Intracellular Growth and Cell Cycle Progression are Dependent on (p)ppGpp Synthetase/Hydrolase in Brucella abortus
Abstract
:1. Introduction
2. Results
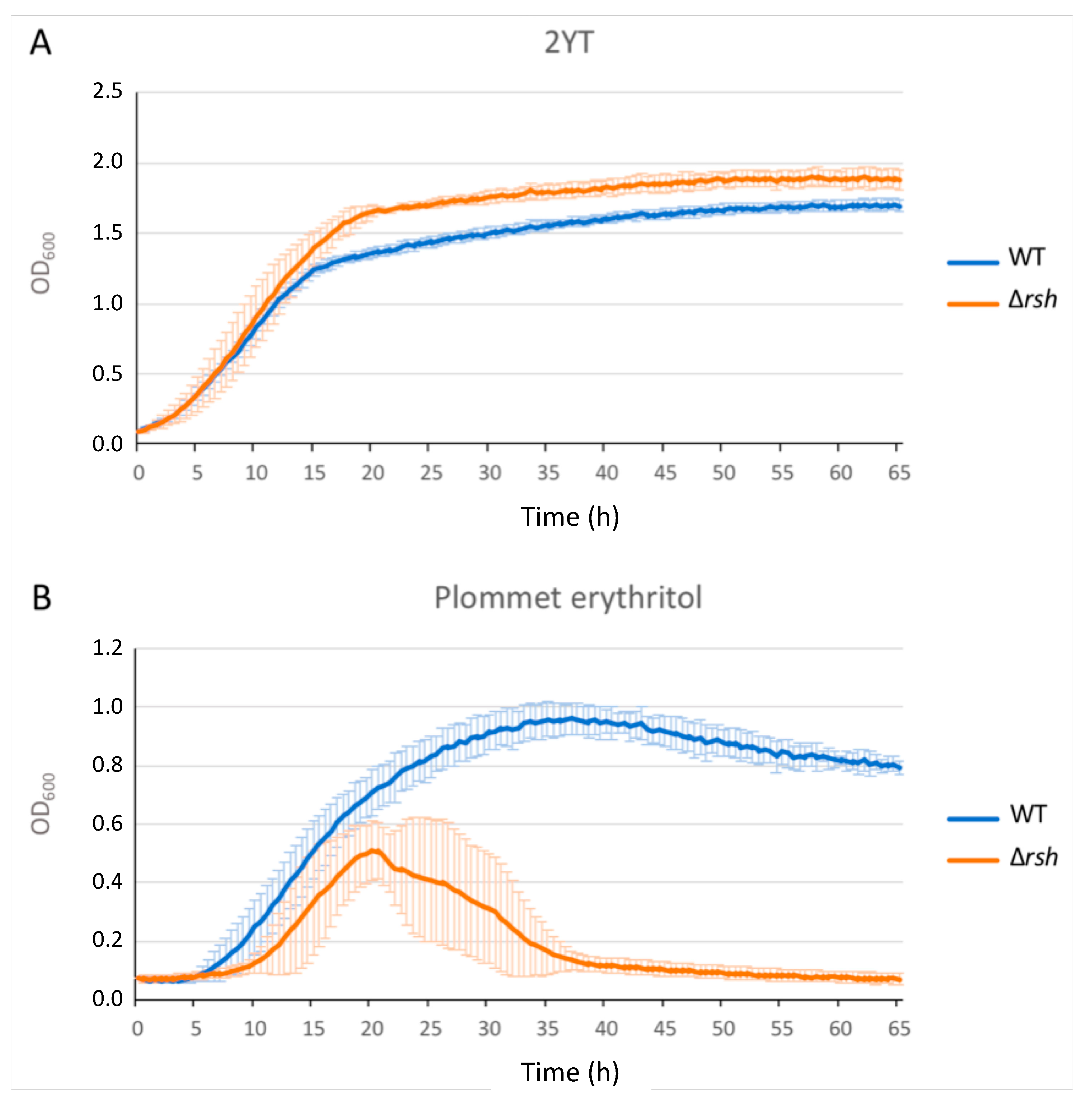
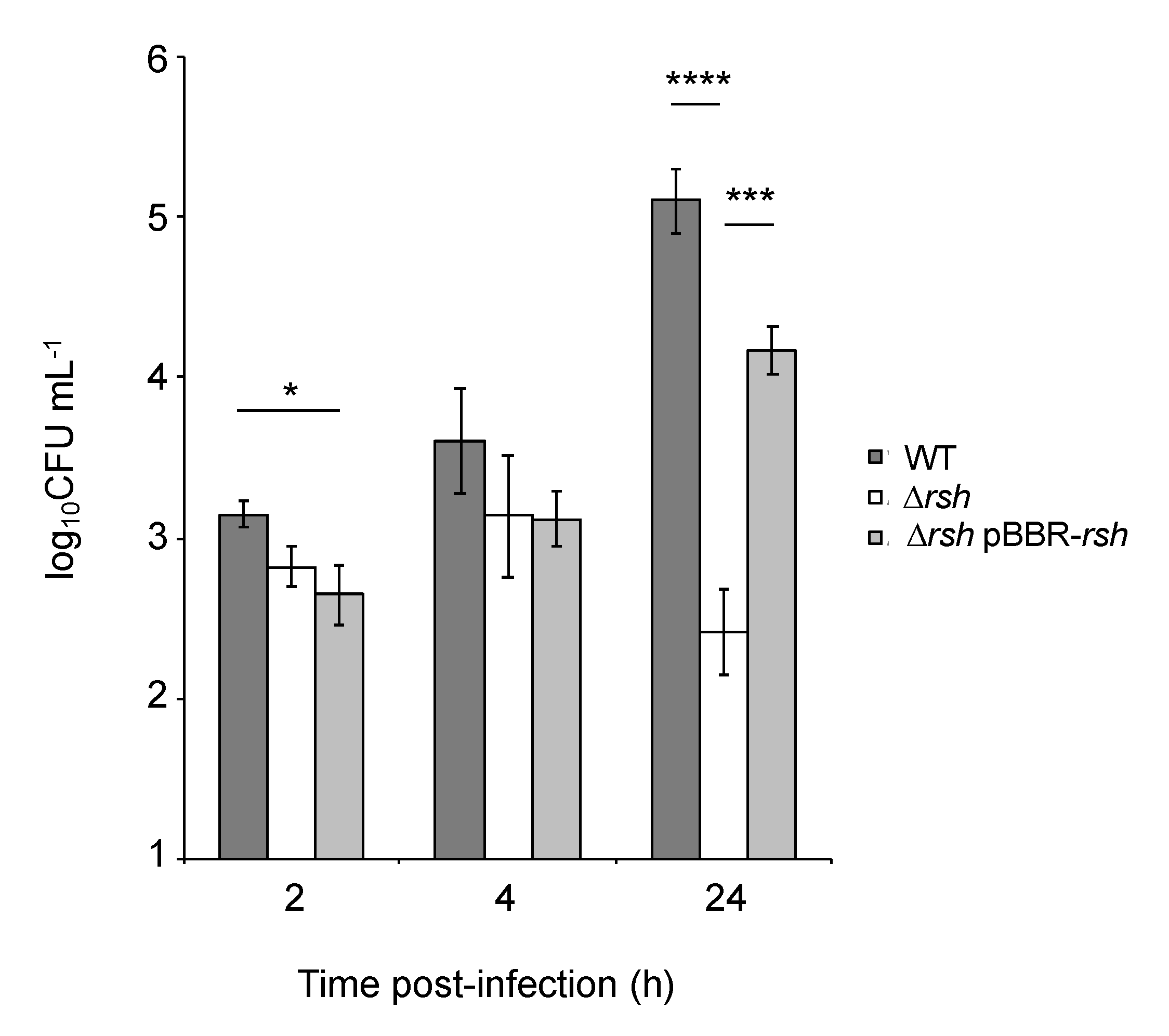
2.1. rsh Deletion Drastically Impacts Growth in Minimal Medium and the Infection Process
2.2. The Artificial Hydrolysis of (p)ppGpp Leads to a Δrsh Phenotype during Infection
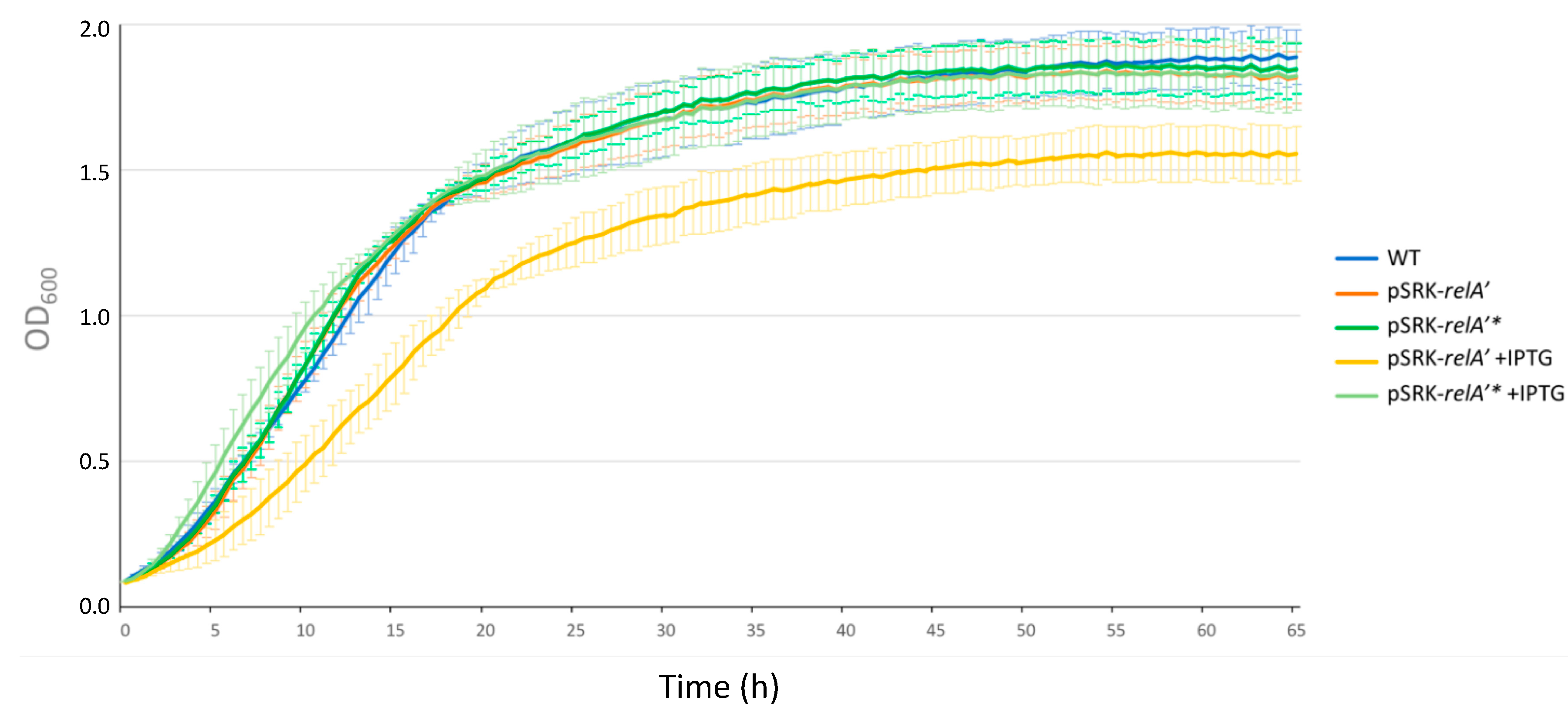
2.3. Expression of a Constitutive Allele for a (p)ppGpp Synthetase Impacts Bacterial Growth and Chromosome Replication
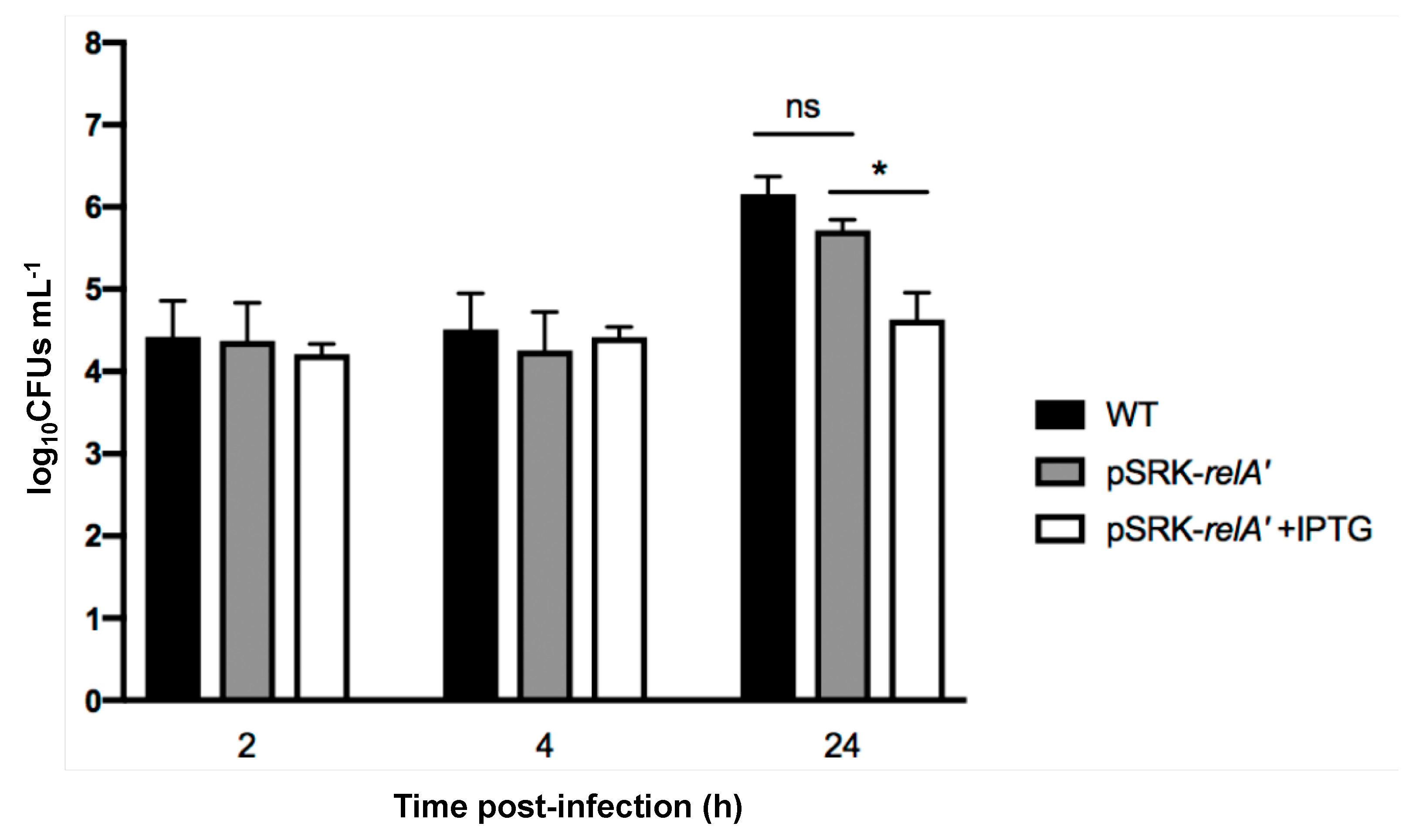
2.4. Induced Production of a Constitutive (p)ppGpp Synthetase Leads to a Proliferation Defect during Infection
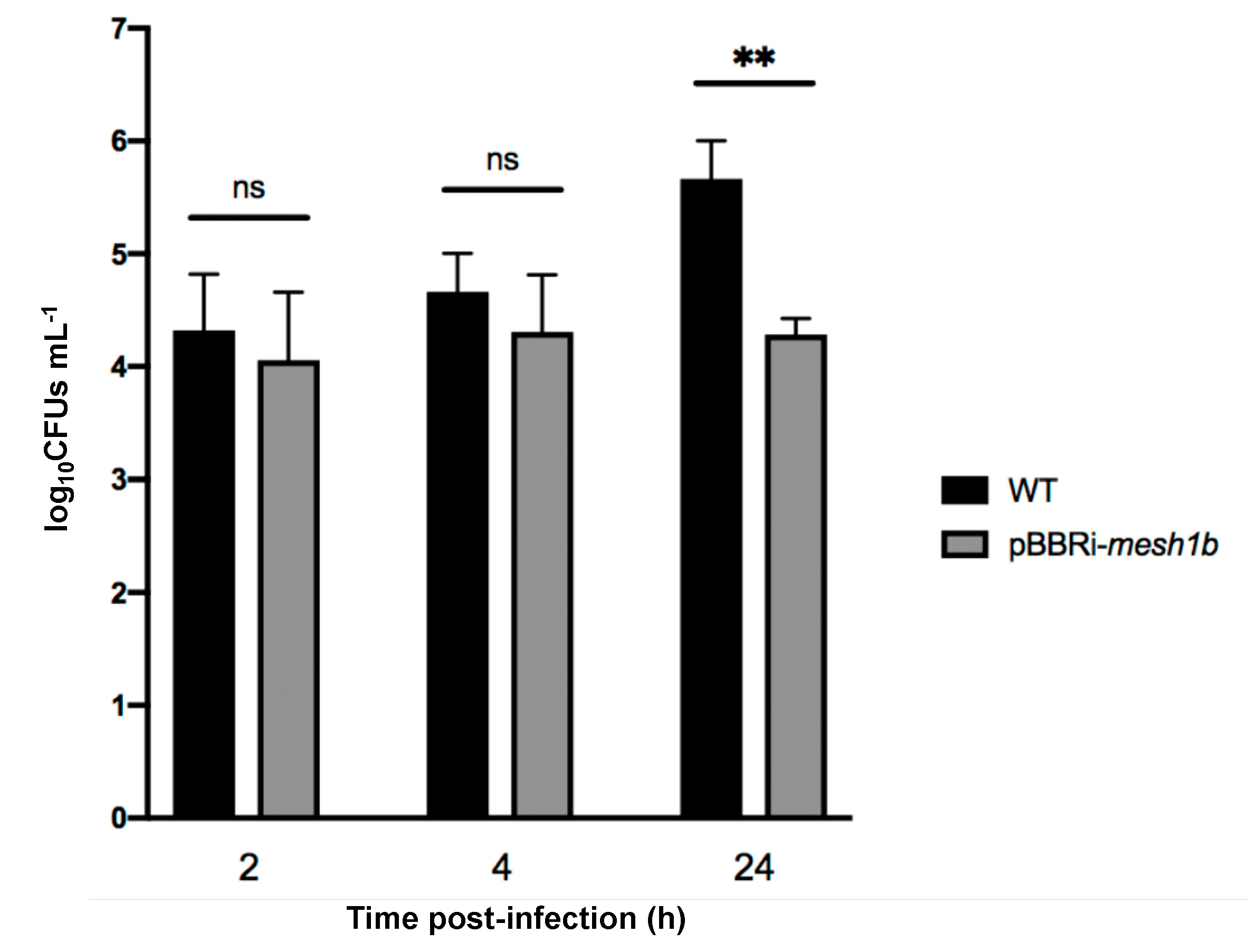
2.5. DksA Is Not Required during the Infection Process
3. Discussion
4. Materials and Methods
4.1. Strains and Growth Conditions
4.2. Strains Construction
4.3. Growth Assays
4.4. Survival Assays
4.5. Infections of RAW 264.7 Macrophages
4.6. Infections of HeLa Cells
4.7. G1 Counting
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moreno, E.; Moriyon, I. The Genus Brucella. Prokaryotes 2006, 5, 315–456. [Google Scholar]
