Pichinde Virus Infection of Outbred Hartley Guinea Pigs as a Surrogate Animal Model for Human Lassa Fever: Histopathological and Immunohistochemical Analyses
Abstract
1. Introduction
2. Results
2.1. Overview of the Viral Strains Used and the Disease Symptoms Produced by Them
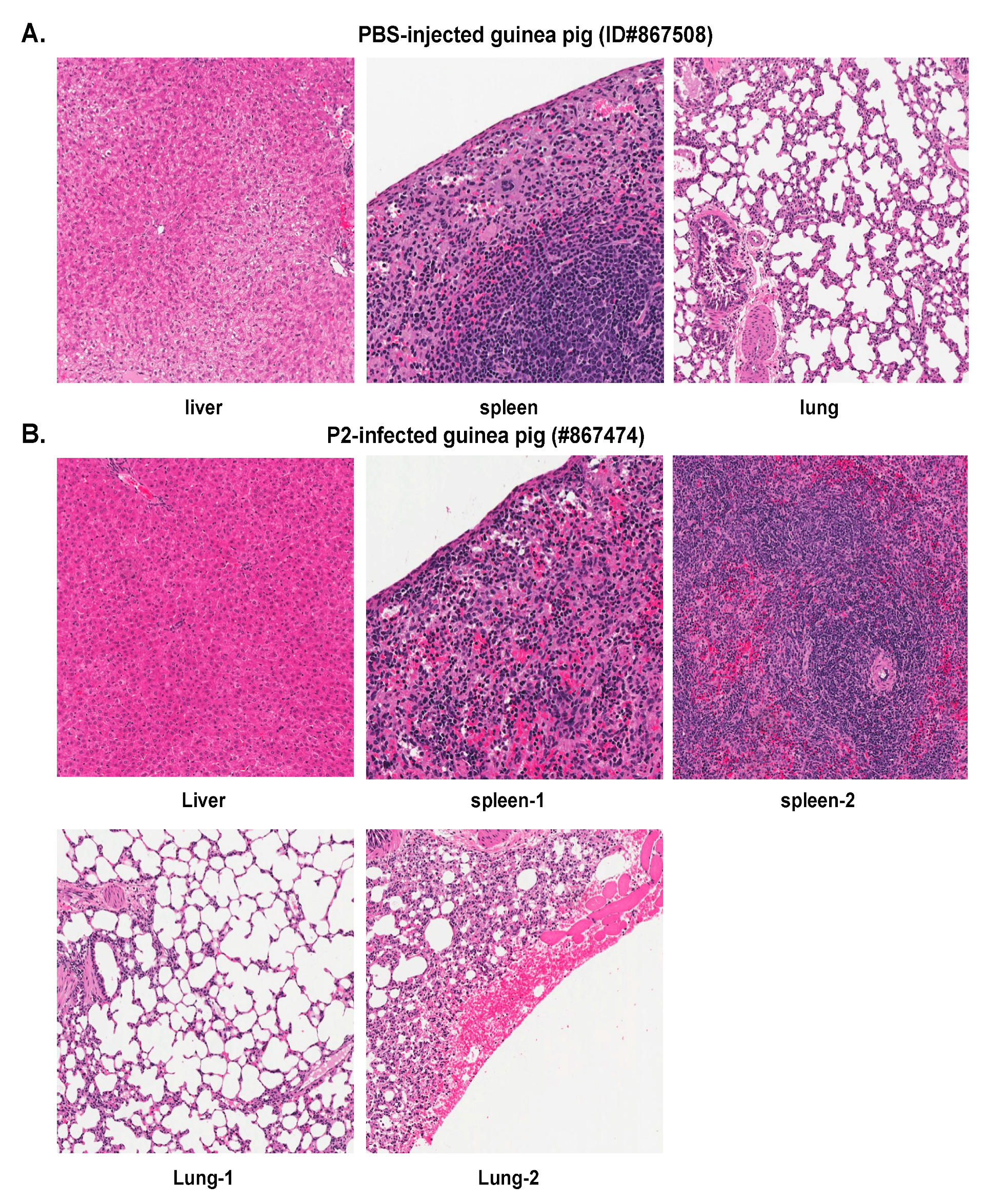
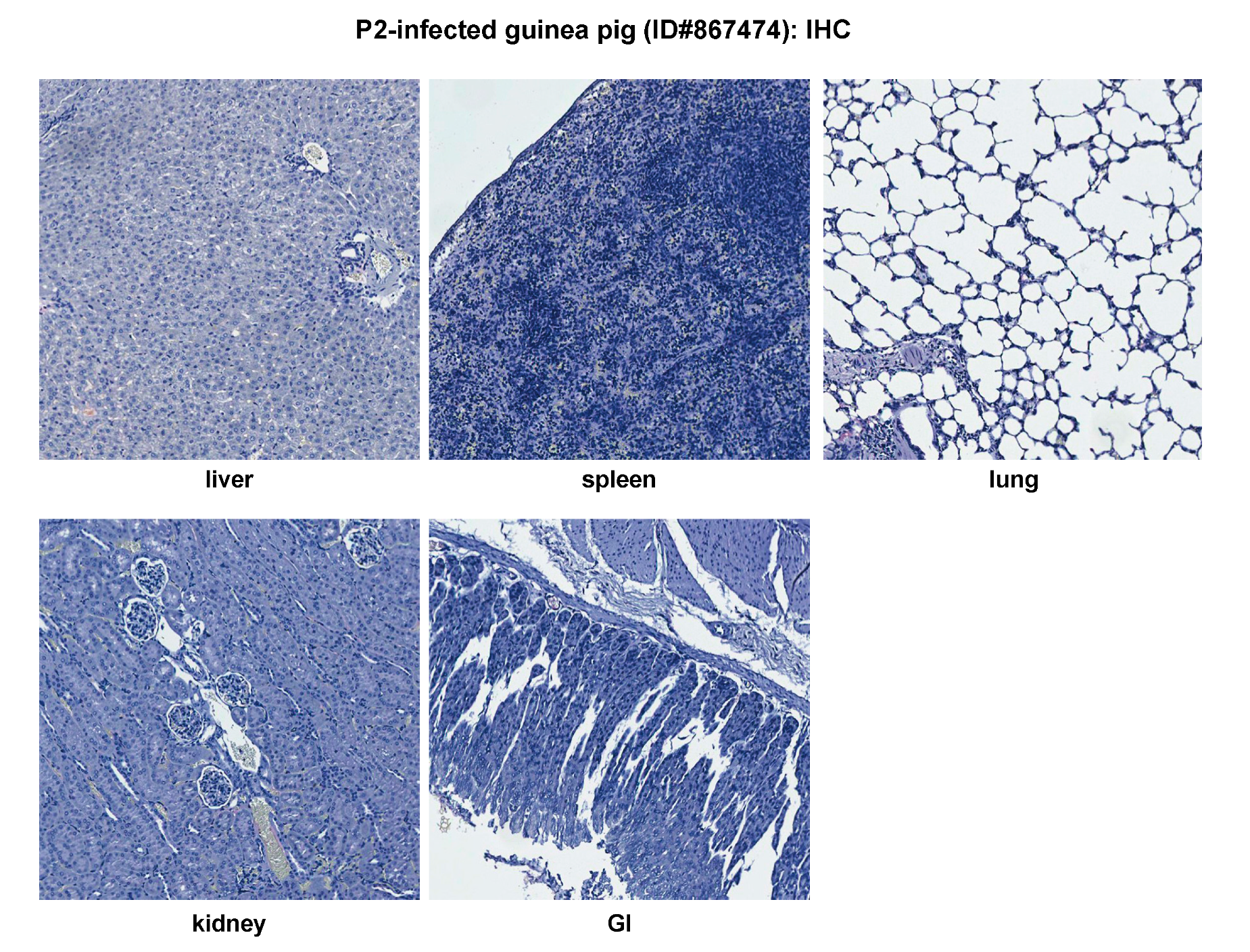
