Legionella Diversity and Spatiotemporal Variation in the Occurrence of Opportunistic Pathogens within a Large Building Water System
Abstract
1. Introduction
2. Results
2.1. Description of Sampling Locations and Water Quality Characteristics
2.2. Legionella Culture Results
2.3. Detection of Water-based Pathogens and Free-living Amoebae (FLA) via Quantitative PCR Analyses
2.3.1. Legionella spp. and L. pneumophila Occurrence
2.3.2. Free-living Amoebae and Mycobacterium intracellulare Occurrence
2.4. Additional Sampling Sites
2.5. Statistical Correlations between Water Quality Characteristics
2.6. Whole Genome Sequencing of Drinking Water Isolates
3. Discussion
4. Materials and Methods
4.1. Sampling Locations
4.2. Sample Collection and Processing
4.3. Water Quality Analysis
4.4. Legionella Enumeration and Presumptive Colony Analysis
4.5. Isolation and Preparation of Total DNA
4.6. Quantitative Polymerase Chain Reaction (qPCR)
4.7. Whole Genome Sequencing and Sequence Analyses
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Disclaimer
Conflicts of Interest
References
- WHO. Legionella and the Prevention of Legionellosis; World Health Organization Press: Geneva, Switzerland, 2007. [Google Scholar]
- Davis, G.S.; Winn, W.C., Jr.; Gump, D.W.; Craighead, J.E.; Beaty, H.N. Legionnaires’ pneumonia after aerosol exposure in guinea pigs and rats. Am. Rev. Respir. Dis. 1982, 126, 1050–1057. [Google Scholar]
- Kaufmann, A.F.; McDade, J.E.; Patton, C.M.; Bennett, J.V.; Skaliy, P.; Feeley, J.C.; Anderson, D.C.; Potter, M.E.; Newhouse, V.F.; Gregg, M.B.; et al. Pontiac fever: Isolation of the etiologic agent (Legionella pneumophilia) and demonstration of its smode of transmission. Am. J. Epidemiol. 1981, 114, 337–347. [Google Scholar] [CrossRef]
- Beer, K.B.; Gargano, J.W.; Roberts, V.A.; Reses, H.E.; Hill, V.R.; Garrison, L.E.; Kutty, P.K.; Hilborn, E.D.; Wade, T.J.; Fullerton, K.E.; et al. Outbreaks associated with environmental and undetermined water exposures—United States, 2011–2012. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 849–851. [Google Scholar] [CrossRef][Green Version]
- Bededict, K.M.; Reses, H.E.; Vigar, M.; Roth, D.M.; Roberts, V.A.; Mattioli, M.; Cooley, L.A.; Hilborn, E.D.; Wade, T.J.; Fullerton, K.E.; et al. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2013–2014. MMWR Morb Mortal. Wkly. Rep. 2017, 66, 1216–1221. [Google Scholar] [CrossRef]
- Fraser, D.W.; Tsai, T.R.; Orenstein, W.; Parkin, W.E.; Beecham, H.J.; Sharrar, R.G. Legionnaires’ disease: Description of an epidemic of pneumonia. N. Engl. J. Med. 1977, 297, 1189–1197. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, S.; Sharaby, Y.; Pecellín, M.; Brettar, I.; Höfle, M.; Halpern, M. Spatial distribution of Legionella pneumophila MLVA-genotypes in a drinking water system. Water Res. 2015, 77, 119–132. [Google Scholar] [CrossRef]
- King, D.N.; Donohue, M.J.; Vesper, S.J.; Villegas, E.N.; Ware, M.W.; Vogel, M.E.; Furlong, E.F.; Kolpin, D.W.; Glassmeyer, S.T.; Pfaller, S. Microbial pathogens in source and treated waters from drinking water treatment plants in the United States and implications for human health. Sci. Total Environ. 2016, 562, 987–995. [Google Scholar] [CrossRef]
- Donohue, M.J.; King, D.; Pfaller, S.; Mistry, J.H. The sporadic nature of Legionella pneumophila, Legionella pneumophila Sg1 and Mycobacterium avium occurrence within residences and office buildings across 36 states in the United States. J. Appl. Microbiol. 2019, 126, 1568–1579. [Google Scholar] [CrossRef]
- Stout, J.E.; Brennen, C.; Muder, R.R. Legionnaires’ disease in a newly constructed long-term care facility. J. Am. Geriatr. Soc. 2000, 48, 1589–1592. [Google Scholar] [CrossRef]
- Orsi, G.B.; Vitali, M.; Marinelli, L.; Ciorba, V.; Tufi, D.; Del Cimmuto, A.; Ursillo, P.; Fabiani, M.; De Santis, S.; Protano, C.; et al. Legionella control in the water system of antiquated hospital buildings by shock and continuous hyperchlorination: 5 years experience. BMC Infect. Dis. 2014, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Perola, O.; Kauppinen, J.; Kusnetsov, J.; Kärkkäinen, U.-M.; Lück, P.C.; Katila, M.-L. Persistent Legionella pneumophila colonization of a hospital water supply: Efficacy of control methods and a molecular epidemiological analysis. APMIS 2005, 113, 45–53. [Google Scholar] [CrossRef]
- Scaturro, M.; Dell’eva, I.; Helfer, F.; Ricci, M.L. Persistence of the same strain of Legionella pneumophila in the water system of an Italian hospital for 15 years. Infect. Control. Hosp. Epidemiol. 2007, 28, 1089–1092. [Google Scholar] [CrossRef]
- Cooper, I.R.; White, J.; Mahenthiralingam, E.; Hanlon, G.W. Long-term persistence of a single Legionella pneumophila strain possessing the mip gene in a municipal shower despite repeated cycles of chlorination. J. Hosp. Infect. 2008, 70, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Nuñez, M.; Sopena, N.; Ragull, S.; Pedro-Botet, M.L.; Morera, J.; Sabria, M. Persistence of Legionella in hospital water supplies and nosocomial Legionnaires’ disease. FEMS Immunol. Med. Microbiol. 2008, 52, 202–206. [Google Scholar] [CrossRef]
- Bédard, E.; Boppe, I.; Kouamé, S.; Martin, P.; Pinsonneault, L.; Valiquette, L.; Racine, J.; Prévost, M. Combination of heat shock and enhanced thermal regime to control the growth of a persistent Legionella pneumophila strain. Pathogens 2016, 5, 35. [Google Scholar] [CrossRef]
- Gomez-Valero, L.; Rusniok, C.; Jarraud, S.; Vacherie, B.; Rouy, Z.; Barbe, V.; Medigue, C.; Etienne, J.; Buchrieser, C. Extensive recombination events and horizontal gene transfer shaped the Legionella pneumophila genomes. BMC Genom. 2011, 12, 536. [Google Scholar] [CrossRef]
- Segal, G.; Shuman, H.A. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 1999, 67, 2117–2124. [Google Scholar] [CrossRef]
