Modulation of Leptin and Leptin Receptor Expression in Mice Acutely Infected with Neospora caninum
Abstract
1. Introduction
2. Results
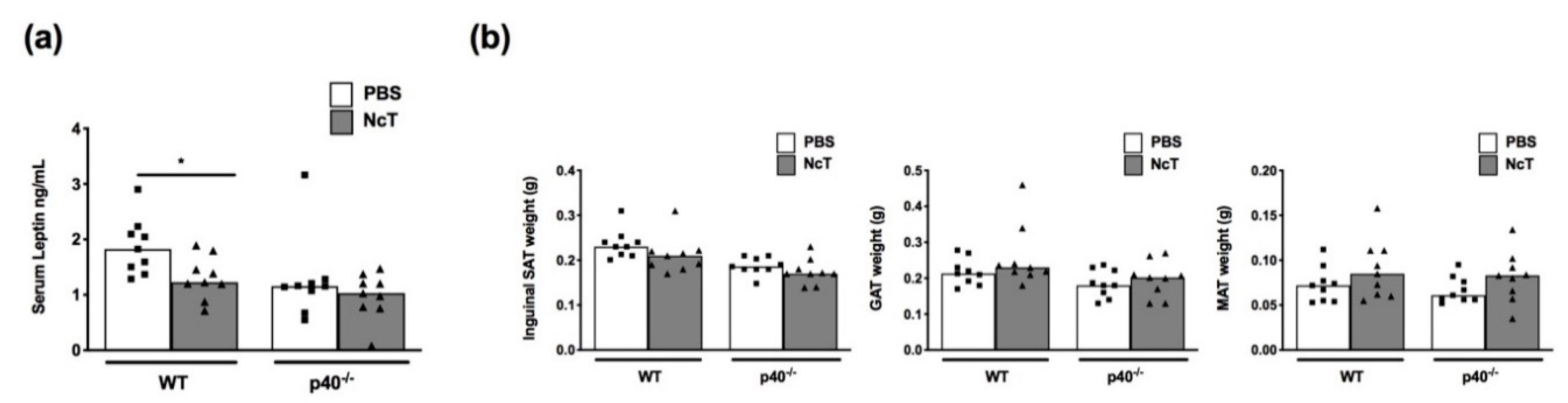
2.1. Leptin Levels in the Serum of N. caninum-Infected Mice
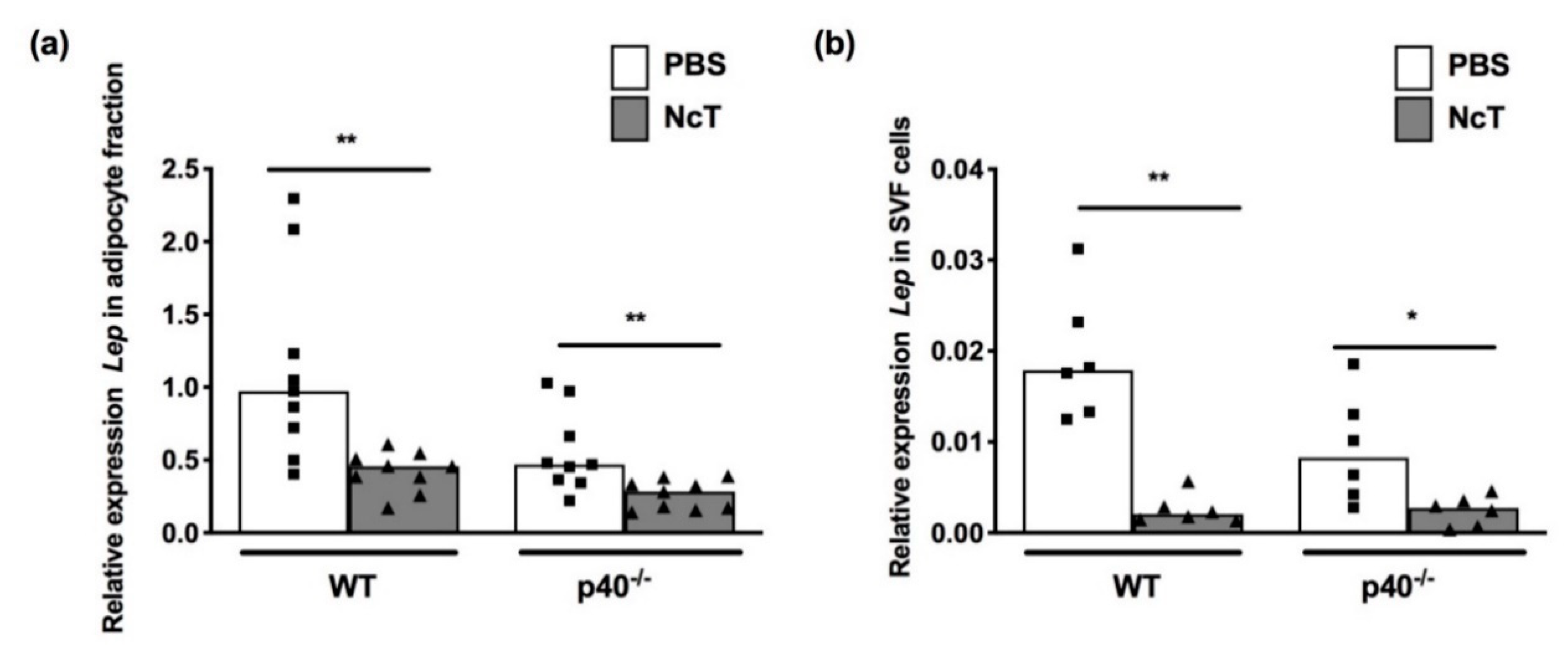
2.2. Leptin mRNA Expression Levels in Adipose Tissue of N. caninum-Infected Mice
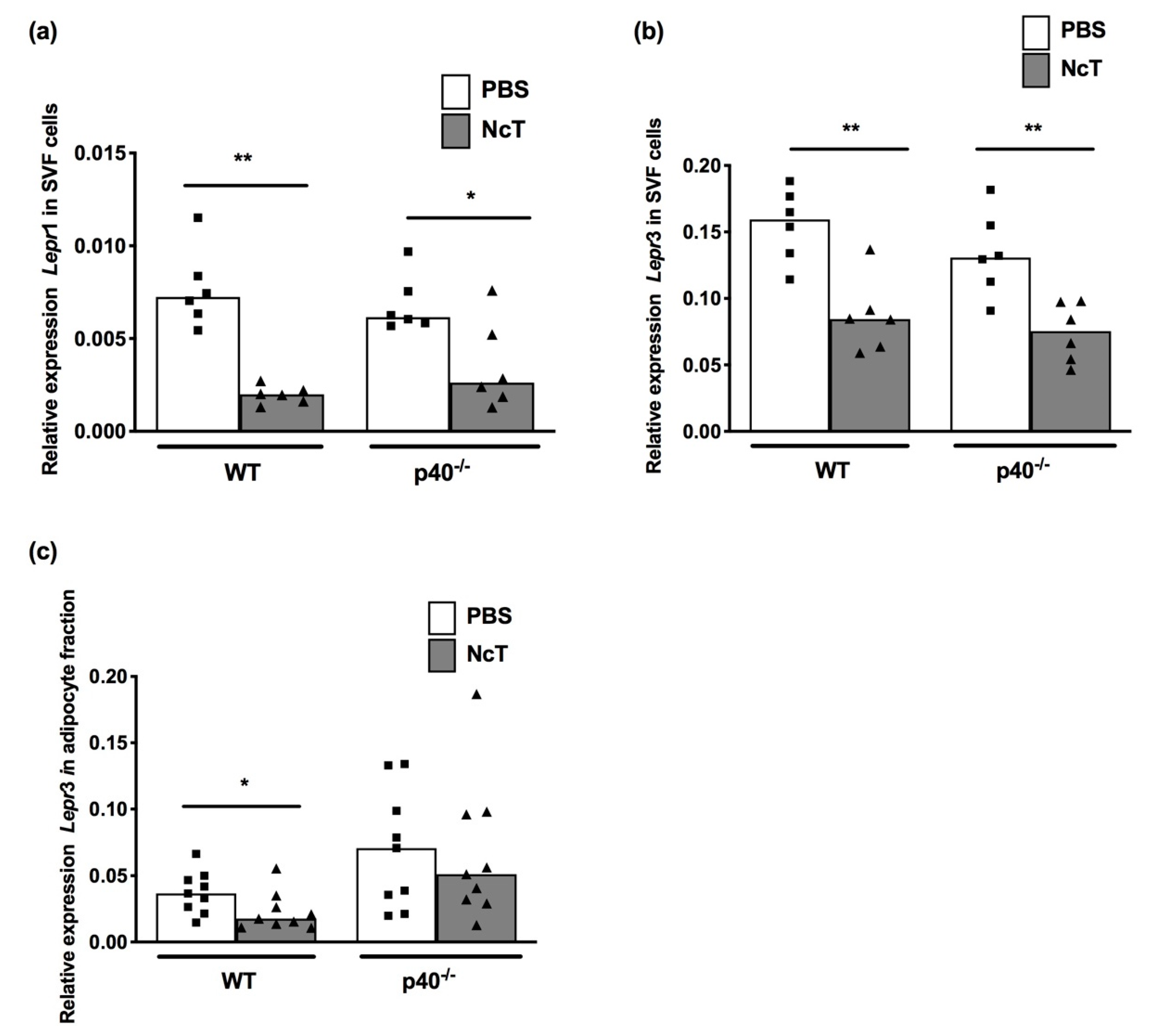
2.3. Leptin Receptor mRNA Expression Levels in Adipose Tissue of N. caninum-Infected Mice
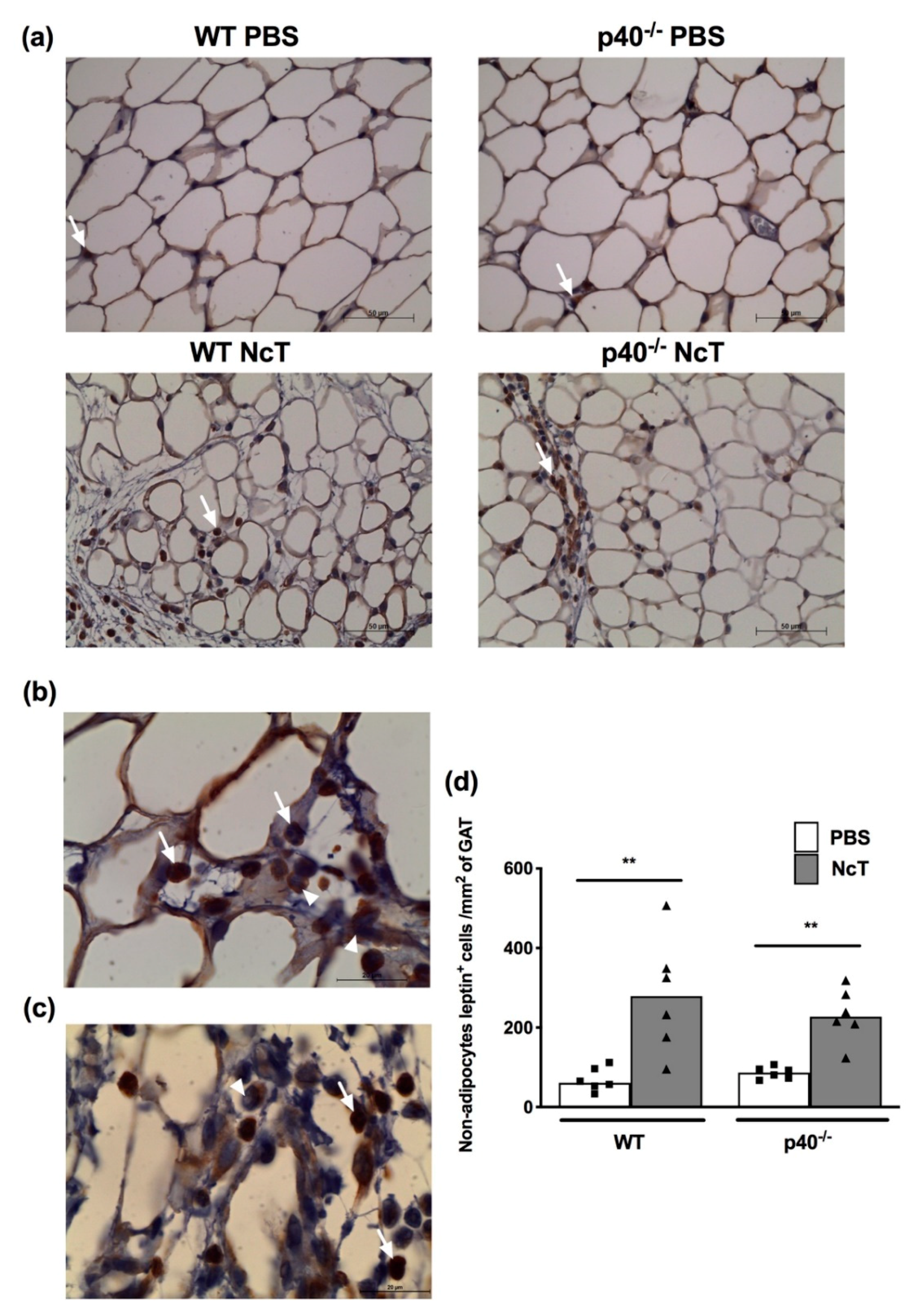
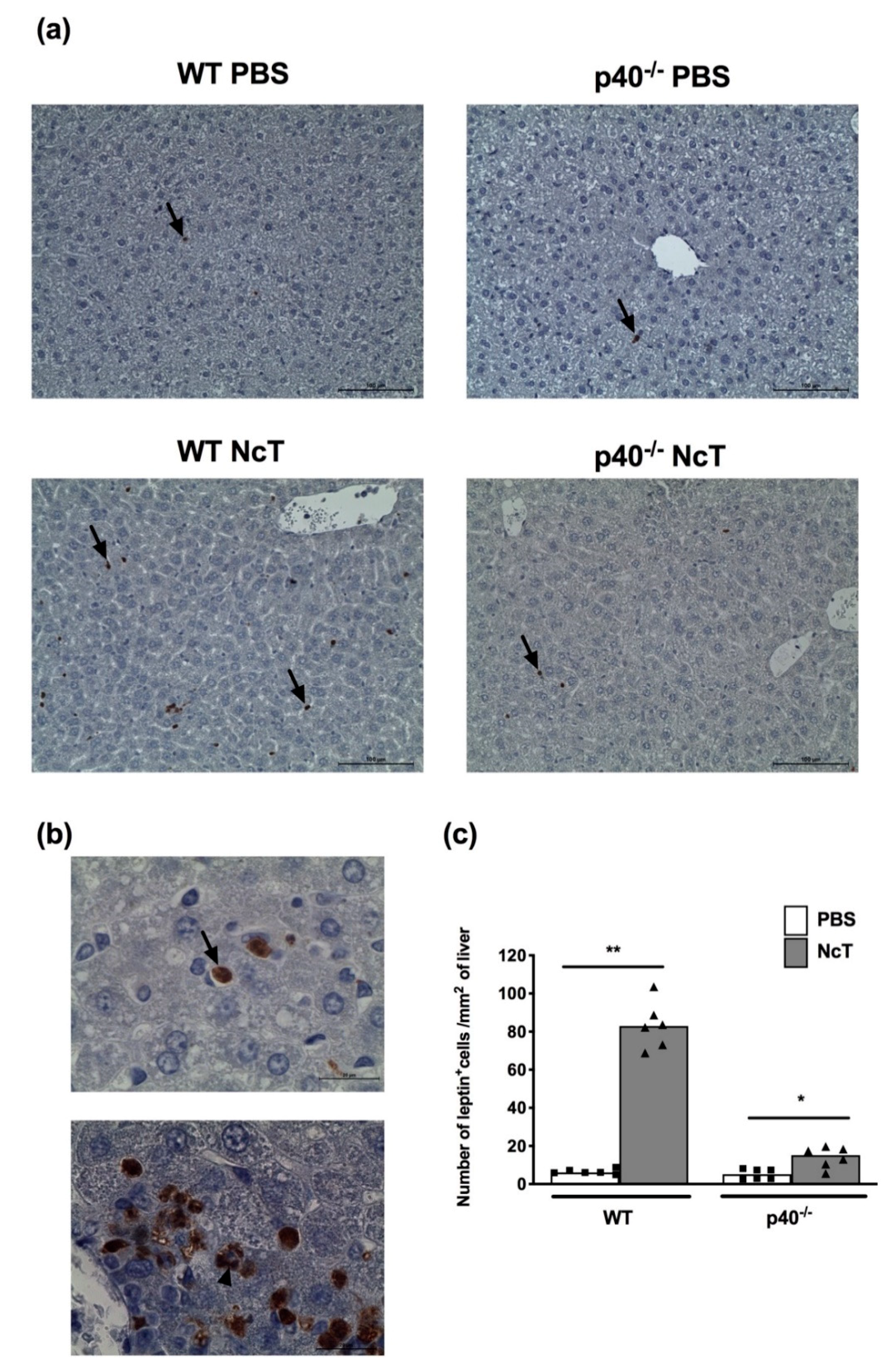
2.4. Leptin Detection by Immunohistochemistry
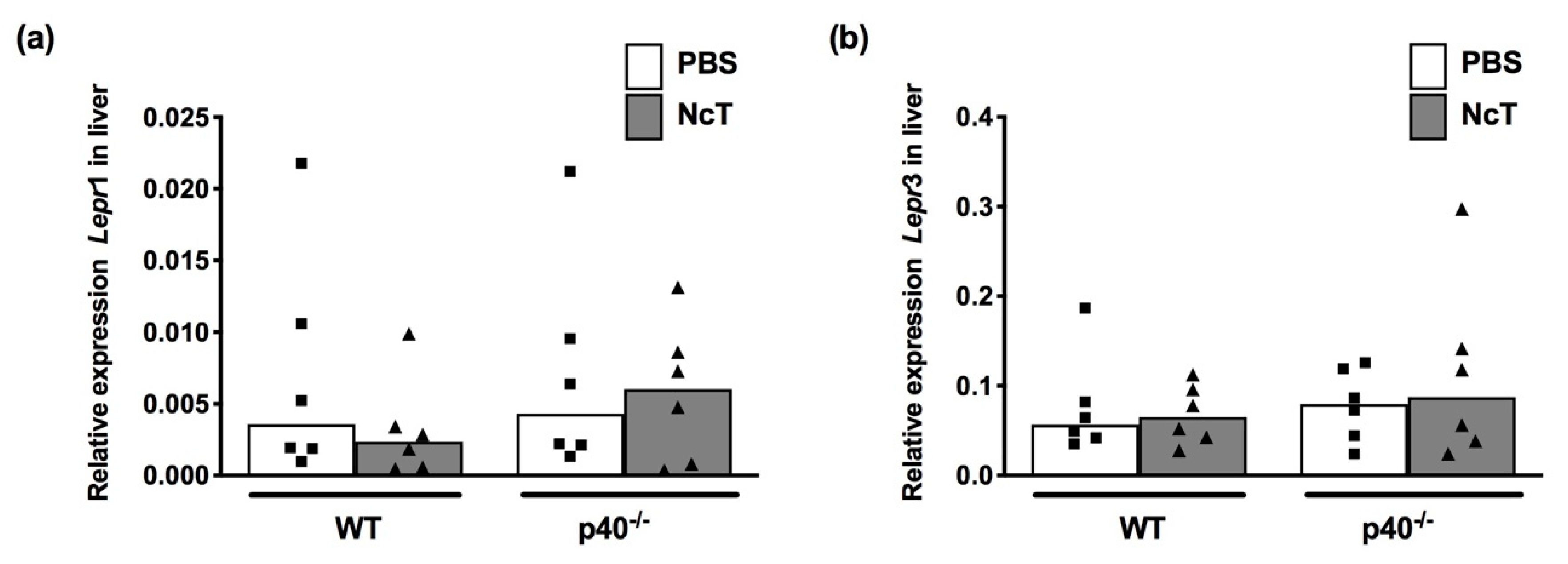
2.5. Leptin and Leptin Receptor mRNA Expression Levels in Liver of N. caninum-Infected Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Parasite Isolation and Challenge Infection
4.3. Collection of Biological Samples
4.4. Isolation of Adipocyte Fraction and Stromal Vascular Fraction Cells
4.5. RNA Isolation and Real-Time PCR Analysis
4.6. Immunohistochemistry and Histopathologic Analysis
4.7. Leptin Measurement in Serum
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P.; Schares, G.; Ortega-Mora, L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007, 20, 323–367. [Google Scholar] [CrossRef]
- Howe, D.K.; Sibley, L.D. Comparison of the major antigens of Neospora caninum and Toxoplasma gondii. Int. J. Parasitol. 1999, 29, 1489–1496. [Google Scholar] [CrossRef]