- Deghelt, M.; Mullier, C.; Sternon, J.F.; Francis, N.; Laloux, G.; Dotreppe, D.; Van der Henst, C.; Jacobs-Wagner, C.; Letesson, J.J.; De Bolle, X. The newborn Brucella abortus blocked at the G1 stage of its cell cycle is the major infectious bacterial subpopulation. Nat. Commun. 2014, 5, 4366. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.; Ng, T.W.; Wehrly, T.D.; Knodler, L.A.; Celli, J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 2008, 9, 678–694. [Google Scholar] [CrossRef] [PubMed]
- Porte, F.; Liautard, J.P.; Kohler, S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 1999, 67, 4041–4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizarro-Cerda, J.; Meresse, S.; Parton, R.G.; van der Goot, G.; Sola-Landa, A.; Lopez-Goni, I.; Moreno, E.; Gorvel, J.P. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 1998, 66, 5711–5724. [Google Scholar] [CrossRef] [Green Version]
- Starr, T.; Child, R.; Wehrly, T.D.; Hansen, B.; Hwang, S.; Lopez-Otin, C.; Virgin, H.W.; Celli, J. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 2012, 11, 33–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roop, R.M., 2nd; Gaines, J.M.; Anderson, E.S.; Caswell, C.C.; Martin, D.W. Survival of the fittest: How Brucella strains adapt to their intracellular niche in the host. Med. Microbiol. Immunol. 2009, 198, 221–238. [Google Scholar] [CrossRef] [Green Version]
- Poncin, K.; Roba, A.; Jimmidi, R.; Potemberg, G.; Fioravanti, A.; Francis, N.; Willemart, K.; Zeippen, N.; Machelart, A.; Biondi, E.G.; et al. Occurrence and repair of alkylating stress in the intracellular pathogen Brucella abortus. Nat. Commun. 2019, 10, 4847. [Google Scholar] [CrossRef] [Green Version]