- Ensminger, A.W. Legionella pneumophila, armed to the hilt: Justifying the largest arsenal of effectors in the bacterial world. Curr. Opin. Microbiol. 2016, 29, 74–80. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Environmental (saprozoic) pathogens of engineered water systems: Understanding their ecology for risk assessment and management. Pathogens 2015, 4, 390–405. [Google Scholar] [CrossRef]
- Ben Salah, I.; Drancourt, M. Surviving within the amoebal exocyst: The Mycobacterium avium complex paradigm. BMC Microbiol. 2010, 10, 99. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O., 3rd; Norton, C.D.; LeChevallier, M.W. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 2001, 67, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Wadowsky, R.M.; Yee, R.B. Satellite growth of Legionella pneumophila with an environmental isolate of Flavobacterium breve. Appl. Environ. Microbiol. 1983, 46, 1447–1449. [Google Scholar] [CrossRef]
- Wadowsky, R.M.; Yee, R.B. Effect of non-Legionellaceae bacteria on the multiplication of Legionella pneumophila in potable water. Appl. Environ. Microb. 1985, 49, 1206–1210. [Google Scholar] [CrossRef]
- Temmerman, R.; Vervaeren, H.; Noseda, B.; Boon, N.; Verstraete, W. Necrotrophic growth of Legionella pneumophila. Appl. Environ. Microbiol. 2006, 72, 4323–4328. [Google Scholar] [CrossRef]
- Tison, D.L.; Pope, D.H.; Cherry, W.B.; Fliermans, C.B. Growth of Legionella pneumophila in association with blue-green algae (cyanobacteria). Appl. Environ. Microb. 1980, 39, 456–459. [Google Scholar] [CrossRef]
- Rogers, J.; Dowsett, A.B.; Dennis, P.J.; Lee, J.V.; Keevil, C.W. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl. Environ. Microbiol. 1994, 60, 1585–1592. [Google Scholar] [CrossRef]
- Cristino, S.; Legnani, P.P.; Leoni, E. Plan for the control of Legionella infections in long-term care facilities: Role of environmental monitoring. Int. J. Hyg. Environ. Health 2012, 215, 279–285. [Google Scholar] [CrossRef]
- CDC. Developing a Water Management Program to Reduce Legionella Growth & Spread in Buildings: A Practical Guide to Implementing Industry Standards; USDHHS, Ed.; CDC: Atlanta, GA, USA, 2017; p. 36.
- ANSI/ASHRAE. Standard 188-2018 Legionellosis: Risk Management for Building Water Systems; ANSI/ASHRAE: Atlanta, GA, USA, 2018. [Google Scholar]
- Szabo, J.G.; Meiners, G.; Heckman, L.; Rice, E.W.; Hall, J. Decontamination of Bacillus spores adhered to iron and cement-mortar drinking water infrastructure in a model system using disinfectants. J. Environ. Manag. 2017, 187, 1–7. [Google Scholar] [CrossRef]
- National Academies of Science, Engineering, and Medicine. Management of Legionella in Water Systems; The National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- Neil, K.; Berkelman, R. Increasing incidence of legionellosis in the United States, 1990–2005: Changing epidemiologic trends. Clin. Infect. Dis. 2008, 47, 591–599. [Google Scholar] [CrossRef]
- Alarcon Falconi, T.M.; Cruz, M.S.; Naumova, E.N. The shift in seasonality of legionellosis in the USA. Epidemiol. Infect. 2018, 146, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Bhopal, R.S.; Fallon, R.J. Seasonal variation of Legionnaires’ disease in Scotland. J. Infect. 1991, 22, 153–160. [Google Scholar] [CrossRef]
- Joseph, C.A.; Watson, J.M.; Harrison, T.G.; Bartlett, C.L.R. Nosocomial Legionnaires’ disease in England and Wales, 1980–92. Epidemiol. Infect. 1994, 112, 329–346. [Google Scholar] [CrossRef]
- Von Baum, H.; Ewig, S.; Marre, R.; Suttorp, N.; Gonschior, S.; Welte, T.; Lück, C.; Group CNfCAPS. Community-acquired Legionella pneumonia: New insights from the German Competence Network for Community Acquired Pneumonia. Clin. Infect. Dis. 2008, 46, 1356–1364. [Google Scholar] [CrossRef]
- Ng, V.; Tang, P.; Jamieson, F.; Drews, S.J.; Brown, S.; Low, D.E.; Johnson, C.C.; Fisman, D.N. Going with the flow: Legionellosis risk in Toronto, Canada is strongly associated with local watershed hydrology. Ecohealth 2008, 5, 482–490. [Google Scholar] [CrossRef]
- Simmering, J.E.; Polgreen, L.A.; Hornick, D.B.; Sewell, D.K.; Polgreen, P.M. Weather-dependent risk for Legionnaires’ Disease, United States. Emerg. Infect. Dis. 2017, 23, 1843–1851. [Google Scholar] [CrossRef]
- Cassell, K.; Gacek, P.; Warren, J.L.; Raymond, P.A.; Cartter, M.; Weinberger, D.M. Association between sporadic legionellosis and river systems in Connecticut. J. Infect. Dis. 2018, 217, 179–187. [Google Scholar] [CrossRef]
- Barskey, A.; Lackraj, D.; Tripathi, P.S.; Cooley, L.A.; Lee, S.; Smith, J.; Edens, C. Legionnaires’ Disease Surveillance Summary Report, United States—2016 and 2017; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- ISO. ISO 12869:2012 Water Quality—Detection and Quantification of Legionella spp. and/or Legionella Pneumophila by Concentration and Genic Amplification by quantitative Polymerase Chain Reaction (qPCR); ISO/TR 13843; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- ISO. ISO 11731:2017 Water Quality—Enumeration of Legionella; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Kozak, N.A.; Lucas, C.E.; Winchell, J.M. Identification of Legionella in the environment. In Legionella Methods and Protocols; Buchrieser, C., Hilbi, H., Eds.; Springer: New York, NY, USA; Humana Press: London, UK, 2013. [Google Scholar]
- Whiley, H.; Taylor, M. Legionella detection by culture and qPCR: Comparing apples and oranges. Crit. Rev. Microbiol. 2016, 42, 65–74. [Google Scholar] [CrossRef]
- Sartory, D.P.; Spies, K.; Lange, B.; Schneider, S.; Langer, B. Evaluation of a most probable number method for the enumeration of Legionella pneumophila from potable and related water samples. Lett. Appl. Microbiol. 2017, 64, 271–275. [Google Scholar] [CrossRef]