2.2. Histopathological and Immunohistochemical Analyses of Tissue Sections Prepared from Representative Healthy Guinea Pigs after Injection with Either PBS or the Parental P2 PICV
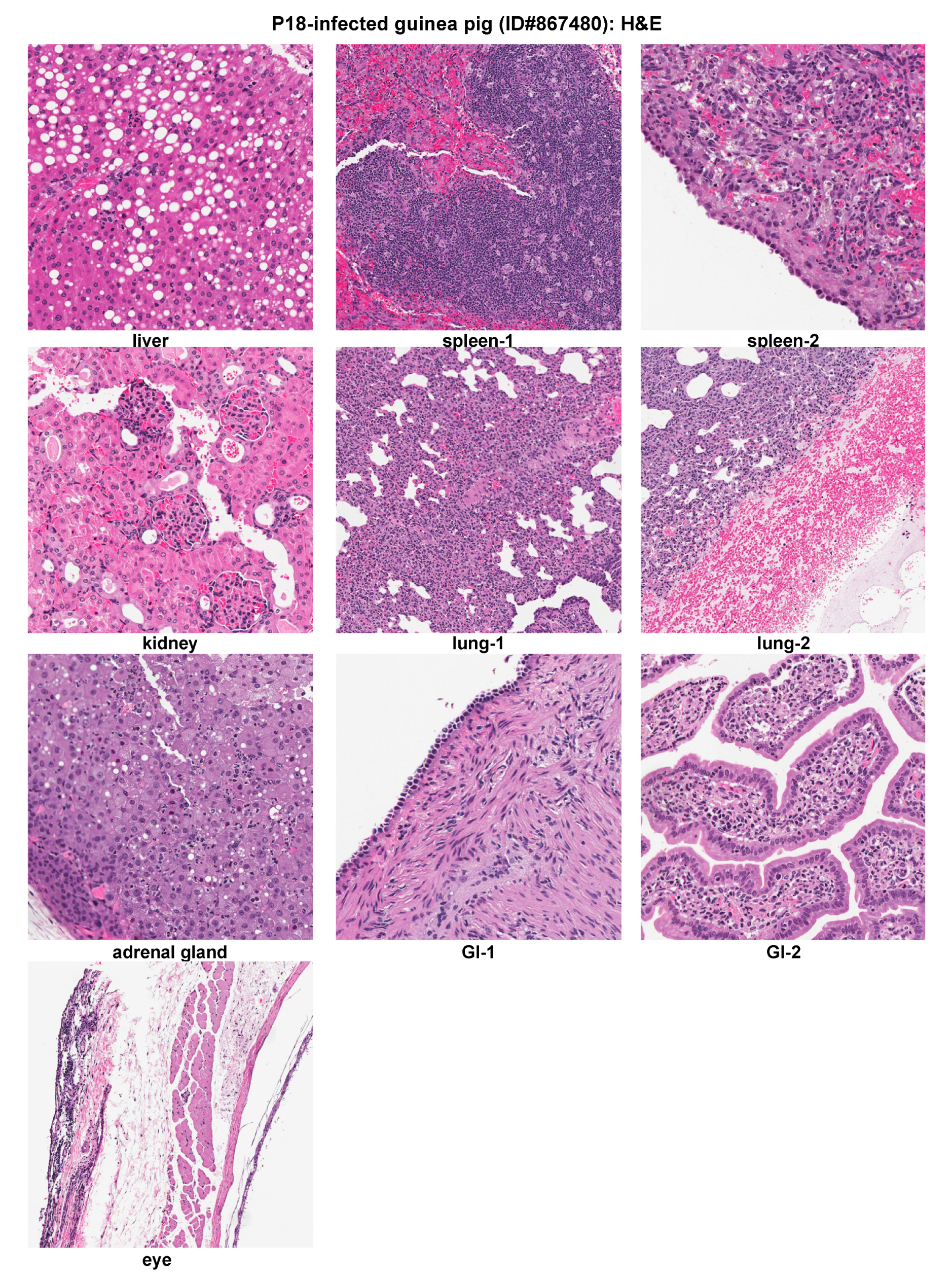
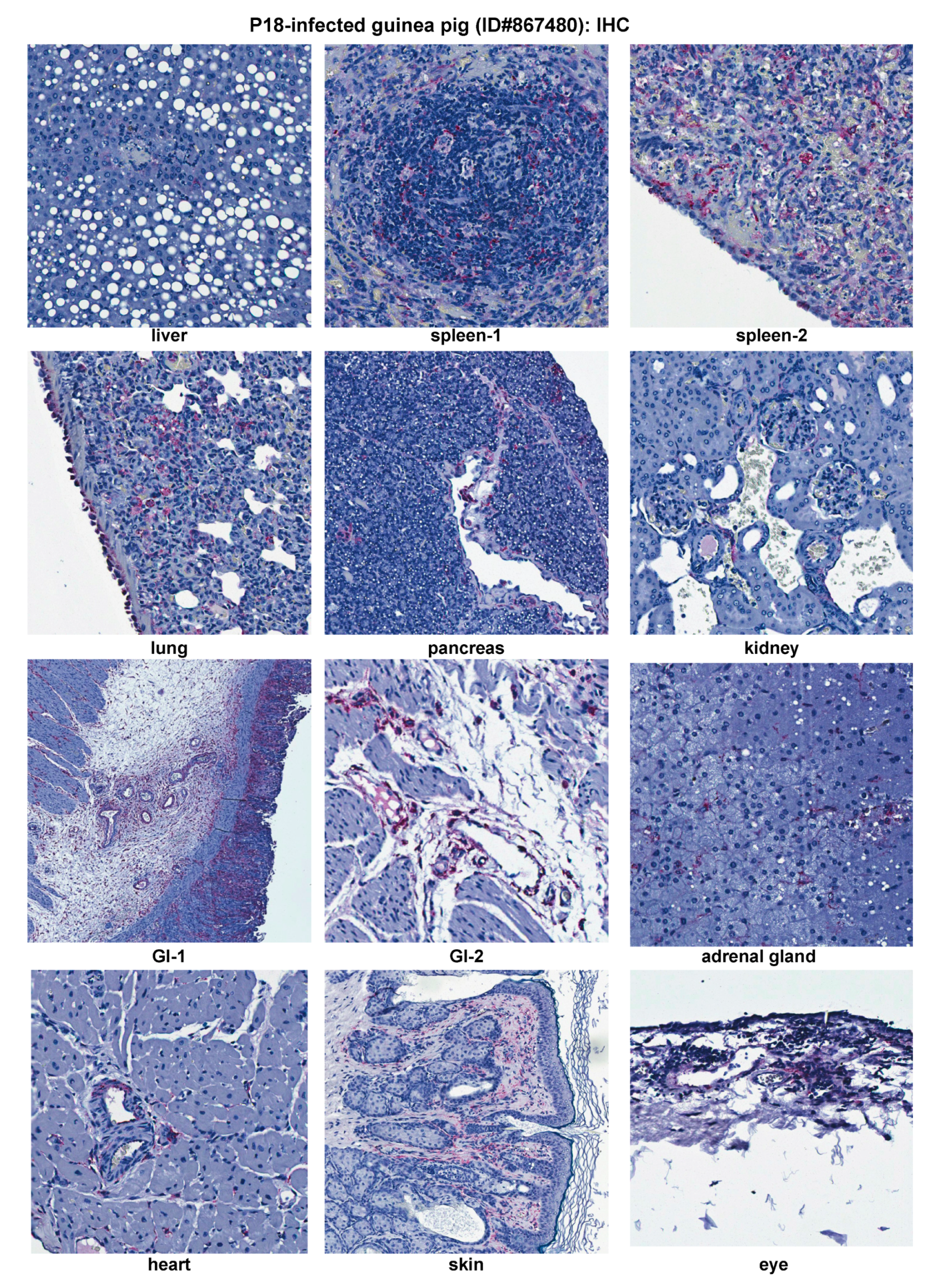
2.3. Histopathological and Immunohistochemical Analyses of Tissue Sections Prepared from a Representative