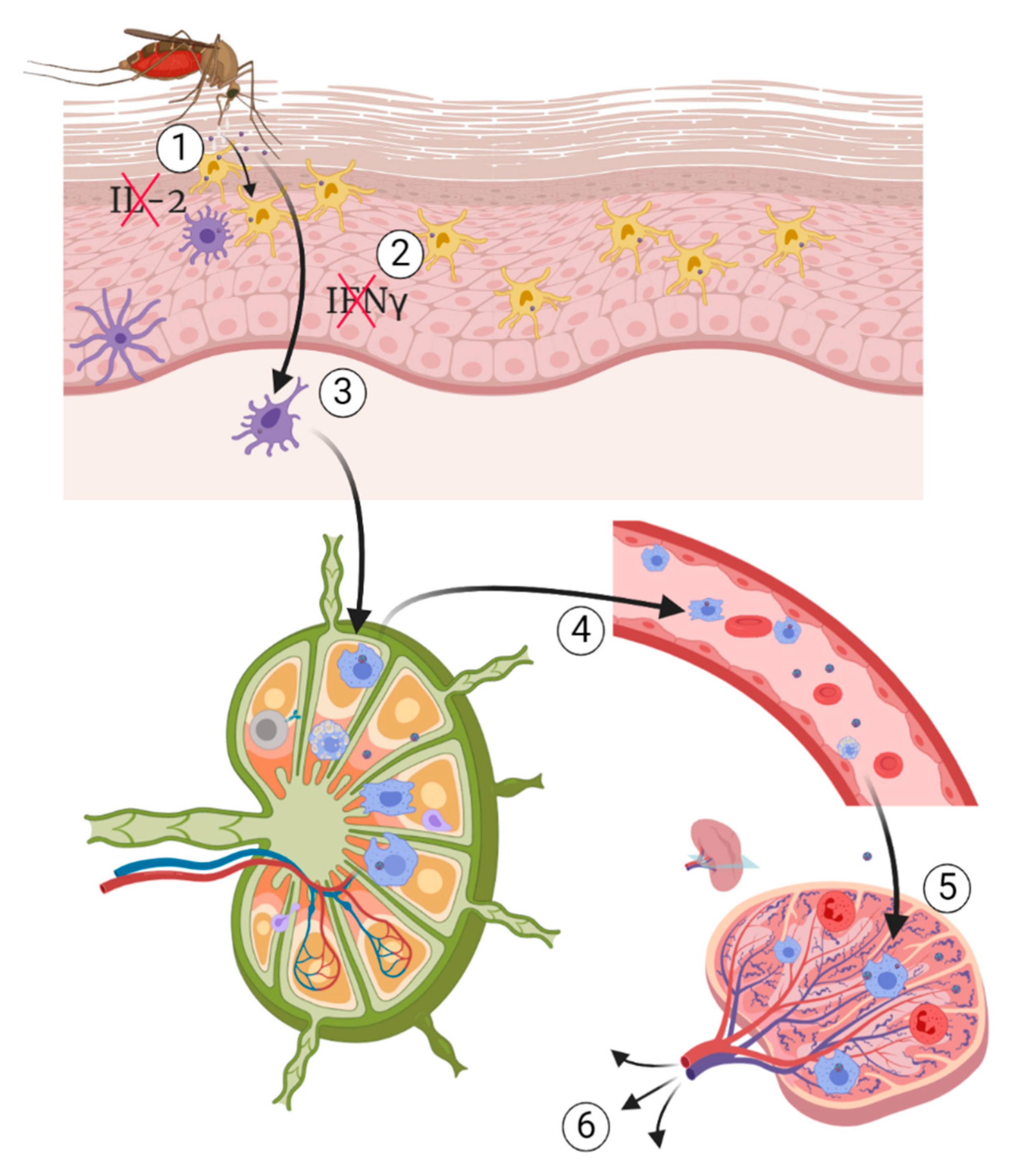
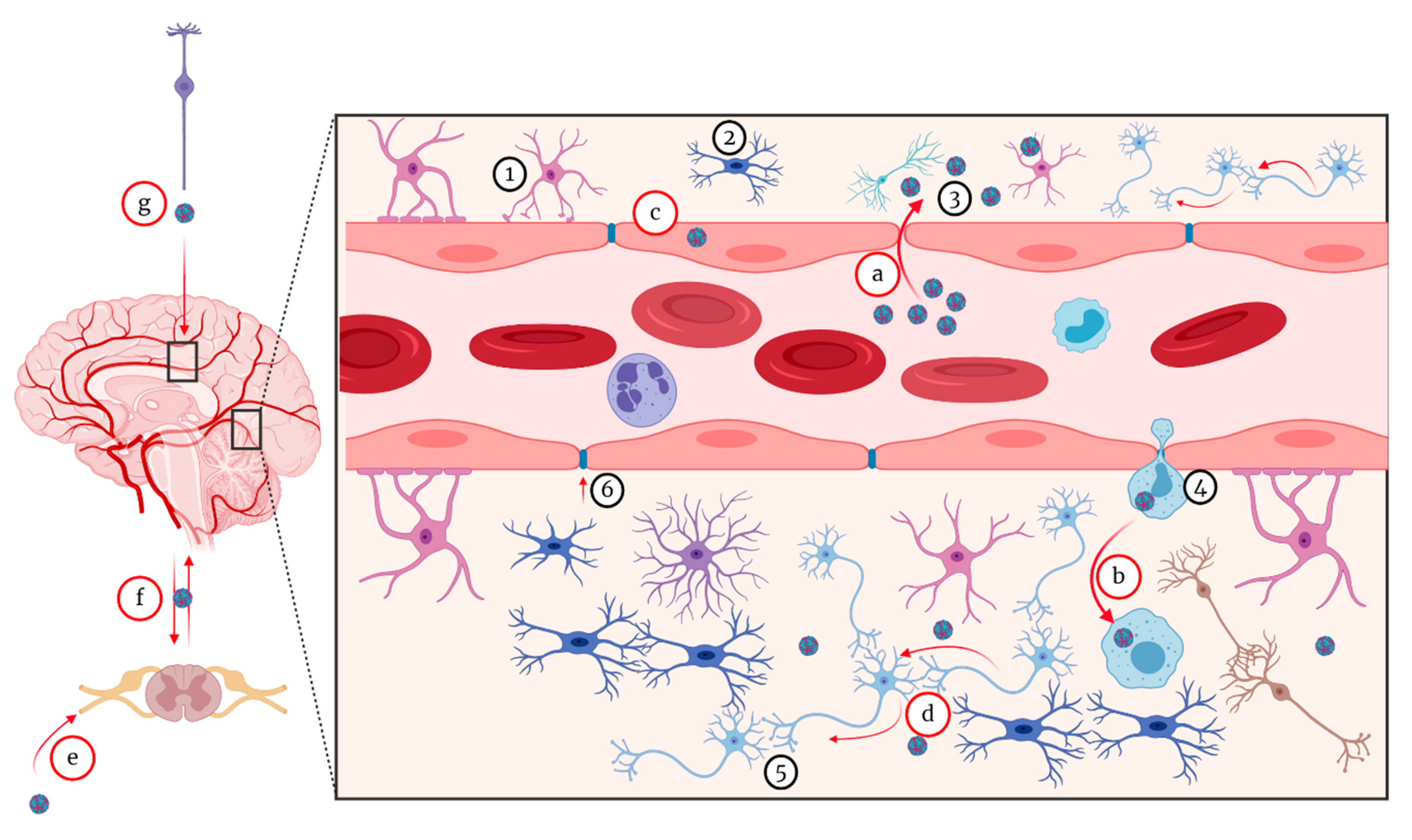
| Country/Continent | Place/Region/City | Year | Host | Form of the Disease | WNV strain | Lineage | Reference |
|---|---|---|---|---|---|---|---|
| USA | New York City | 1999 | Human | Neuroinvasive | WNVNY99 | Lineage 1 | [153,228,301,319,320] |
| USA | New York | 1999 | Horse | Neuroinvasive | WNVNY99 | Lineage 1 | [319] |
| USA | Georgia | 2001–2004 | Birds | Neuroinvasive | WNVNY99 | Lineage 1 | [321,322,323,324] |
| USA | North America | 2002 | Birds | - | WNVNY99 | Lineage 1 | [325] |
| USA | Southern California | 2004 | Birds | Neuroinvasive and gastrointestinal | WNVNY99 | Lineage 1 | [326,327] |
| USA | District of Columbia California | 2013 | Horse | Neuroinvasive | WNVNY99 | Lineage 1 | [328] |
| Australia | New South Wales | 2011 | Horse | Neuroinvasive | WNVNSW2011 | Lineage I | [329] |
| Romania | Several districts | Human | Neuroinvasive WNV Fever syndrome | Various strains of lineage 2 | Lineage 2 | [330] | |
| Russia | Volgograd Region | 1999 | Human | Neuroinvasive | Lineage 1 | [331] | |
| Israel | Various geographical areas | 2000 | Human | Neuroinvasive Gastrointestinal | [287] | ||
