Inflammatory Immune Responses and Gut Microbiota Changes Following Campylobacter coli Infection of IL-10-/- Mice with Chronic Colitis
Abstract
1. Introduction
2. Results
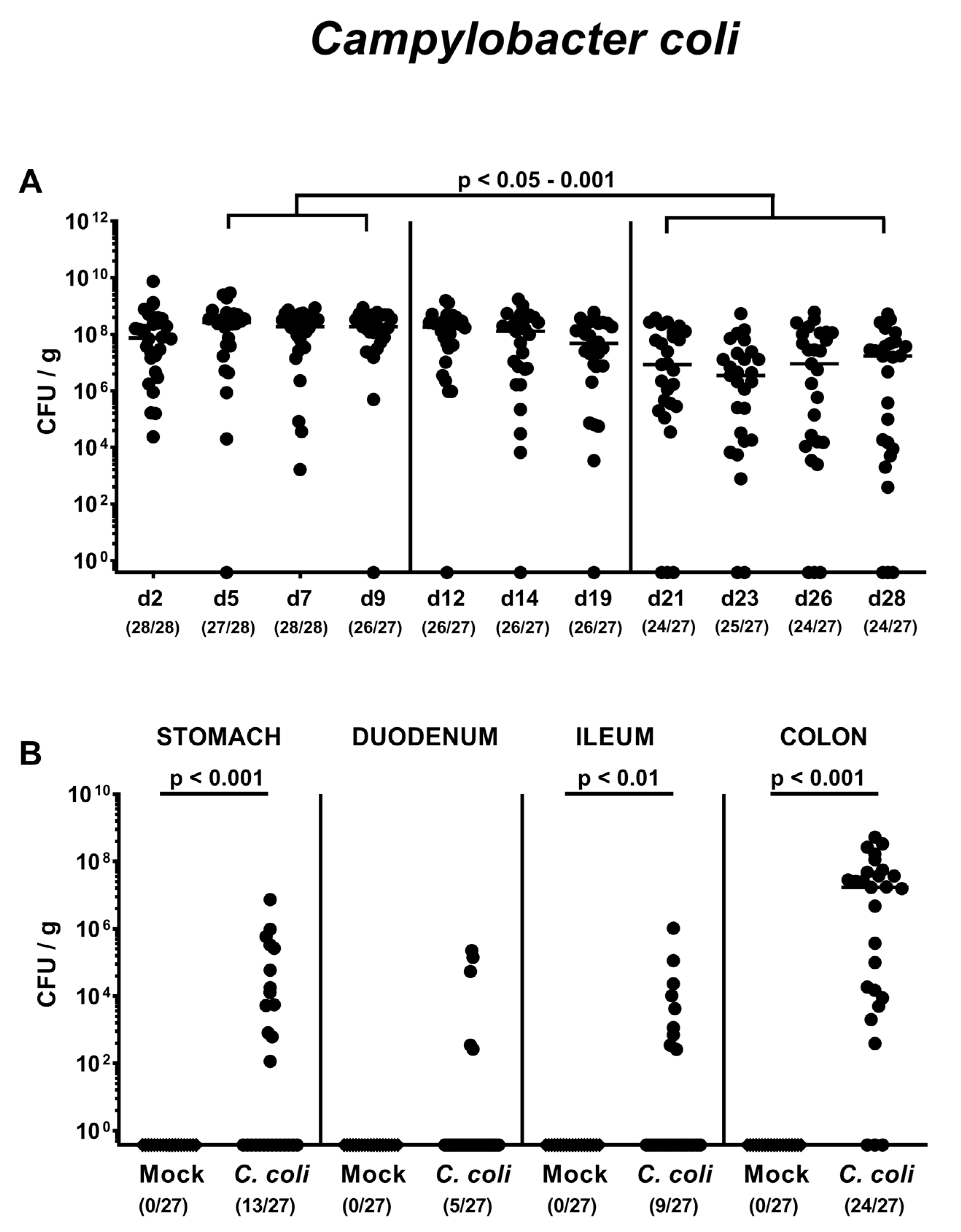
2.1. Gastrointestinal Campylobacter coli Colonization Following Peroral Infection of Aged Conventional IL-10-/- mice