- Petrisek, R.; Hall, J. Evaluation of a most probable number method for the enumeration of Legionella pneumophila from North American potable and nonpotable water samples. J. Water Health 2018, 16, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rech, M.M.; Swalla, B.M.; Dobranic, J.K. Evaluation of Legiolert for quantification of Legionella pneumophila from non-potable water. Curr. Microbiol. 2018, 75, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Levi, K.; Smedley, J.; Towner, K.J. Evaluation of a real-time PCR hybridization assay for rapid detection of Legionella pneumophila in hospital and environmental water samples. Clin. Microbiol Infect. 2003, 9, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.V.; Lai, S.; Exner, M.; Lenz, J.; Gaia, V.; Casati, S.; Hartemann, P.; Luck, C.; Pangon, B.; Ricci, M.L.; et al. An international trial of quantitative PCR for monitoring Legionella in artificial water systems. J. Appl. Microbiol. 2011, 110, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Toplitsch, D.; Platzer, S.; Pfeifer, B.; Hautz, J.; Mascher, F.; Kittinger, C. Legionella detection in environmental samples as an example for successful implementation of qPCR. Water 2018, 10, 1012. [Google Scholar] [CrossRef]
- Joly, P.; Falconnet, P.A.; André, J.; Weill, N.; Reyrolle, M.; Vandenesch, F.; Maurin, M.; Etienne, J.; Jarraud, S. Quantitative real-time Legionella PCR for environmental water samples: Data interpretation. Appl. Environ. Microbiol. 2006, 72, 2801–2808. [Google Scholar] [CrossRef]
- ISO. ISO 19458:2006 Water Quality—Sampling for Microbiological Analysis; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- The European Guidelines Working Group. Infections EWGfL: European Technical Guidelines 2017: Minimising the Risk from Legionella Infections in Building Water Systems; The European Guidelines Working Group: London, UK, 2017. [Google Scholar]
- Lu, J.; Buse, H.; Struewing, I.; Zhao, A.; Lytle, D.; Ashbolt, N. Annual variations and effects of temperature on Legionella spp. and other potential opportunistic pathogens in a bathroom. Environ. Sci. Pollut. Res. Int. 2017, 24, 2326–2336. [Google Scholar] [CrossRef]
- Bédard, E.; Laferrière, C.; Déziel, E.; Prévost, M. Impact of stagnation and sampling volume on water microbial quality monitoring in large buildings. PLoS ONE 2018, 13, e0199429. [Google Scholar] [CrossRef]
- Flannery, B.; Gelling, L.B.; Vugia, D.J.; Weintraub, J.M.; Salerno, J.J.; Conroy, M.J.; Stevens, V.A.; Rose, C.E.; Moore, M.R.; Fields, B.S.; et al. Reducing Legionella colonization in water systems with monochloramine. Emerg. Infect. Dis. 2006, 12, 588–596. [Google Scholar] [CrossRef]
- Duda, S.; Kandiah, S.; Stout, J.E.; Baron, J.L.; Yassin, M.; Fabrizio, M.; Ferrelli, J.; Hariri, R.; Wagener, M.M.; Goepfert, J.; et al. Evaluation of a new monochloramine generation system for controlling Legionella in building hot water systems. Infect. Control. Hosp. Epidemiol. 2014, 35, 1356–1363. [Google Scholar] [CrossRef]
- Mancini, B.; Scurti, M.; Dormi, A.; Grottola, A.; Zanotti, A.; Cristino, S. Effect of monochloramine treatment on colonization of a hospital water distribution system by Legionella spp.: A 1 year experience study. Environ. Sci. Technol. 2015, 49, 4551–4558. [Google Scholar] [CrossRef]
- Waak, M.B.; LaPara, T.M.; Hallé, C.; Hozalski, R.M. Occurrence of Legionella spp. in water-main biofilms from two drinking water distribution systems. Environ. Sci. Technol. 2018, 52, 7630–7639. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Pressman, J.G.; Wahman, D.G. Three-dimensional free chlorine and monochloramine biofilm penetration: Correlating penetration with biofilm activity and viability. Environ. Sci. Technol. 2018, 52, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Buse, H.Y.; Morris, B.J.; Struewing, I.T.; Szabo, J.G. Chlorine and monochloramine disinfection of Legionella pneumophila colonizing copper and polyvinyl chloride drinking water biofilms. Appl. Environ. Microbiol. 2019, 85, e02956-18. [Google Scholar] [CrossRef] [PubMed]
- Jacangelo, J.G.; Olivieri, V.P.; Kawata, K. Investigating the mechanism of inactivation of Escherichia coli B by monochloramine. J. AWWA 1991, 83, 80–87. [Google Scholar] [CrossRef]
- Xue, Z.; Hessler, C.M.; Panmanee, W.; Hassett, D.J.; Seo, Y. Pseudomonas aeruginosa inactivation mechanism is affected by capsular extracellular polymeric substances reactivity with chlorine and monochloramine. FEMS Microbiol. Ecol. 2013, 83, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Herrera-Rimann, K.; Blanc, D.S.; Greub, G. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 2006, 72, 2428–2438. [Google Scholar] [CrossRef]
- Pagnier, I.; Valles, C.; Raoult, D.; Scola, B. Isolation of Vermamoeba vermiformis and associated bacteria in hospital water. Microb. Pathog. 2015, 80, 14–20. [Google Scholar] [CrossRef]
- Le Dantec, C.; Duguet, J.P.; Montiel, A.; Dumoutier, N.; Dubrou, S.; Vincent, V. Occurrence of mycobacteria in water treatment lines and in water distribution systems. Appl. Environ. Microbiol. 2002, 68, 5318–5325. [Google Scholar] [CrossRef]
- Garcia, A.; Goni, P.; Cieloszyk, J.; Fernandez, M.T.; Calvo-Begueria, L.; Rubio, E.; Fillat, M.F.; Peleato, M.L.; Clavel, A. Identification of free-living amoebae and amoeba-associated bacteria from reservoirs and water treatment plants by molecular techniques. Environ. Sci. Technol. 2013, 47, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- Buse, H.Y.; Schoen, M.E.; Ashbolt, N.J. Legionellae in engineered systems and use of quantitative microbial risk assessment to predict exposure. Water Res. 2012, 46, 921–933. [Google Scholar] [CrossRef]