| Serbia | - | 2012 | Human | Neuroinvasive WNV Fever syndrome Gastrointestinal | - | - | [2] |
| Romania | Several districts | 2016–2017 | Human | Neuroinvasive WNV Fever syndrome | WN-Romania-1996 | Lineage 2 | [306] |
| Greece | Various geographical areas | 2010 | Human | Neuroinvasive WNV Fever syndrome | Nea Santa-Greece-2010 | Lineage 2 | [150,332] |
| Hungary | Various geographical areas | Horse | Neuroinvasive | Various strains of lineage 2 | Lineage 2 | [333] | |
| Italy | Various geographical areas | Horse Human | Neuroinvasive | Various strains of lineage 1 and 2 | Lineage 1 and 2 | [334] | |
| Israel | Central Israel | 2015 | Human | Neuroinvasive WNV Fever syndrome | - | - | [335] |
| Israel | Eilat | 1998 | Birds | Neuroinvasive | - | lineage 1 and 2 | [220] |
| Tunisia | Sfax area (South Eastern Tunisia) | 1997 | Human | Neuroinvasive | - | - | [336] |
| Democratic Republic of the Congo | Kisangani Kapalata military camp | 1998 | Humans | Neuroinvasive WNV Fever syndrome Gastrointestinal | - | - | [337] |
| Sudan | Nuba Mountains | 2002 | Human | Neuroinvasive | - | - | [338] |
| Tunisia | Monastir, Mahdia and Sousse | 2003 | Human | Neuroinvasive WNV Fever syndrome Gastrointestinal | - | - | [339] |