- Reichel, M.P.; McAllister, M.M.; Pomroy, W.E.; Campero, C.; Ortega-Mora, L.M.; Ellis, J.T. Control options for Neospora caninum—Is there anything new or are we going backwards? Parasitology 2014, 141, 1455–1470. [Google Scholar] [CrossRef]
- Aguado-Martinez, A.; Basto, A.P.; Leitao, A.; Hemphill, A. Neospora caninum in non-pregnant and pregnant mouse models: Cross-talk between infection and immunity. Int. J. Parasitol. 2017, 47, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Tragoolpua, K.; Inoue, N.; Makala, L.; Nagasawa, H.; Otsuka, H.; Mikami, T. In the absence of endogenous gamma interferon, mice acutely infected with Neospora caninum succumb to a lethal immune response characterized by inactivation of peritoneal macrophages. Clin. Diagn. Lab. Immunol. 2001, 8, 811–816. [Google Scholar] [CrossRef]
- Mineo, T.W.; Benevides, L.; Silva, N.M.; Silva, J.S. Myeloid differentiation factor 88 is required for resistance to Neospora caninum infection. Vet. Res. 2009, 40, 32. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Schwartzman, J.D.; Fonseka, S.; Kasper, L.H. Neospora caninum: Role for immune cytokines in host immunity. Exp. Parasitol. 1997, 85, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.; Marques, R.M.; Ferreirinha, P.; Bezerra, F.; Melo, J.; Moreira, J.; Pinto, A.; Correia, A.; Ferreira, P.G.; Vilanova, M. Enrichment of IFN-gamma producing cells in different murine adipose tissue depots upon infection with an apicomplexan parasite. Sci. Rep. 2016, 6, 23475. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.; Moreira, J.; Melo, J.; Bezerra, F.; Marques, R.M.; Ferreirinha, P.; Correia, A.; Monteiro, M.P.; Ferreira, P.G.; Vilanova, M. Immune response in the adipose tissue of lean mice infected with the protozoan parasite Neospora caninum. Immunology 2015, 145, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Desruisseaux, M.S.; Nagajyothi; Trujillo, M.E.; Tanowitz, H.B.; Scherer, P.E. Adipocyte, adipose tissue, and infectious disease. Infect. Immun. 2007, 75, 1066–1078. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Procaccini, C.; La Rocca, C.; Carbone, F.; De Rosa, V.; Galgani, M.; Matarese, G. Leptin as immune mediator: Interaction between neuroendocrine and immune system. Dev. Comp. Immunol. 2017, 66, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Naylor, C.; Petri, W.A., Jr. Leptin Regulation of Immune Responses. Trends Mol. Med. 2016, 22, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef]
- Saucillo, D.C.; Gerriets, V.A.; Sheng, J.; Rathmell, J.C.; Maciver, N.J. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 2014, 192, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Papathanassoglou, E.; El-Haschimi, K.; Li, X.C.; Matarese, G.; Strom, T.; Mantzoros, C. Leptin receptor expression and signaling in lymphocytes: Kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J. Immunol. 2006, 176, 7745–7752. [Google Scholar] [CrossRef]