- Kohler, S.; Foulongne, V.; Ouahrani-Bettache, S.; Bourg, G.; Teyssier, J.; Ramuz, M.; Liautard, J.P. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. USA 2002, 99, 15711–15716. [Google Scholar] [CrossRef] [Green Version]
- Dozot, M.; Boigegrain, R.A.; Delrue, R.M.; Hallez, R.; Ouahrani-Bettache, S.; Danese, I.; Letesson, J.J.; De Bolle, X.; Kohler, S. The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system virB. Cell Microbiol. 2006, 8, 1791–1802. [Google Scholar] [CrossRef]
- Kim, S.; Watanabe, K.; Suzuki, H.; Watarai, M. Roles of Brucella abortus SpoT in morphological differentiation and intramacrophagic replication. Microbiology 2005, 151, 1607–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, D.; Collier, J. Effects of (p)ppGpp on the progression of the cell cycle of Caulobacter crescentus. J. Bacteriol. 2014, 196, 2514–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesley, J.A.; Shapiro, L. SpoT regulates DnaA stability and initiation of DNA replication in carbon-starved Caulobacter crescentus. J. Bacteriol. 2008, 190, 6867–6880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronneau, S.; Petit, K.; De Bolle, X.; Hallez, R. Phosphotransferase-dependent accumulation of (p)ppGpp in response to glutamine deprivation in Caulobacter crescentus. Nat. Commun. 