Moribund Guinea Pig Due to the Parental P18 PICV Infection
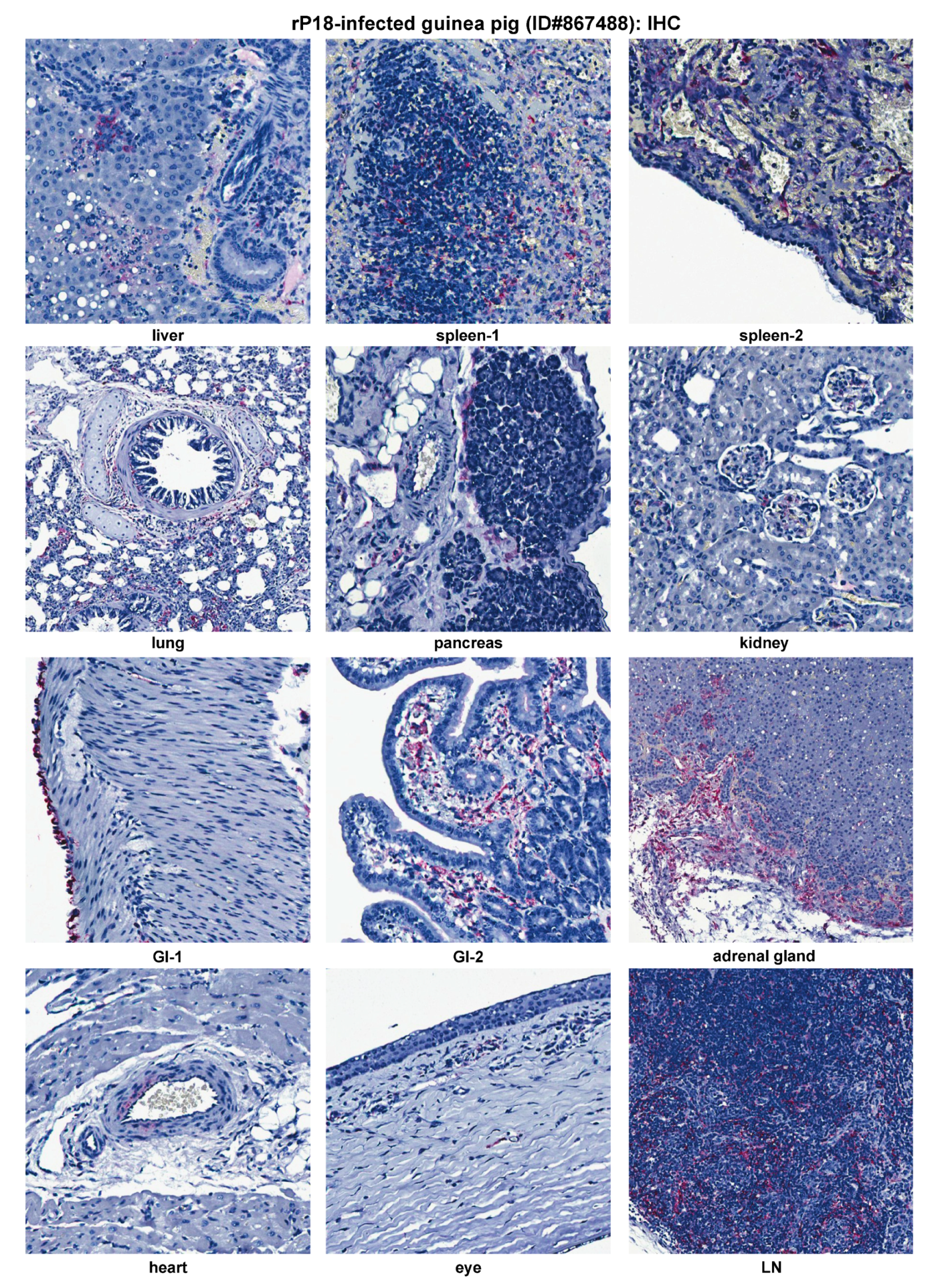
2.4. Histopathological and Immunohistochemical Analyses of Tissue Sections Prepared from a Representative Moribund Guinea Pig Due to the Recombinant rP18 PICV Infection
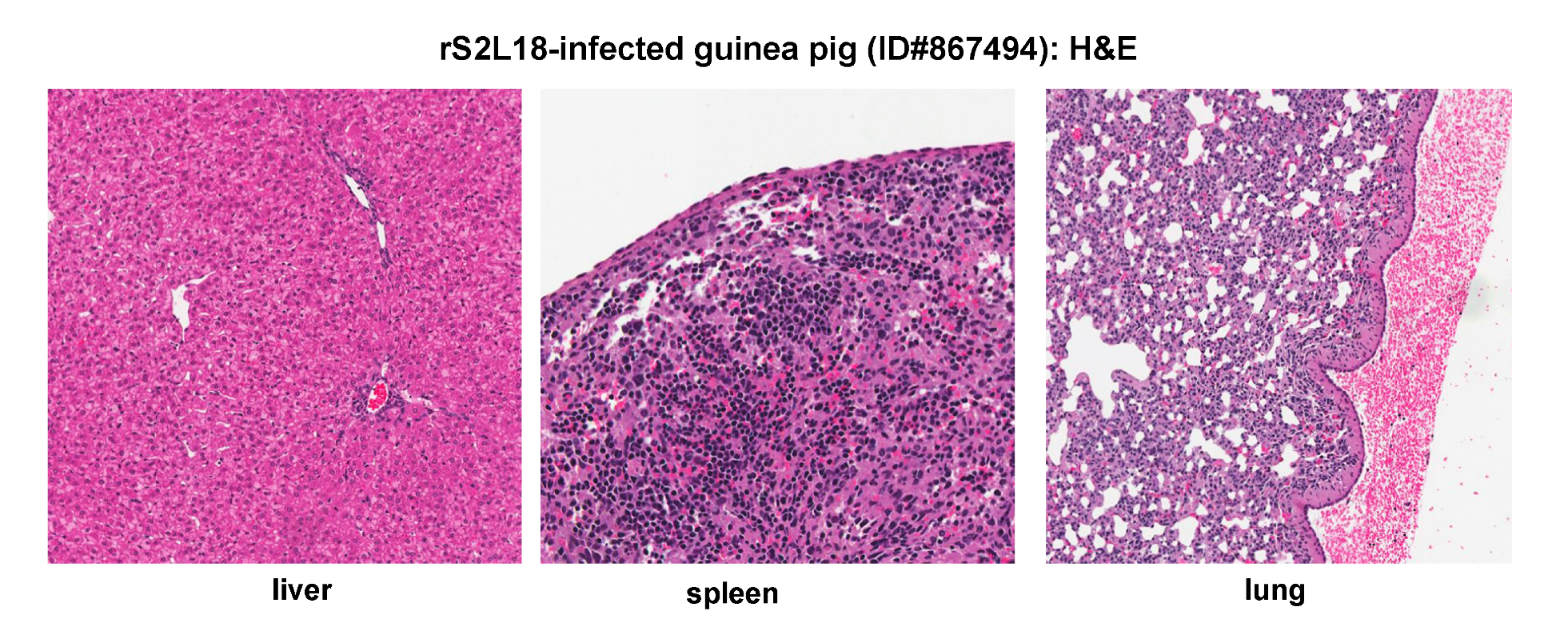