| South Africa | Pretoria | 2008–2009 | Human | Neuroinvasive | - | Lineage 1 | [340] |
| Gabon | Libreville | 2009 | Human | Neuroinvasive | - | - | [341] |
| Madagascar | 2010 | Horse | [342] | ||||
| South Africa | Ceres in the Western Cape | 2010 | Horse | SAE75/10 | Lineage 1 | [343] | |
| Zambia | Southern Province | 2019 | Nile Crocodile | Neuroinvasive Cutaneous | Croc110/2019/ZM | Lineage 1a | [344] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. https://doi.org/10.3390/pathogens9070589
Habarugira G, Suen WW, Hobson-Peters J, Hall RA, Bielefeldt-Ohmann H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens. 2020; 9(7):589. https://doi.org/10.3390/pathogens9070589
Chicago/Turabian StyleHabarugira, Gervais, Willy W. Suen, Jody Hobson-Peters, Roy A. Hall, and Helle Bielefeldt-Ohmann. 2020. "West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications" Pathogens 9, no. 7: 589. https://doi.org/10.3390/pathogens9070589
APA StyleHabarugira, G., Suen, W. W., Hobson-Peters, J., Hall, R. A., & Bielefeldt-Ohmann, H. (2020). West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens, 9(7), 589. https://doi.org/10.3390/pathogens9070589