- Maurya, R.; Bhattacharya, P.; Dey, R.; Nakhasi, H.L. Leptin Functions in Infectious Diseases. Front. Immunol. 2018, 9, 2741. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000Prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef]
- Bjorbaek, C.; Uotani, S.; da Silva, B.; Flier, J.S. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 1997, 272, 32686–32695. [Google Scholar] [CrossRef]
- Li, Z.; Ceccarini, G.; Eisenstein, M.; Tan, K.; Friedman, J.M. Phenotypic effects of an induced mutation of the ObRa isoform of the leptin receptor. Mol. Metab. 2013, 2, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Odle, A.K.; Haney, A.; Allensworth-James, M.; Akhter, N.; Childs, G.V. Adipocyte versus pituitary leptin in the regulation of pituitary hormones: Somatotropes develop normally in the absence of circulating leptin. Endocrinology 2014, 155, 4316–4328. [Google Scholar] [CrossRef][Green Version]
- Collantes-Fernandez, E.; Lopez-Perez, I.; Alvarez-Garcia, G.; Ortega-Mora, L.M. Temporal distribution and parasite load kinetics in blood and tissues during Neospora caninum infection in mice. Infect. Immun. 2006, 74, 2491–2494. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R. Plasma leptin levels in rats with induced Toxoplasma gondii infection. Bratisl. Lek. Listy 2012, 113, 67–69. [Google Scholar] [CrossRef] [PubMed]