2.5. Histopathological and Immunohistochemical Analyses of Tissue Sections Prepared from a Representative Guinea Pig Infected with a Recombinant rS2L18 Reassortant Virus
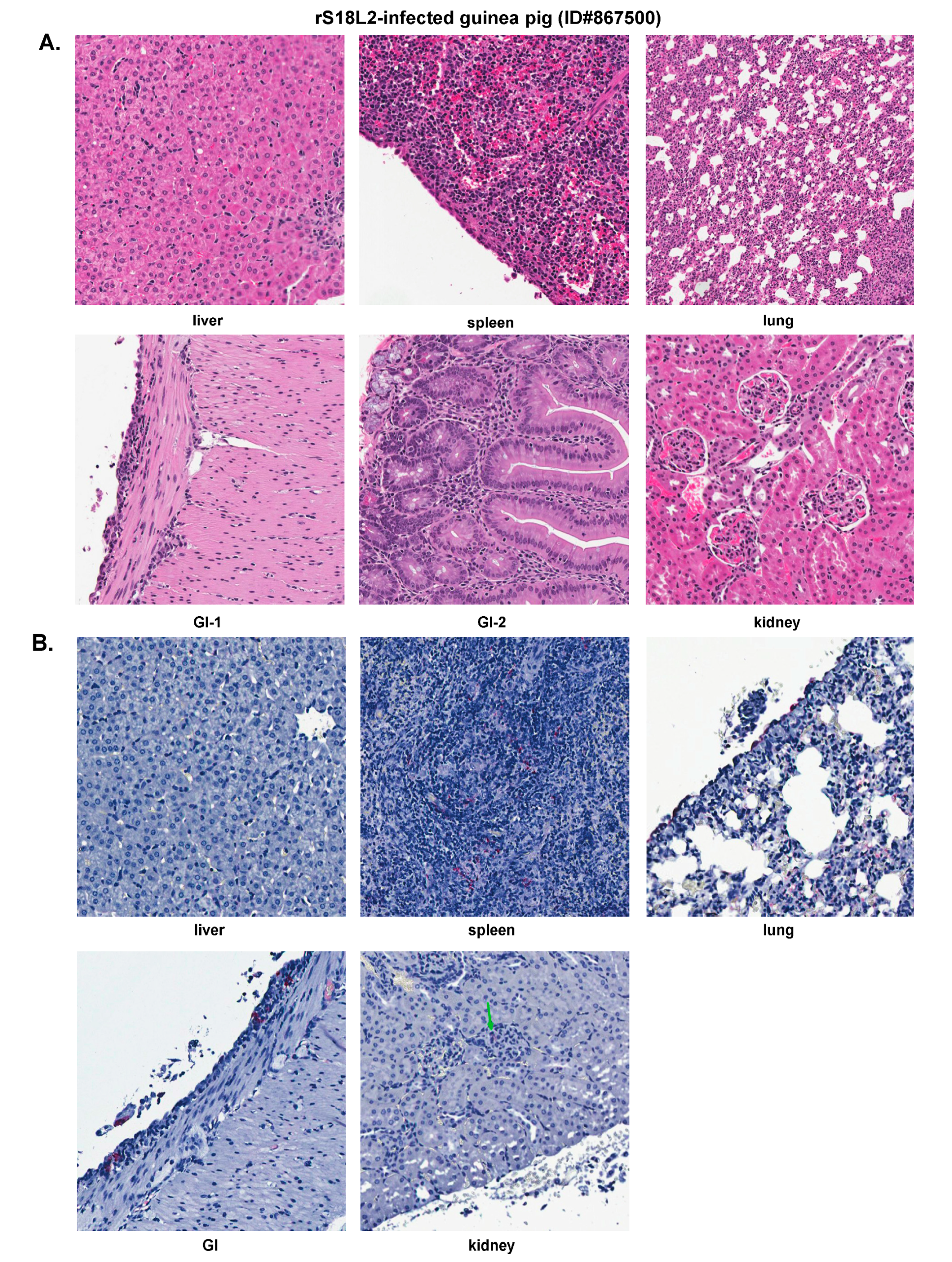
2.6. Histopathological and Immunohistochemical Analyses of Tissue Sections Prepared from a Representative Guinea Pig Infected with a Recombinant rS18L2 Reassortant Virus
3. Discussion
4. Materials and Methods
4.1. In Vivo Experiment
4.2. Histopathological and Immunohistochemical Analyses
4.3. PICV Plaque Assay
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anonymous. Lassa fever. Br. Med. J. 1972, 4, 253–254. [Google Scholar] [CrossRef]
- CDC. Lassa Fever. 2019. Available online: https://www.cdc.gov/vhf/lassa/index.html (accessed on 3 February 2020).