- Lesnik, R.; Brettar, I.; Höfle, M.G. Legionella species diversity and dynamics from surface reservoir to tap water: From cold adaptation to thermophily. ISME J. 2016, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.L.; Plouffe, J.F.; Pastoris, M.C.; Stout, J.E.; Schousboe, M.; Widmer, A.; Summersgill, J.; File, T.; Heath, C.M.; Paterson, D.L.; et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: An international collaborative survey. J. Infect. Dis. 2002, 186, 127–128. [Google Scholar] [CrossRef]
- Harrison, T.G.; Doshi, N.; Fry, N.K.; Joseph, C.A. Comparison of clinical and environmental isolates of Legionella pneumophila obtained in the UK over 19 years. Clin. Microbiol. Infect. 2007, 13, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, C.A.; Blaser, M.J.; Wang, W.L. Serogroup specificity of Legionella pneumophila is related to lipopolysaccharide characteristics. Infect. Immun. 1986, 51, 397–404. [Google Scholar] [CrossRef]
- Otten, S.; Iyer, S.; Johnson, W.; Montgomery, R. Serospecific antigens of Legionella pneumophila. J. Bacteriol. 1986, 167, 893–904. [Google Scholar] [CrossRef]
- Helbig, J.H.; Amemura-Maekawa, J. Serotyping of Legionella pneumophila and epidemiological investigations. Nowa Medycyna 2009, 16, 69–75. [Google Scholar]
- Kozak-Muiznieks, N.A.; Morrison, S.S.; Mercante, J.W.; Ishaq, M.K.; Johnson, T.; Caravas, J.; Lucas, C.E.; Brown, E.; Raphael, B.H.; Winchell, J.M. Comparative genome analysis reveals a complex population structure of Legionella pneumophila subspecies. Infect. Genet. Evol. 2018, 59, 172–185. [Google Scholar] [CrossRef]
- Carvalho, F.R.; Vazoller, R.F.; Foronda, A.S.; Pellizari, V.H. Phylogenetic study of legionella species in pristine and polluted aquatic samples from a tropical Atlantic forest ecosystem. Curr. Microbiol. 2007, 55, 288–293. [Google Scholar] [CrossRef]
- Kozak-Muiznieks, N.A.; Lucas, C.E.; Brown, E.; Pondo, T.; Taylor, T.H., Jr.; Frace, M.; Miskowski, D.; Winchell, J.M. Prevalence of sequence types among clinical and environmental isolates of Legionella pneumophila serogroup 1 in the United States from 1982 to 2012. J. Clin. Microbiol. 2014, 52, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Quero, S.; Párraga-Niño, N.; Barrabeig, I.; Sala, M.R.; Pedro-Botet, M.L.; Monsó, E.; Jané, M.; Sabrià, M.; Garcia-Núñez, M. Population structure of environmental and clinical Legionella pneumophila isolates in Catalonia. Sci. Rep. 2018, 8, 6241. [Google Scholar] [CrossRef]
- Edagawa, A.; Kimura, A.; Doi, H.; Tanaka, H.; Tomioka, K.; Sakabe, K.; Nakajima, C.; Suzuki, Y. Detection of culturable and nonculturable Legionella species from hot water systems of public buildings in Japan. J. Appl. Microbiol. 2008, 105, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Leoni, E.; Legnani, P.P. Comparison of selective procedures for isolation and enumeration of Legionella species from hot water systems. J. Appl. Microbiol. 2001, 90, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Asghari, F.B.; Nikaeen, M.; Hatamzadeh, M.; Hassanzadeh, A. Surveillance of Legionella species in hospital water systems: The significance of detection method for environmental surveillance data. J. Water Health 2013, 11, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Duda, S.; Baron, J.L.; Wagener, M.M.; Vidic, R.D.; Stout, J.E. Lack of correlation between Legionella colonization and microbial population quantification using heterotrophic plate count and adenosine triphosphate bioluminescence measurement. Environ. Monit. Assess. 2015, 187, 393. [Google Scholar] [CrossRef]
- Pierre, D.; Baron, J.L.; Ma, X.; Sidari, F.P., 3rd; Wagener, M.M.; Stout, J.E. Water quality as a predictor of Legionella positivity of building water systems. Pathogens 2019, 8, 295. [Google Scholar] [CrossRef]
- Rakić, A.; Perić, J.; Foglar, L. Influence of temperature, chlorine residual and heavy metals on the presence of Legionella pneumophila in hot water distribution systems. Ann. Agric. Environ. Med. 2012, 19, 431–436. [Google Scholar]
- Rakić, A.; Štambuk-Giljanović, N.; Foglar, L. Monitoring Legionella pneumophila in drinking water distribution systems in southern Croatia. Fresenius Environ. Bull. 2013, 22, 3390–3397. [Google Scholar]
- Revetta, R.P.; Gomez-Alvarez, V.; Gerke, T.L.; Curioso, C.; Santo Domingo, J.W.; Ashbolt, N.J. Establishment and early succession of bacterial communities in monochloramine-treated drinking water biofilms. FEMS Microbiol. Ecol. 2013, 86, 404–414. [Google Scholar] [CrossRef]
- Chern, E.C.; King, D.; Haugland, R.; Pfaller, S. Evaluation of quantitative polymerase chain reaction assays targeting Mycobacterium avium, M. intracellulare, and M. avium subspecies paratuberculosis in drinking water biofilms. J. Water Health 2015, 13, 131–139. [Google Scholar] [CrossRef]
- Lu, J.; Struewing, I.; Yelton, S.; Ashbolt, N. Molecular survey of occurrence and quantity of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in municipal drinking water storage tank sediments. J. Appl. Microbiol. 2015, 119, 278–288. [Google Scholar] [CrossRef]
- Kuiper, M.W.; Valster, R.M.; Wullings, B.A.; Boonstra, H.; Smidt, H.; van der Kooij, D. Quantitative detection of the free-living amoeba Hartmannella vermiformis in surface water by using real-time PCR. Appl. Environ. Microbiol. 2006, 72, 5750–5756. [Google Scholar] [CrossRef]
- Riviére, D.; Szczebara, F.M.; Berjeaud, J.M.; Frére, J.; Héchard, Y. Development of a real-time PCR assay for quantification of Acanthamoeba trophozoites and cysts. J. Microbiol. Methods 2006, 64, 78–83. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Alvarez, V.; Boczek, L.; King, D.; Pemberton, A.; Pfaller, S.; Rodgers, M.; SantoDomingo, J.; Revetta, R.P. Draft genome sequences of seven Legionella pneumophila isolates from a hot water system of a large building. Microbiol. Resour. Announc. 2019, 8, e00384-19. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Team RC. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 1 April 2020).