2016, 7, 11423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, G.; Ron, E.Z.; Glaser, G. ppGpp-mediated regulation of DNA replication and cell division in Escherichia coli. Curr. Microbiol. 1995, 30, 27–32. [Google Scholar] [CrossRef]
- Dalebroux, Z.D.; Edwards, R.L.; Swanson, M.S. SpoT governs Legionella pneumophila differentiation in host macrophages. Mol. Microbiol. 2009, 71, 640–658. [Google Scholar] [CrossRef]
- Das, B.; Pal, R.R.; Bag, S.; Bhadra, R.K. Stringent response in Vibrio cholerae: Genetic analysis of spoT gene function and identification of a novel (p)ppGpp synthetase gene. Mol. Microbiol. 2009, 72, 380–398. [Google Scholar] [CrossRef]
- Dahl, J.L.; Kraus, C.N.; Boshoff, H.I.; Doan, B.; Foley, K.; Avarbock, D.; Kaplan, G.; Mizrahi, V.; Rubin, H.; Barry, C.E., 3rd. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10026–10031. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.J.; Barker, M.M.; Ross, W.; Schneider, D.A.; Webb, C.; Foster, J.W.; Gourse, R.L. DksA: A critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 2004, 118, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Ross, W.; Sanchez-Vazquez, P.; Chen, A.Y.; Lee, J.H.; Burgos, H.L.; Gourse, R.L. ppGpp Binding to a Site at the RNAP-DksA Interface Accounts for Its Dramatic Effects on Transcription Initiation during the Stringent Response. Mol. Cell 2016, 62, 811–823. [Google Scholar] [CrossRef] [Green Version]