- Ibekwe, T.S.; Okokhere, P.O.; Asogun, D.; Blackie, F.F.; Nwegbu, M.M.; Wahab, K.; Omilabu, S.A.; Akpede, G.O. Early-onset sensorineural hearing loss in Lassa fever. Eur. Arch. Oto-Rhino-Laryngology 2010, 268, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Sattler, R.A.; Paessler, S.; Ly, H.; Huang, C. Animal models of lassa fever. Pathogens 2020, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Wulff, H.; Lange, J.V.; Murphy, F.A. Comparative pathology of Lassa virus infection in monkeys, guinea-pigs, and Mastomys natalensis*. Bull. World Heal. Organ. 1975, 52, 523–534. [Google Scholar]
- Oestereich, L.; Lüdtke, A.; Ruibal, P.; Pallasch, E.; Kerber, R.; Rieger, T.; Wurr, S.; Bockholt, S.; Pérez-Girón, J.V.; Krasemann, S.; et al. Chimeric mice with competent hematopoietic immunity reproduce key features of severe lassa fever. PLoS Pathog. 2016, 12, e1005656. [Google Scholar] [CrossRef]
- Rieger, T.; Merkler, D.; Günther, S. Infection of type I interferon receptor-deficient mice with Various Old World Arenaviruses: A model for studying virulence and host species barriers. PLoS ONE 2013, 8, e72290. [Google Scholar] [CrossRef]
- Yun, N.E.; Poussard, A.L.; Seregin, A.V.; Walker, A.G.; Smith, J.K.; Aronson, J.F.; Soong, L.; Paessler, S.; Smith, J.K.; Smith, J.N. Functional interferon system is required for clearance of Lassa Virus. J. Virol. 2012, 86, 3389–3392. [Google Scholar] [CrossRef]
- Yun, N.E.; Seregin, A.V.; Walker, D.H.; Popov, V.L.; Walker, A.G.; Smith, J.N.; Miller, M.; De La Torre, J.C.; Borisevich, V.; Fair, J.N.; et al. Mice lacking functional STAT1 are highly susceptible to lethal infection with Lassa Virus. J. Virol. 2013, 87, 10908–10911. [Google Scholar] [CrossRef]
- Yun, N.E.; Ronca, S.; Tamura, A.; Koma, T.; Seregin, A.V.; Dineley, K.T.; Miller, M.; Cook, R.; Shimizu, N.; Walker, A.G.; et al. Animal model of Sensorineural hearing loss associated with Lassa Virus infection. J. Virol. 2015, 90, 2920–2927. [Google Scholar] [CrossRef]
- Oestereich, L.; Rieger, T.; Lüdtke, A.; Ruibal, P.; Wurr, S.; Pallasch, E.; Bockholt, S.; Krasemann, S.; Muñoz-Fontela, C.; Günther, S. Efficacy of Favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of Lassa Fever. J. Infect. Dis. 2015, 213, 934–938. [Google Scholar] [CrossRef]
- Flatz, L.; Rieger, T.; Merkler, D.; Bergthaler, A.; Regen, T.; Schedensack, M.; Bestmann, L.; Verschoor, A.; Kreutzfeldt, M.; Bruck, W.; et al. T Cell-dependence of lassa fever pathogenesis. PLoS Pathog. 2010, 6, e1000836. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.M.; Shaia, C.; Bearss, J.J.; Mattix, M.E.; Koistinen, K.A.; Honnold, S.P.; Zeng, X.; Blancett, C.D.; Donnelly, G.C.; Shamblin, J.D.; et al. Temporal progression of lesions in guinea pigs infected with lassa virus. Veter Pathol. 2016, 54, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.A.; Smith, M.A.; Twenhafel, N.A.; Larson, R.A.; Jones, K.F.; Allen, R.D.; Dai, D.; Chinsangaram, J.; Bolken, T.C.; Hruby, D.E.; et al. Evaluation of Lassa antiviral compound ST-193 in a guinea pig model. Antivir. Res. 2011, 90, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Gary, J.M.; Welch, S.R.; Ritter, J.M.; Coleman-McCray, J.; Huynh, T.; Kainulainen, M.H.; Bollweg, B.C.; Parihar, V.; Nichol, S.T.; Zaki, S.R.; et al. Lassa Virus Targeting of Anterior Uvea and Endothelium of Cornea and Conjunctiva in Eye of Guinea Pig Model. Emerg. Infect. Dis. 2019, 25, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, J.; Manning, J.T.; Mateer, E.J.; Sattler, R.; Bukreyeva, N.; Huang, C.; Paessler, S. Lethal infection of lassa virus isolated from a human clinical sample in outbred guinea pigs without adaptation. mSphere 2019, 4, e00428-19. [Google Scholar] [CrossRef]