- MacDougald, O.A.; Hwang, C.S.; Fan, H.; Lane, M.D. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 1995, 92, 9034–9037. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, X.; Chen, H.; Sjoberg, S.; Roux, J.; Zhang, L.; Ivoulsou, A.H.; Bensaid, F.; Liu, C.L.; Liu, J.; et al. Leptin Deficiency Shifts Mast Cells toward Anti-Inflammatory Actions and Protects Mice from Obesity and Diabetes by Polarizing M2 Macrophages. Cell Metab. 2015, 22, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Abanni, L.; Cali, G.; Porcellini, A.; Carbone, F.; Fontana, S.; Horvath, T.L.; La Cava, A.; et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity 2010, 33, 929–941. [Google Scholar] [CrossRef]
- Cinti, S.; Frederich, R.C.; Zingaretti, M.C.; De Matteis, R.; Flier, J.S.; Lowell, B.B. Immunohistochemical localization of leptin and uncoupling protein in white and brown adipose tissue. Endocrinology 1997, 138, 797–804. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Abu-Asab, M.; Glasow, A.; Path, G.; Hauner, H.; Tsokos, M.; Chrousos, G.P.; Scherbaum, W.A. Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture. Diabetes 2000, 49, 532–538. [Google Scholar] [CrossRef]
- Fei, H.; Okano, H.J.; Li, C.; Lee, G.H.; Zhao, C.; Darnell, R.; Friedman, J.M. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl. Acad. Sci. USA 1997, 94, 7001–7005. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, F.; Zhao, D.; Machado, F.S.; Weiss, L.M.; Schwartz, G.J.; Desruisseaux, M.S.; Zhao, Y.; Factor, S.M.; Huang, H.; Albanese, C.; et al. Crucial role of the central leptin receptor in murine Trypanosoma cruzi (Brazil strain) infection. J. Infect. Dis. 2010, 202, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Otte, J.M.; Strodthoff, D.; Bornstein, S.R.; Folsch, U.R.; Monig, H.; Kloehn, S. Expression of leptin and leptin receptor during the development of liver fibrosis and cirrhosis. Exp. Clin. Endocrinol. Diabetes 2004, 112, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Alessi, M.; Soresi, S.; Bonanno, A.; Riccobono, L.; Montalbano, A.M.; Albano, G.D.; Gjomarkaj, M.; Profita, M. Increased leptin/leptin receptor pathway affects systemic and airway inflammation in COPD former smokers. J. Inflamm. Res. 2011, 4, 51–59. [Google Scholar] [CrossRef][Green Version]
- Caldefie-Chezet, F.; Poulin, A.; Tridon, A.; Sion, B.; Vasson, M.P. Leptin: A potential regulator of polymorphonuclear neutrophil bactericidal action? J. Leukoc. Biol. 2001, 69, 414–418. [Google Scholar]
- Fischer, J.; Gutierrez, S.; Ganesan, R.; Calabrese, C.; Ranjan, R.; Cildir, G.; Hos, N.J.; Rybniker, J.; Wolke, M.; Fries, J.W.U.; et al. Leptin signaling impairs macrophage defenses against Salmonella Typhimurium. Proc. Natl. Acad. Sci. USA 2019, 116, 16551–16560. [Google Scholar] [CrossRef]