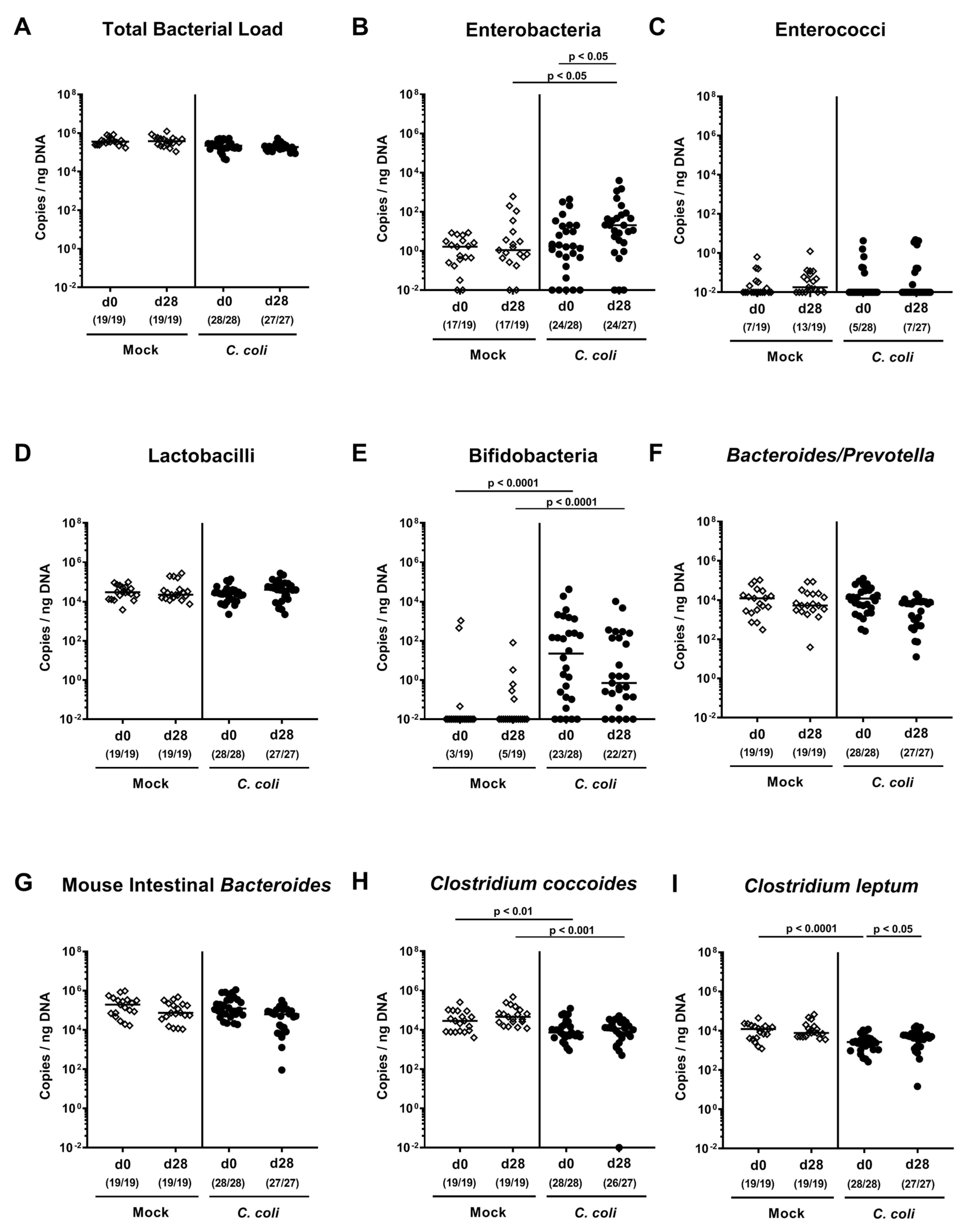
2.2. Changes in Gut Microbiota Composition Following Peroral C. coli Infection of Aged Conventional IL-10-/- mice