- Zapata, J.C.; Pauza, C.D.; Djavani, M.M.; Rodas, J.D.; Moshkoff, D.; Bryant, J.; Ateh, E.; Garcia, C.; Lukashevich, I.S.; Salvato, M.S. Lymphocytic choriomeningitis virus (LCMV) infection of macaques: A model for Lassa fever. Antivir. Res. 2011, 92, 125–138. [Google Scholar] [CrossRef]
- Remy, M.M.; Şahin, M.; Flatz, L.; Regen, T.; Xu, L.; Kreutzfeldt, M.; Fallet, B.; Doras, C.; Rieger, T.; Bestmann, L.; et al. Interferon-γ-Driven iNOS: A molecular pathway to terminal shock in Arenavirus Hemorrhagic Fever. Cell Host Microbe 2017, 22, 354–365.e5. [Google Scholar] [CrossRef]
- Trapido, H.; Sanmartín, C. Pichindé virus, a new virus of the Tacaribe group from Colombia. Am. J. Trop. Med. Hyg. 1971, 20, 631–641. [Google Scholar] [CrossRef]
- Aronson, J.F.; Herzog, N.K.; Jerrells, T.R. Pathological and virological features of arenavirus disease in guinea pigs. Comparison of two Pichinde virus strains. Am. J. Pathol. 1994, 145, 228–235. [Google Scholar]
- Connolly, B.M.; Geyer, S.; Peters, C.J.; McPherson, R.A.; Barth, J.F.; Jenson, A.B. Pathogenesis of pichinde virus infection in strain 13 guinea Pigs: An immunocytochemical, virologic, and clinical chemistry study. Am. J. Trop. Med. Hyg. 1993, 49, 10–24. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Hesse, R.A.; Rhoderick, J.B.; Elwell, M.A.; Moe, J.B. Pathogenesis of a pichinde virus strain adapted to produce lethal infections in guinea pigs. Infect. Immun. 1981, 32, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Cosgriff, T.M.; Jahrling, P.B.; Chen, J.P.; Hodgson, L.A.; Lewis, R.M.; Green, D.E.; Smith, J.I. Studies of the coagulation system in arenaviral hemorrhagic fever: Experimental infection of strain 13 Guinea Pigs with Pichinde Virus. Am. J. Trop. Med. Hyg. 1987, 36, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Schelde, L.M.; Wang, J.; Kumar, N.; Ly, H.; Liang, Y. Development of infectious clones for virulent and avirulent pichinde viruses: A model virus to study arenavirus-induced hemorrhagic fevers. J. Virol. 2009, 83, 6357–6362. [Google Scholar] [CrossRef] [PubMed]
- Dhanwani, R.; Huang, Q.; Lan, S.; Zhou, Y.; Shao, J.; Liang, Y.; Ly, H. Establishment of bisegmented and trisegmented reverse genetics systems to generate recombinant Pichindé viruses. Breast Cancer 2018, 1604, 247–253. [Google Scholar] [CrossRef]
- Liang, Y.; Lan, S.; Ly, H. Molecular determinants of pichinde virus infection of guinea pigs-a small animal model system for arenaviral hemorrhagic fevers. Ann. N. Y. Acad. Sci. 2009, 1171, E65–E74. [Google Scholar] [CrossRef]
- Lan, S.; Shieh, W.J.; Huang, Q.; Zaki, S.R.; Ly, H.; Liang, Y. Recombinant Pichinde virus infection of outbred Hartley guinea pigs as an improved surrogate small animal model for Lassa fever. Virulence 2020. (submitted). [Google Scholar]
- Walker, D.H.; McCormick, J.B.; Johnson, K.M.; Webb, P.A.; Komba-Kono, G.; Elliott, L.H.; Gardner, J.J. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 1982, 107, 349–356. [Google Scholar]
- McLay, L.; Lan, S.; Ansari, A.; Liang, Y.; Ly, H. Identification of Virulence Determinants within the L Genomic Segment of the Pichinde Arenavirus. J. Virol. 2013, 87, 6635–6643. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Smith, S.A.; Hesse, R.; Rhoderick, J.B. Pathogenesis of Lassa virus infection in guinea pigs. Infect. Immun. 1982, 37, 771–778. [Google Scholar] [CrossRef]
- Safronetz, D.; Mire, C.; Rosenke, K.; Feldmann, F.; Haddock, E.; Geisbert, T.; Feldmann, H. A Recombinant vesicular stomatitis virus-based Lassa fever vaccine protects guinea pigs and macaques against challenge with geographically and genetically distinct Lassa Viruses. PLoS Neglected Trop. Dis. 2015, 9, e0003736. [Google Scholar] [CrossRef]