- Ronneau, S.; Hallez, R. Make and break the alarmone: Regulation of (p)ppGpp synthetase/hydrolase enzymes in bacteria. FEMS Microbiol. Rev. 2019, 43, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Mittenhuber, G. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins). J. Mol. Microbiol. Biotechnol. 2001, 3, 585–600. [Google Scholar] [PubMed]
- Plommet, M. Minimal requirements for growth of Brucella suis and other Brucella species. Zentralblatt Bakteriologie 1991, 275, 436–450. [Google Scholar] [CrossRef]
- Ronneau, S.; Caballero-Montes, J.; Coppine, J.; Mayard, A.; Garcia-Pino, A.; Hallez, R. Regulation of (p)ppGpp hydrolysis by a conserved archetypal regulatory domain. Nucleic Acids Res. 2019, 47, 843–854. [Google Scholar] [CrossRef] [Green Version]
- Sternon, J.F.; Godessart, P.; Goncalves de Freitas, R.; Van der Henst, M.; Poncin, K.; Francis, N.; Willemart, K.; Christen, M.; Christen, B.; Letesson, J.J.; et al. Transposon Sequencing of Brucella abortus Uncovers Essential Genes for Growth In Vitro and Inside Macrophages. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Lee, G.; Lee, J.H.; Kim, H.Y.; Rhee, H.W.; Park, S.Y.; Kim, K.J.; Kim, Y.; Kim, B.Y.; Hong, J.I.; et al. A metazoan ortholog of SpoT hydrolyzes ppGpp and functions in starvation responses. Nat. Struct. Mol. Biol. 2010, 17, 1188–1194. [Google Scholar] [CrossRef]