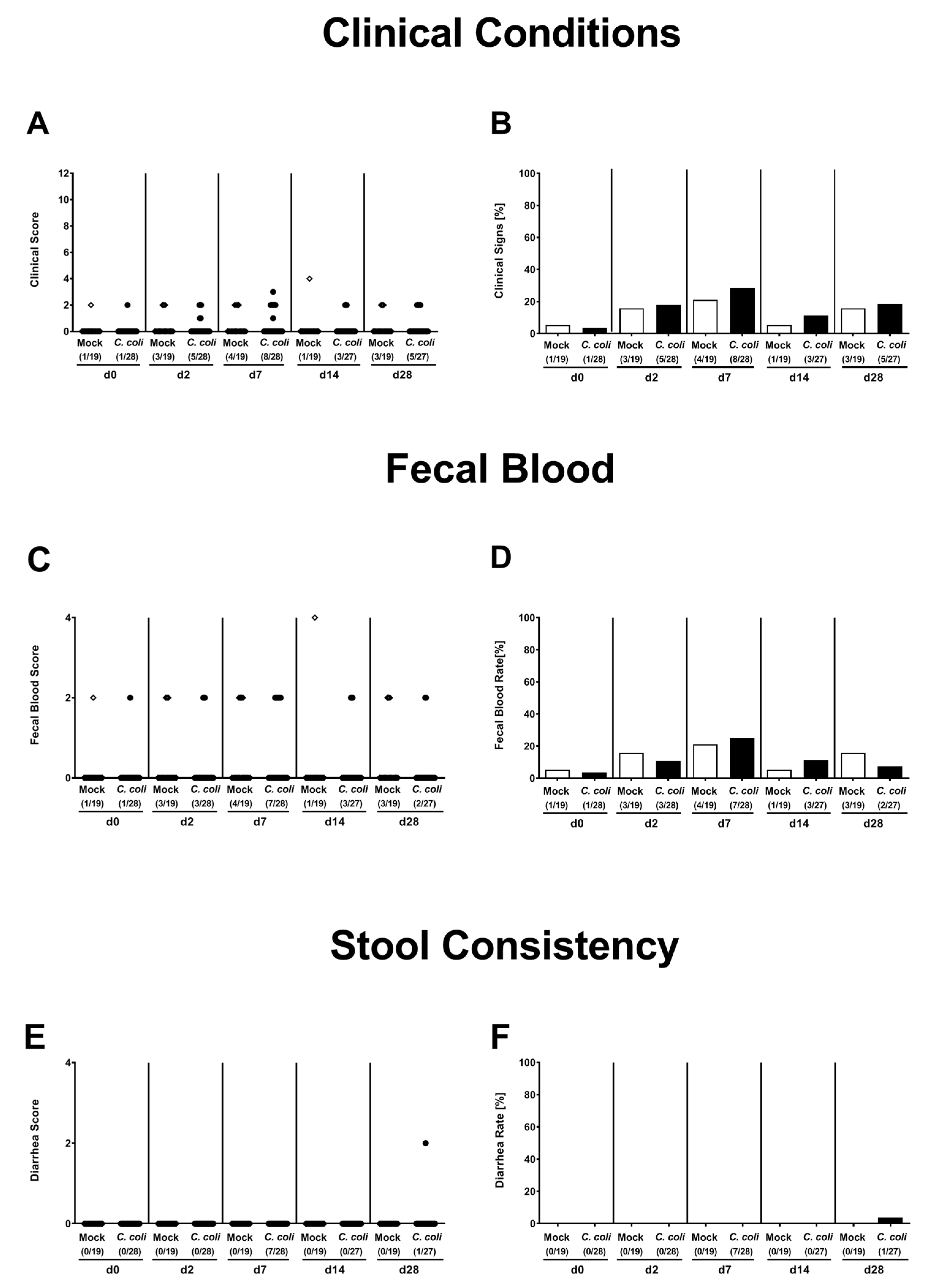
2.3. Clinical Conditions Over Time Following Peroral C. coli Infection of Aged Conventional IL-10-/- mice
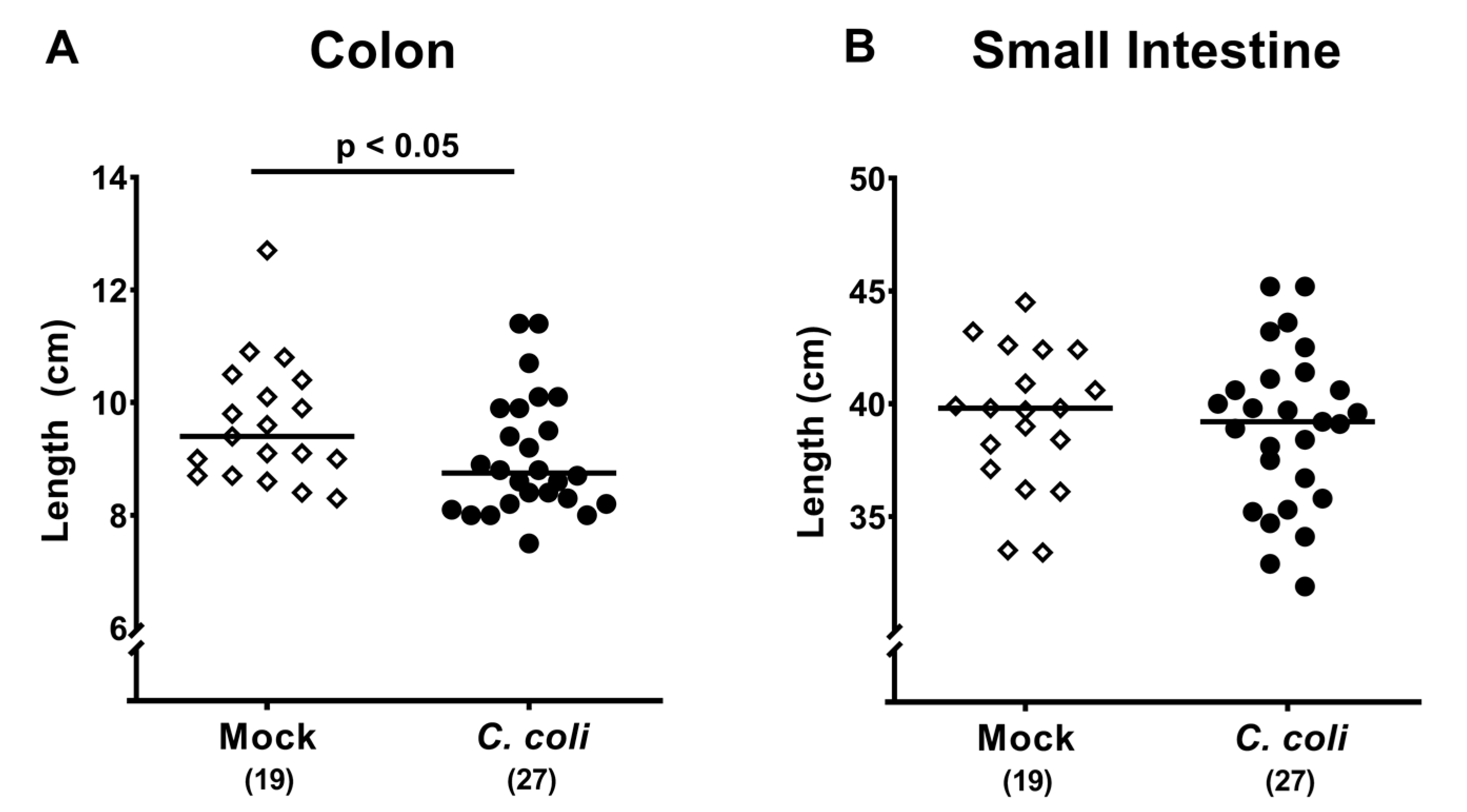