- Safronetz, D.; Strong, J.E.; Feldmann, F.; Haddock, E.; Sogoba, N.; Brining, U.; Geisbert, T.W.; Scott, D.P.; Feldmann, H. A recently isolated Lassa virus from Mali demonstrates atypical clinical disease manifestations and decreased virulence in Cynomolgus macaques. J. Infect. Dis. 2013, 207, 1316–1327. [Google Scholar] [CrossRef]
- Walker, D.H.; Johnson, K.M.; Lange, J.V.; Gardner, J.J.; Kiley, M.P.; McCormick, J.B. Experimental infection of rhesus monkeys with Lassa virus and a closely related arenavirus, mozambique virus. J. Infect. Dis. 1982, 146, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.W.; Mire, C.E.; Branco, L.M.; Geisbert, J.B.; Rowland, M.M.; Heinrich, M.L.; Goba, A.; Momoh, M.; Grant, D.S.; Fullah, M.; et al. Treatment of Lassa virus infection in outbred guinea pigs with first-in-class human monoclonal antibodies. Antivir. Res. 2016, 133, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Safronetz, D.; Rosenke, K.; Westover, J.B.; Martellaro, C.; Okumura, A.; Furuta, Y.; Geisbert, J.; Saturday, G.; Komeno, T.; Geisbert, T.W.; et al. The broad-spectrum antiviral favipiravir protects guinea pigs from lethal Lassa virus infection post-disease onset. Sci. Rep. 2015, 5, 14775. [Google Scholar] [CrossRef]
- Stein, D.R.; Warner, B.M.; Soule, G.; Tierney, K.; Frost, K.L.; Booth, S.; Safronetz, D. A recombinant vesicular stomatitis-based Lassa fever vaccine elicits rapid and long-term protection from lethal Lassa virus infection in guinea pigs. npj Vaccines 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Butler, J.C.; Zaki, S.R.; Khabbaz, R.F.; Peters, C.J. Hantavirus pulmonary syndrome. Infect. Dis. Clin. Pr. 1995, 4, 189–193. [Google Scholar] [CrossRef][Green Version]
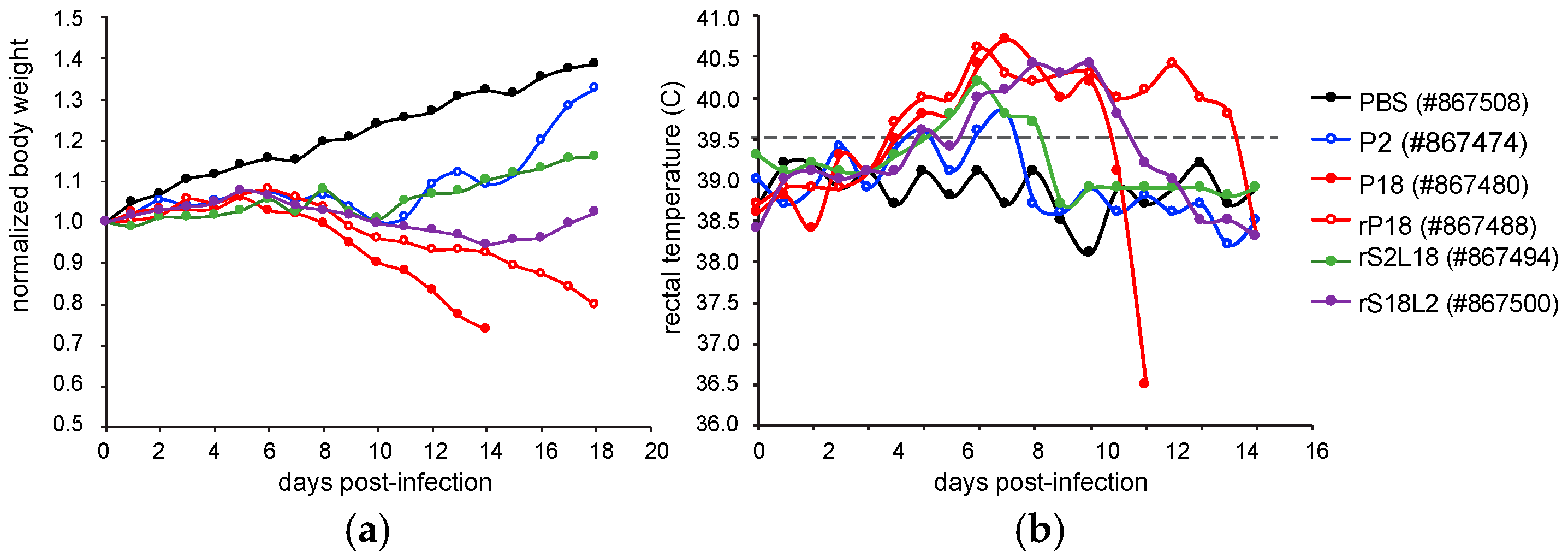

| Guinea Pig ID | Viruses | Fever, BW Loss 1 | DoE 2 | Health, BW at DoE 3 |
|---|---|---|---|---|
| 867508 | PBS | No fever, no BW loss | 18 dpi | Healthy, BW 139% |
| 867474 | P2 | Fever d6 to d9, BW loss d9 to d10 | 18 dpi | Healthy, BW 132% |
| 867480 | P18 | Fever d5 to d13, BW loss since d8 | 14 dpi | Moribund, BW 74% |
| 867488 | rP18 | Fever d5 to d17 BW loss since d9 | 18 dpi | Moribund BW 80% |
| 867494 | rS2L18 | Fever d6 to d10 BW loss d9 to d10 | 18 dpi | Healthy, BW 116% |
| 867500 | rS18L2 | Fever d6 to d13 BW loss d9 to d16 | 18 dpi | Healthy, BW 102% |
| Guinea Pig ID | Viruses | 6 dpi (PFU/mL) | 12 dpi (PFU/mL) | TP 1 or 18 dpi (PFU/mL) |
|---|---|---|---|---|
| 867474 | P2 | BD | BD | BD |
| 867480 | P18 | 360 | 3.4 × 104 | 4 × 106 |
| 867488 | rP18 | NA | NA | 1.8 × 106 |
| 867494 | rS2L18 | BD | BD | BD |
| 867500 | rS18L2 | BD | BD | BD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shieh, W.-J.; Lan, S.; Zaki, S.R.; Ly, H.; Liang, Y. Pichinde Virus Infection of Outbred Hartley Guinea Pigs as a Surrogate Animal Model for Human Lassa Fever: Histopathological and Immunohistochemical Analyses. Pathogens 2020, 9, 579. https://doi.org/10.3390/pathogens9070579
Shieh W-J, Lan S, Zaki SR, Ly H, Liang Y. Pichinde Virus Infection of Outbred Hartley Guinea Pigs as a Surrogate Animal Model for Human Lassa Fever: Histopathological and Immunohistochemical Analyses. Pathogens. 2020; 9(7):579. https://doi.org/10.3390/pathogens9070579
Chicago/Turabian StyleShieh, Wun-Ju, Shuiyun Lan, Sherif R. Zaki, Hinh Ly, and Yuying Liang. 2020. "Pichinde Virus Infection of Outbred Hartley Guinea Pigs as a Surrogate Animal Model for Human Lassa Fever: Histopathological and Immunohistochemical Analyses" Pathogens 9, no. 7: 579. https://doi.org/10.3390/pathogens9070579
APA StyleShieh, W.-J., Lan, S., Zaki, S. R., Ly, H., & Liang, Y. (2020). Pichinde Virus Infection of Outbred Hartley Guinea Pigs as a Surrogate Animal Model for Human Lassa Fever: Histopathological and Immunohistochemical Analyses. Pathogens, 9(7), 579. https://doi.org/10.3390/pathogens9070579