- Khan, S.R.; Gaines, J.; Roop, R.M., 2nd; Farrand, S.K. Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl. Environ. Microbiol. 2008, 74, 5053–5062. [Google Scholar] [CrossRef] [Green Version]
- Hanna, N.; Ouahrani-Bettache, S.; Drake, K.L.; Adams, L.G.; Kohler, S.; Occhialini, A. Global Rsh-dependent transcription profile of Brucella suis during stringent response unravels adaptation to nutrient starvation and cross-talk with other stress responses. BMC Genom. 2013, 14, 459. [Google Scholar] [CrossRef] [Green Version]
- Dozot, M.; Poncet, S.; Nicolas, C.; Copin, R.; Bouraoui, H.; Maze, A.; Deutscher, J.; De Bolle, X.; Letesson, J.J. Functional characterization of the incomplete phosphotransferase system (PTS) of the intracellular pathogen Brucella melitensis. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Nunez, C.; Altamirano-Silva, P.; Alvarado-Guillen, F.; Moreno, E.; Guzman-Verri, C.; Chaves-Olarte, E. The two-component system BvrR/BvrS regulates the expression of the type IV secretion system VirB in Brucella abortus. J. Bacteriol. 2010, 192, 5603–5608. [Google Scholar] [CrossRef] [Green Version]
- Lacerda, T.L.; Salcedo, S.P.; Gorvel, J.P. Brucella T4SS: The VIP pass inside host cells. Curr. Opin. Microbiol. 