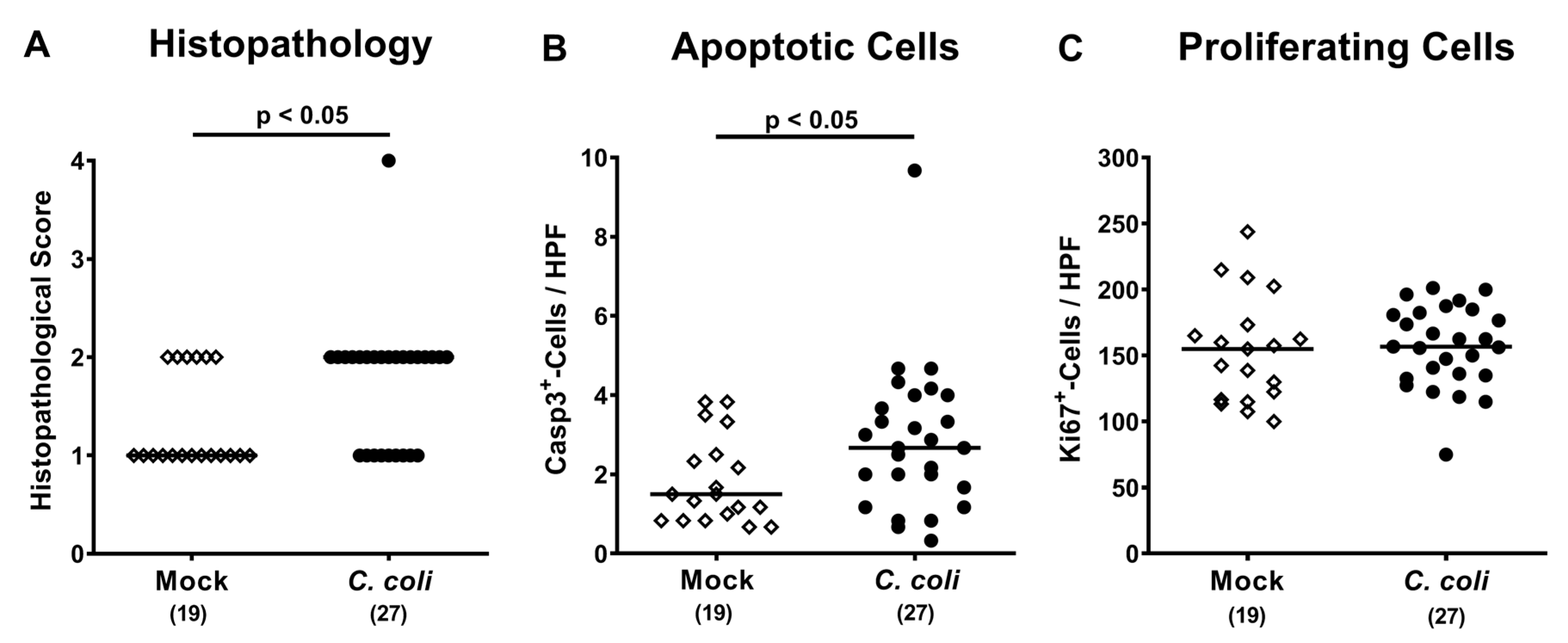
2.4. Macroscopic and Microscopic Inflammatory Sequelae of Peroral C. coli Infection in Aged Conventional IL-10-/- mice