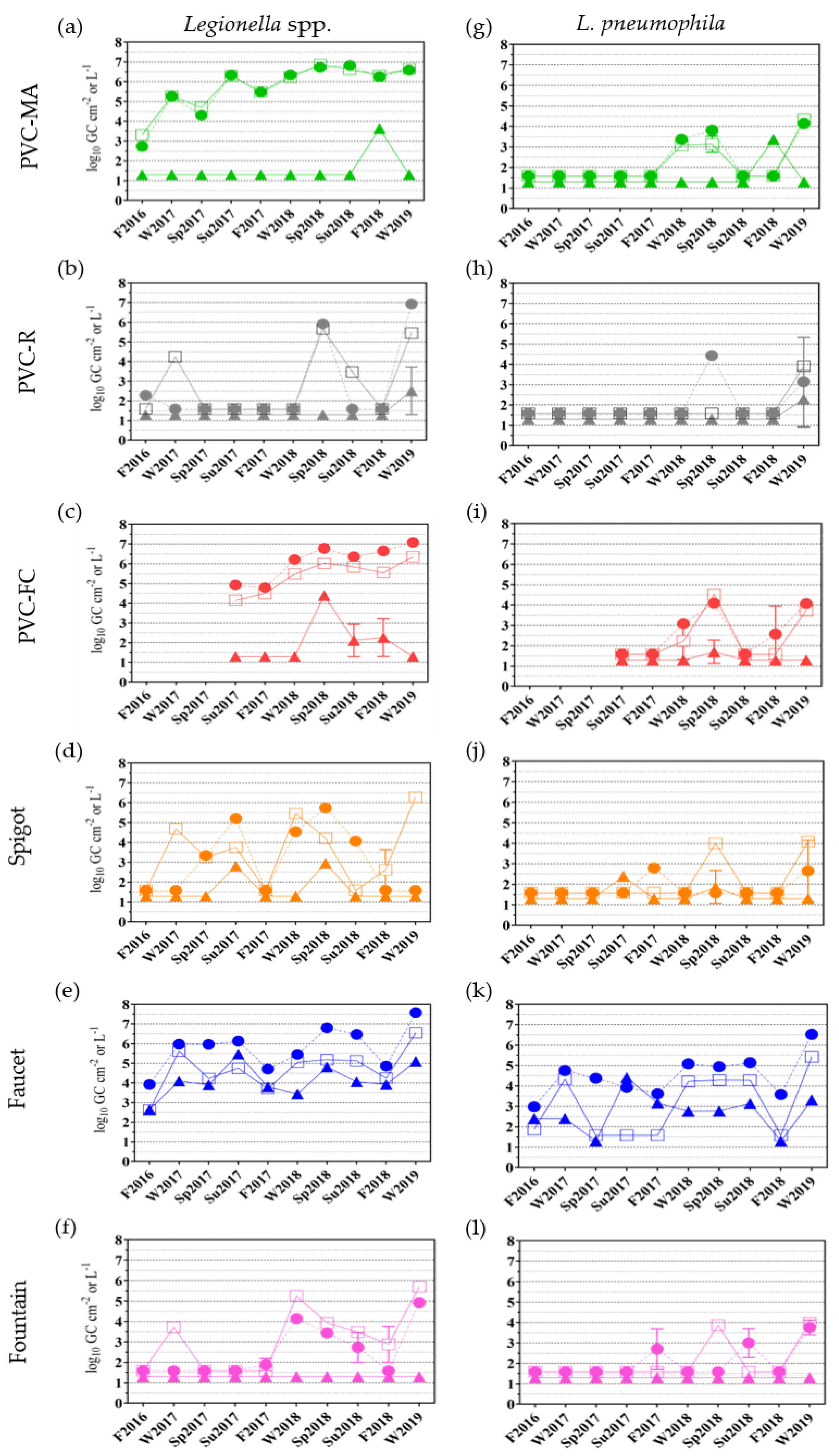
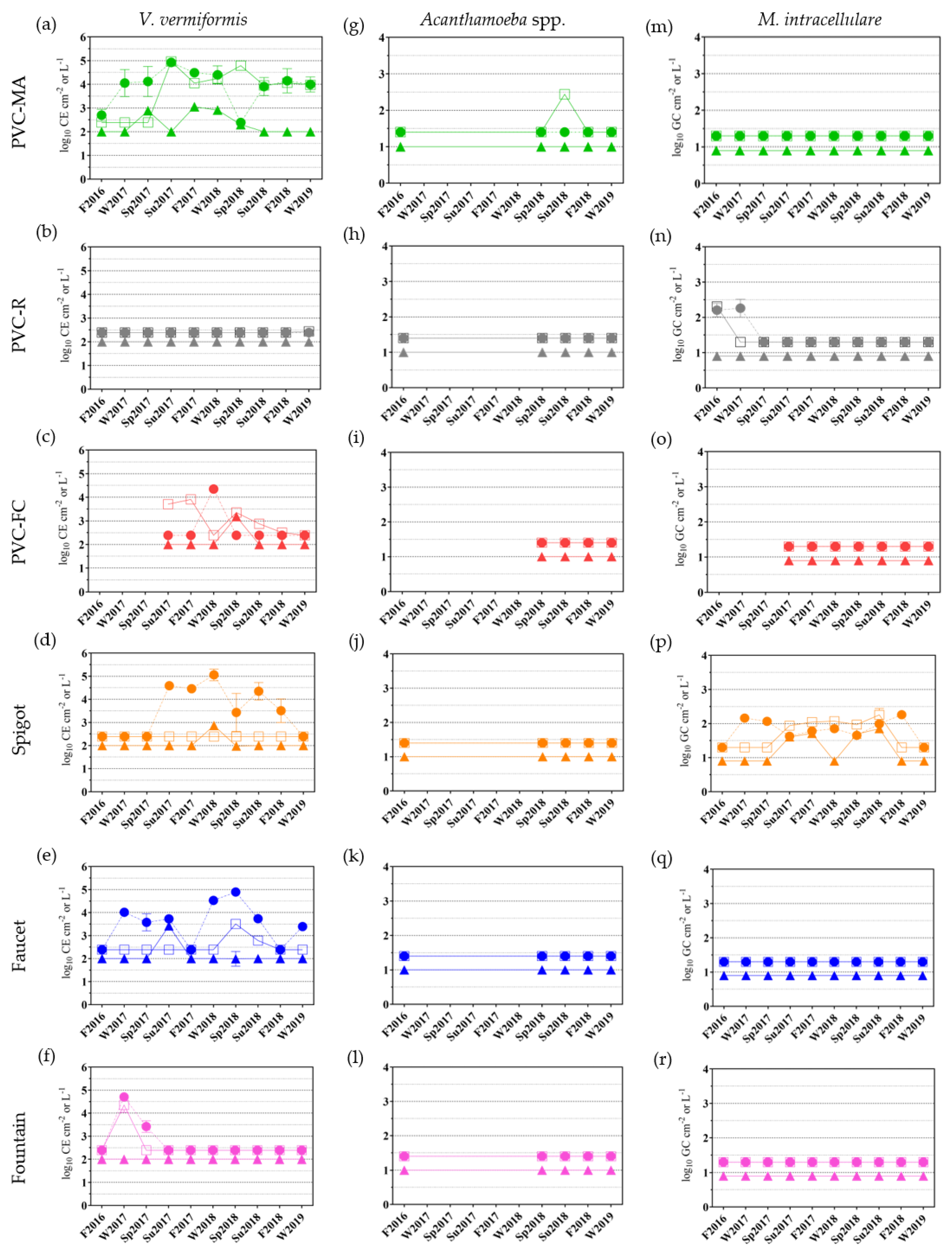
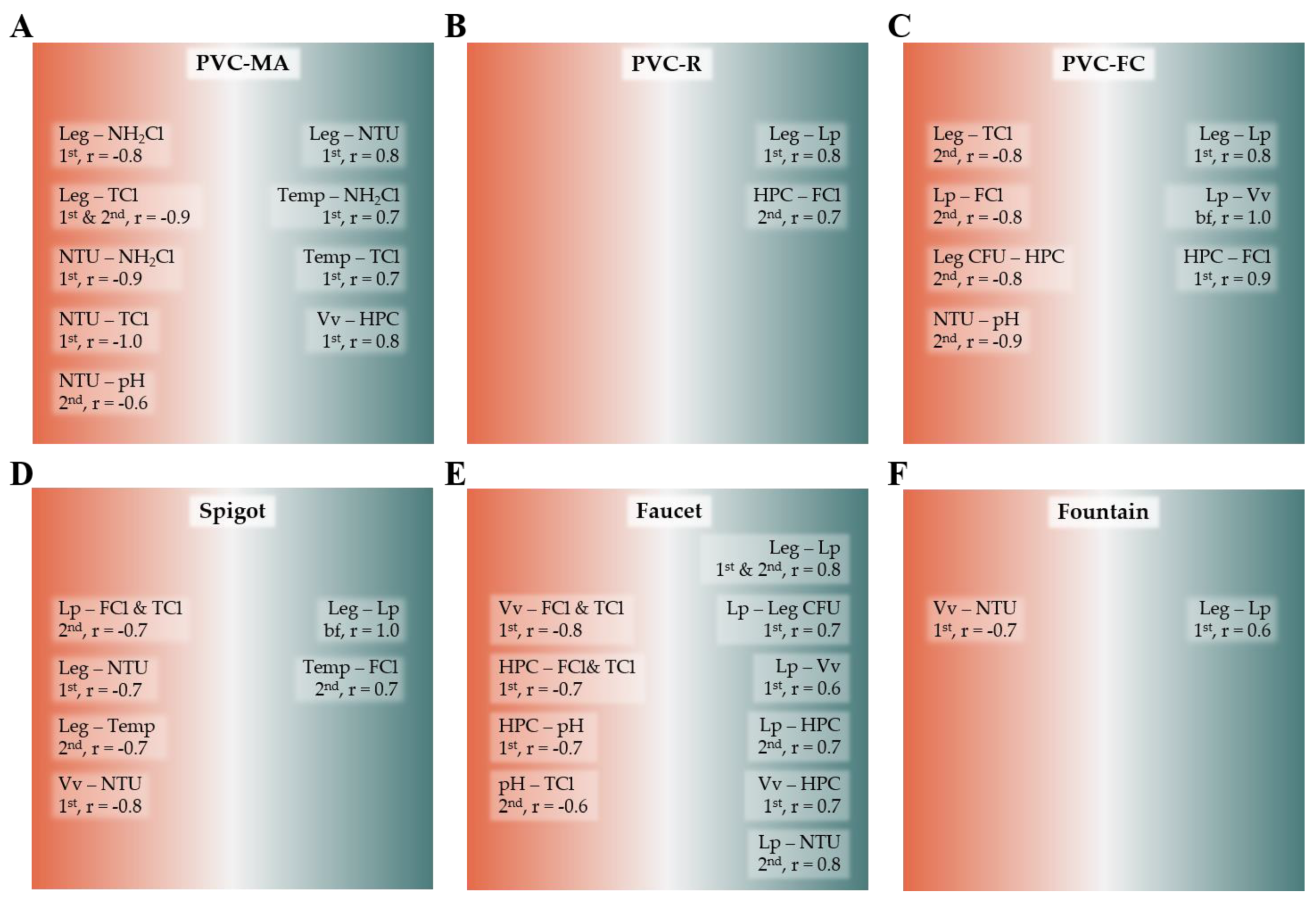

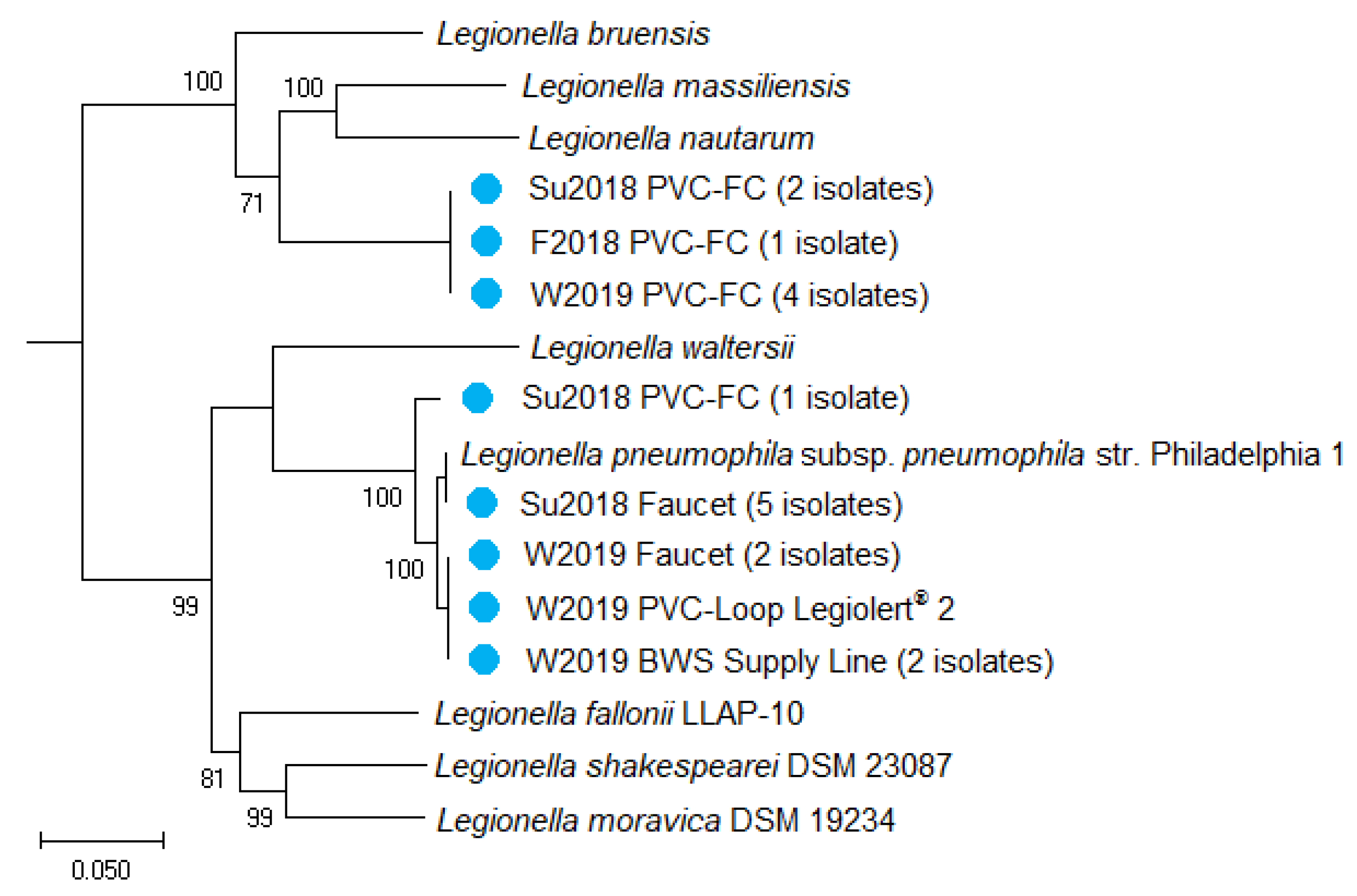
| Sampling Locations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site Name | PVC-MA | PVC-R | PVC-FC | Spigot | Faucet | Fountain | ||||||
| Disinfectant type | monochloramine | chlorine | chlorine | chlorine | chlorine | chlorine | ||||||
| Water type | potable water | chiller water | potable water | potable water | potable water | potable water | ||||||
| Approx. usage per season | 172,709 L | 1,492,209 L | 5 L | 1703 L | 864 L | 689 L | ||||||
| Outlet type | valve | valve | valve | spigot | faucet | bubbler | ||||||
| Outlet material | PVC80; PharMed BPT1 | PVC80 | PVC80 | cast brass | chrome plated, forged brass | SS/EDPM rubber | ||||||
| Pipe material | PVC80 | FDI and PPT2 | PVC80 and copper2 | copper | copper | copper | ||||||
| Total distance to feed water | 14 m | 46 ft | 396 m | 1299 ft | 21 m | 70 ft | 102 m | 334 ft | 20 m | 65 ft | 9.7 m | 32 ft |
| Section 1 | 14 m | 46 ft | 366 m | 1201 ft | 9 m | 30 ft | 5 m | 16 ft | 8 m | 26 ft | 9.1 m | 30 ft |
| pipe diameter | 15 cm | 6 in | 7.6 cm | 3 in | 2.5 cm | 1 in | 1.9 cm | 0.75 in | 1.9 cm | 0.75 in | 1.3 cm | 0.5 in |
| Section 2 | - | 30 m | 98 ft | 12 m | 40 ft | 97 m | 318 ft | 12 m | 39 ft | 0.6 m | 2 ft | |
| pipe diameter | - | 1.3 cm | 0.5 in | 2.5 cm | 1 in | 5.1 cm | 2 in | 5.1 cm | 2 in | 0.6 cm | 0.25 in | |
| Location Name & Sample Type | HPC | pH | Turbidity | Temp | Free Cl2 | Total Cl2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| PVC-MA a | First Draw | 5.5 | 0.1 | 8.21 | 0.08 | 0.23 | 0.03 | 20.2 | 0.3 | 0.06 | 0.01 | 1.21 | 0.11 |
| Second Draw | 4.9 | 0.2 | 8.19 | 0.07 | 0.26 | 0.04 | 20.2 | 0.3 | 0.06 | 0.01 | 1.22 | 0.11 | |