- Ramirez, O.; Garza, K.M. Leptin deficiency in vivo enhances the ability of splenic dendritic cells to activate T cells. Int. Immunol. 2014, 26, 627–636. [Google Scholar] [CrossRef]
- Oliveira, B.M.; Pinto, A.; Correia, A.; Ferreira, P.G.; Vilanova, M.; Teixeira, L. Characterization of Myeloid Cellular Populations in Mesenteric and Subcutaneous Adipose Tissue of Holstein-Friesian Cows. Sci. Rep. 2020, 10, 1771. [Google Scholar] [CrossRef]
- Forbes, A.B.; Warren, M.; Upjohn, M.; Jackson, B.; Jones, J.; Charlier, J.; Fox, M.T. Associations between blood gastrin, ghrelin, leptin, pepsinogen and Ostertagia ostertagi antibody concentrations and voluntary feed intake in calves exposed to a trickle infection with O. ostertagi. Vet. Parasitol. 2009, 162, 295–305. [Google Scholar] [CrossRef]
- Teixeira, L.; Botelho, A.S.; Mesquita, S.D.; Correia, A.; Cerca, F.; Costa, R.; Sampaio, P.; Castro, A.G.; Vilanova, M. Plasmacytoid and conventional dendritic cells are early producers of IL-12 in Neospora caninum-infected mice. Immunol. Cell Biol. 2010, 88, 79–86. [Google Scholar] [CrossRef]
- Hemphill, A. Subcellular localization and functional characterization of Nc-p43, a major Neospora caninum tachyzoite surface protein. Infect. Immun. 1996, 64, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Bartley, P.M.; Wright, S.; Sales, J.; Chianini, F.; Buxton, D.; Innes, E.A. Long-term passage of tachyzoites in tissue culture can attenuate virulence of Neospora caninum in vivo. Parasitology 2006, 133, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Kitchen, C.M. Nonparametric vs parametric tests of location in biomedical research. Am. J. Ophthalmol. 2009, 147, 571–572. [Google Scholar] [CrossRef]