2013, 16, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Terwagne, M.; Mirabella, A.; Lemaire, J.; Deschamps, C.; De Bolle, X.; Letesson, J.J. Quorum sensing and self-quorum quenching in the intracellular pathogen Brucella melitensis. PLoS ONE 2013, 8, e82514. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Gaynor, J.B.; White, K.G.; Lopez, C.; Bosio, C.M.; Karkhoff-Schweizer, R.R.; Schweizer, H.P. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2005, 2, 443–448. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van der Henst, M.; Carlier, E.; De Bolle, X. Intracellular Growth and Cell Cycle Progression are Dependent on (p)ppGpp Synthetase/Hydrolase in Brucella abortus. Pathogens 2020, 9, 571. https://doi.org/10.3390/pathogens9070571
Van der Henst M, Carlier E, De Bolle X. Intracellular Growth and Cell Cycle Progression are Dependent on (p)ppGpp Synthetase/Hydrolase in Brucella abortus. Pathogens. 2020; 9(7):571. https://doi.org/10.3390/pathogens9070571
Chicago/Turabian StyleVan der Henst, Mathilde, Elodie Carlier, and Xavier De Bolle. 2020. "Intracellular Growth and Cell Cycle Progression are Dependent on (p)ppGpp Synthetase/Hydrolase in Brucella abortus" Pathogens 9, no. 7: 571. https://doi.org/10.3390/pathogens9070571
APA StyleVan der Henst, M., Carlier, E., & De Bolle, X. (2020). Intracellular Growth and Cell Cycle Progression are Dependent on (p)ppGpp Synthetase/Hydrolase in Brucella abortus. Pathogens, 9(7), 571. https://doi.org/10.3390/pathogens9070571