| Biofilm | 3.4 | 0.2 | - | - | - | - | - | - | - | - | - | - | |
| PVC-R | First Draw | 5.4 | 0.2 | 8.53 | 0.07 | 6.94 | 3.23 | 24.8 | 1.4 | 1.04 | 0.10 | 1.13 | 0.09 |
| Second Draw | 4.5 | 0.2 | 8.68 | 0.05 | 2.13 | 0.96 | 24.8 | 1.4 | 0.90 | 0.06 | 1.02 | 0.06 | |
| Biofilm | 2.6 | 0.1 | - | - | - | - | - | - | - | - | - | - | |
| PVC-FC | First Draw | 4.2 | 0.6 | 8.47 | 0.15 | 0.42 | 0.15 | 20.7 | 2.5 | 0.83 | 0.07 | 0.92 | 0.08 |
| Second Draw | 4.0 | 0.1 | 8.60 | 0.17 | 0.22 | 0.02 | 18.6 | 3.0 | 0.99 | 0.04 | 1.10 | 0.05 | |
| Biofilm | 2.0 | 0.6 | - | - | - | - | - | - | - | - | - | - | |
| Spigot | First Draw | 5.4 | 0.1 | 8.67 | 0.06 | 0.81 | 0.35 | 24.8 | 1.2 | 0.53 | 0.12 | 0.59 | 0.12 |
| Second Draw | 2.8 | 0.2 | 8.73 | 0.06 | 0.23 | 0.08 | 18.4 | 2.3 | 1.00 | 0.06 | 1.12 | 0.04 | |
| Biofilm | 2.5 | 0.2 | - | - | - | - | - | - | - | - | - | - | |
| Faucet | First Draw | 5.5 | 0.2 | 8.67 | 0.06 | 0.17 | 0.02 | 24.0 | 0.7 | 0.35 | 0.10 | 0.40 | 0.11 |
| Second Draw | 4.2 | 0.2 | 8.72 | 0.06 | 0.18 | 0.02 | 20.0 | 1.7 | 1.05 | 0.04 | 1.13 | 0.04 | |
| Biofilm | 4.2 | 0.2 | - | - | - | - | - | - | - | - | - | - | |
| Fountain | First Draw | 4.0 | 0.2 | 8.75 | 0.06 | 0.25 | 0.06 | 13.5 | 0.4 | 0.59 | 0.07 | 0.69 | 0.07 |
| Second Draw | 3.8 | 0.3 | 8.78 | 0.07 | 0.18 | 0.02 | 15.4 | 1.0 | 0.89 | 0.03 | 0.98 | 0.03 | |
| Biofilm | 4.0 | 0.5 | - | - | - | - | - | - | - | - | - | - | |
| Time Point | PVC-R | PVC-FC | Faucet | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Draw | First Draw | Second Draw | First Draw | Second Draw | Biofilm | |||||||||||||
| CFU | sg | PCR | CFU | sg | PCR | CFU | sg | PCR | CFU | sg | PCR | CFU | sg | PCR | CFU | sg | PCR | |
| F2016 | - | - | - | nd | nd | nd | nd | nd | nd | - | - | - | - | - | - | 1.9 | 1 & 2-14 | Leg/Lp |
| W2017 | - | - | - | nd | nd | nd | nd | nd | nd | - | - | - | - | - | - | - | - | - |