- Grech, V.; Calleja, N. WASP (Write a Scientific Paper): Parametric vs. non-parametric tests. Early Hum. Dev. 2018, 123, 48–49. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, L.; Correia, A.; Oliveira, B.M.; Pinto, A.; Ferreira, P.G.; Vilanova, M. Modulation of Leptin and Leptin Receptor Expression in Mice Acutely Infected with Neospora caninum. Pathogens 2020, 9, 587. https://doi.org/10.3390/pathogens9070587
Teixeira L, Correia A, Oliveira BM, Pinto A, Ferreira PG, Vilanova M. Modulation of Leptin and Leptin Receptor Expression in Mice Acutely Infected with Neospora caninum. Pathogens. 2020; 9(7):587. https://doi.org/10.3390/pathogens9070587
Chicago/Turabian StyleTeixeira, Luzia, Alexandra Correia, Bárbara M. Oliveira, Ana Pinto, Paula G. Ferreira, and Manuel Vilanova. 2020. "Modulation of Leptin and Leptin Receptor Expression in Mice Acutely Infected with Neospora caninum" Pathogens 9, no. 7: 587. https://doi.org/10.3390/pathogens9070587
APA StyleTeixeira, L., Correia, A., Oliveira, B. M., Pinto, A., Ferreira, P. G., & Vilanova, M. (2020). Modulation of Leptin and Leptin Receptor Expression in Mice Acutely Infected with Neospora caninum. Pathogens, 9(7), 587. https://doi.org/10.3390/pathogens9070587