| Sp2017 | - | - | - | nd | nd | nd | nd | nd | nd | 1.2 | 2-14 | Leg/Lp | - | - | - | - | - | - |
| Su2017 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| F2017 | - | - | - | - | - | - | - | - | - | 1.2 | neg | Leg | - | - | - | - | - | - |
| W2018 | 2.6 | neg | Leg | 4.5 | neg | Leg | 2.6 | neg | Leg | 2.1 | 1 & 2-14 | Leg/Lp | - | - | - | - | - | - |
| Sp2018 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Su2018 | - | - | - | 3.8 | neg & 2-14 | Leg & Leg/Lp | 2.8 | neg & 2-14 | Leg & Leg/Lp | 2.7 | 1 | Leg/Lp | - | - | - | - | - | - |
| F2018 | - | - | - | - | - | 3.0 | neg | Leg | - | - | - | - | - | - | - | - | - | |
| W2019 | - | - | - | 2.1 | neg | Leg | 2.6 | neg | Leg | 2.8 | 1 | Leg/Lp | 2.1 | 1 | Leg/Lp | - | - | - |
| Isolate | Lineage | Genome Size (bp) | No. of Contigs | Contig N50 (bp) | G+C Content (%) | No. Predicted Genes | MLST |
|---|---|---|---|---|---|---|---|
| Su2018 Faucet 1 | L. pneumophila | 3,589,286 | 72 | 160,018 | 38 | 3240 | 1 |
| Su2018 Faucet 2 | L. pneumophila | 3,589,059 | 75 | 160,010 | 38 | 3239 | 1 |
| Su2018 Faucet 3 | L. pneumophila | 3,589,228 | 71 | 160,018 | 38 | 3236 | 1 |
| Su2018 Faucet 4 | L. pneumophila | 3,574,346 | 99 | 88,934 | 38 | 3228 | 1 |
| Su2018 Faucet 5 | L. pneumophila | 3,590,303 | 69 | 160,010 | 38 | 3240 | 1 |
| W2019 Faucet Legiolert® 1 | L. pneumophila | 3,564,531 | 124 | 59,538 | 38 | 3229 | 1 |
| W2019 Faucet 2 | L. pneumophila | 3,562,418 | 77 | 123,108 | 38 | 3224 | 1 |
| Su2018 PVC-FC 1 | L. pneumophila | 3,510,698 | 39 | 413,452 | 38 | 3151 | 2037 |
| Su2018 PVC-FC 2 | L. pneumophila | 3,498,273 | 40 | 223,695 | 38 | 3138 | 2037 |
| Su2018 PVC-FC 3 | Legionella | 3,285,021 | 57 | 260,915 | 41 | 3028 | - |
| F2018 PVC-FC | Legionella | 3,287,868 | 49 | 348,799 | 41 | 3028 | - |
| W2019 PVC-FC 1 | Legionella | 3,063,339 | 43 | 348,799 | 41 | 2812 | - |
| W2019 PVC-FC 2 | Legionella | 3,288,042 | 53 | 321,108 | 41 | 3033 | - |
| W2019 PVC-FC 3 | Legionella | 3,286,784 | 53 | 283,321 | 41 | 3031 | - |
| W2019 PVC-FC 4 | Legionella | 3,285,778 | 45 | 466,387 | 41 | 3022 | - |
| W2019 PVC-Loop Legiolert® 1 | Ochrobactrum | 4,764,477 | 55 | 478,728 | 58 | 4621 | - |
| W2019 PVC-Loop Legiolert® 2 | L. pneumophila | 3,388,353 | 42 | 255,126 | 38 | 3057 | 1950 |
| W2019 BWS Supply Line 1 | L. pneumophila | 3,589,200 | 67 | 176,930 | 38 | 3237 | 1 |
| W2019 BWS Supply Line 2 | L. pneumophila | 3,588,658 | 70 | 160,010 | 38 | 3242 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buse, H.Y.; Morris, B.J.; Gomez-Alvarez, V.; Szabo, J.G.; Hall, J.S. Legionella Diversity and Spatiotemporal Variation in the Occurrence of Opportunistic Pathogens within a Large Building Water System. Pathogens 2020, 9, 567. https://doi.org/10.3390/pathogens9070567
Buse HY, Morris BJ, Gomez-Alvarez V, Szabo JG, Hall JS. Legionella Diversity and Spatiotemporal Variation in the Occurrence of Opportunistic Pathogens within a Large Building Water System. Pathogens. 2020; 9(7):567. https://doi.org/10.3390/pathogens9070567
Chicago/Turabian StyleBuse, Helen Y., Brian J. Morris, Vicente Gomez-Alvarez, Jeffrey G. Szabo, and John S. Hall. 2020. "Legionella Diversity and Spatiotemporal Variation in the Occurrence of Opportunistic Pathogens within a Large Building Water System" Pathogens 9, no. 7: 567. https://doi.org/10.3390/pathogens9070567
APA StyleBuse, H. Y., Morris, B. J., Gomez-Alvarez, V., Szabo, J. G., & Hall, J. S. (2020). Legionella Diversity and Spatiotemporal Variation in the Occurrence of Opportunistic Pathogens within a Large Building Water System. Pathogens, 9(7), 567. https://doi.org/10.3390/pathogens9070567