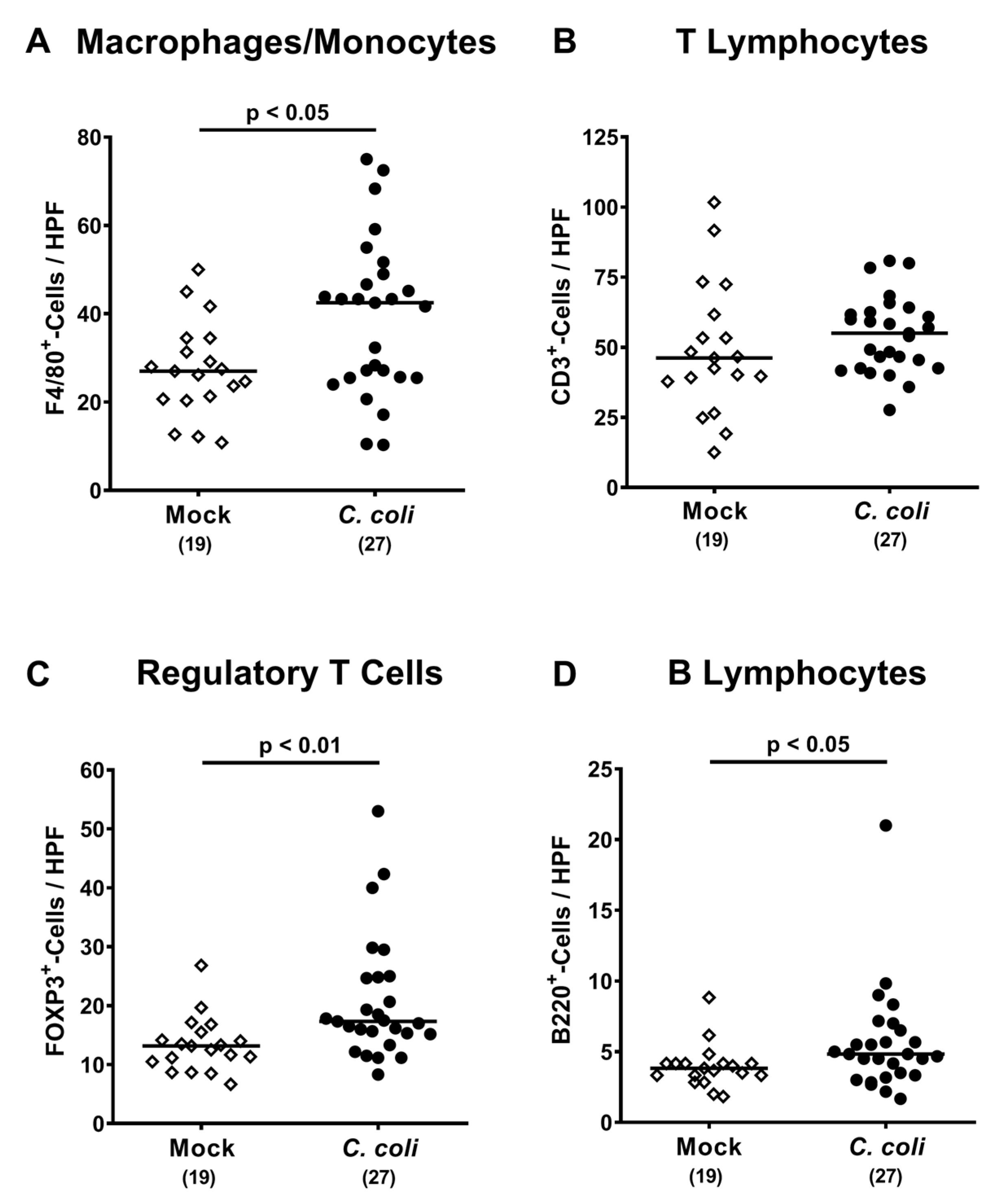
2.5. Colonic Immune Cell Responses Following Peroral C. coli Infection of Aged Conventional IL-10-/- mice
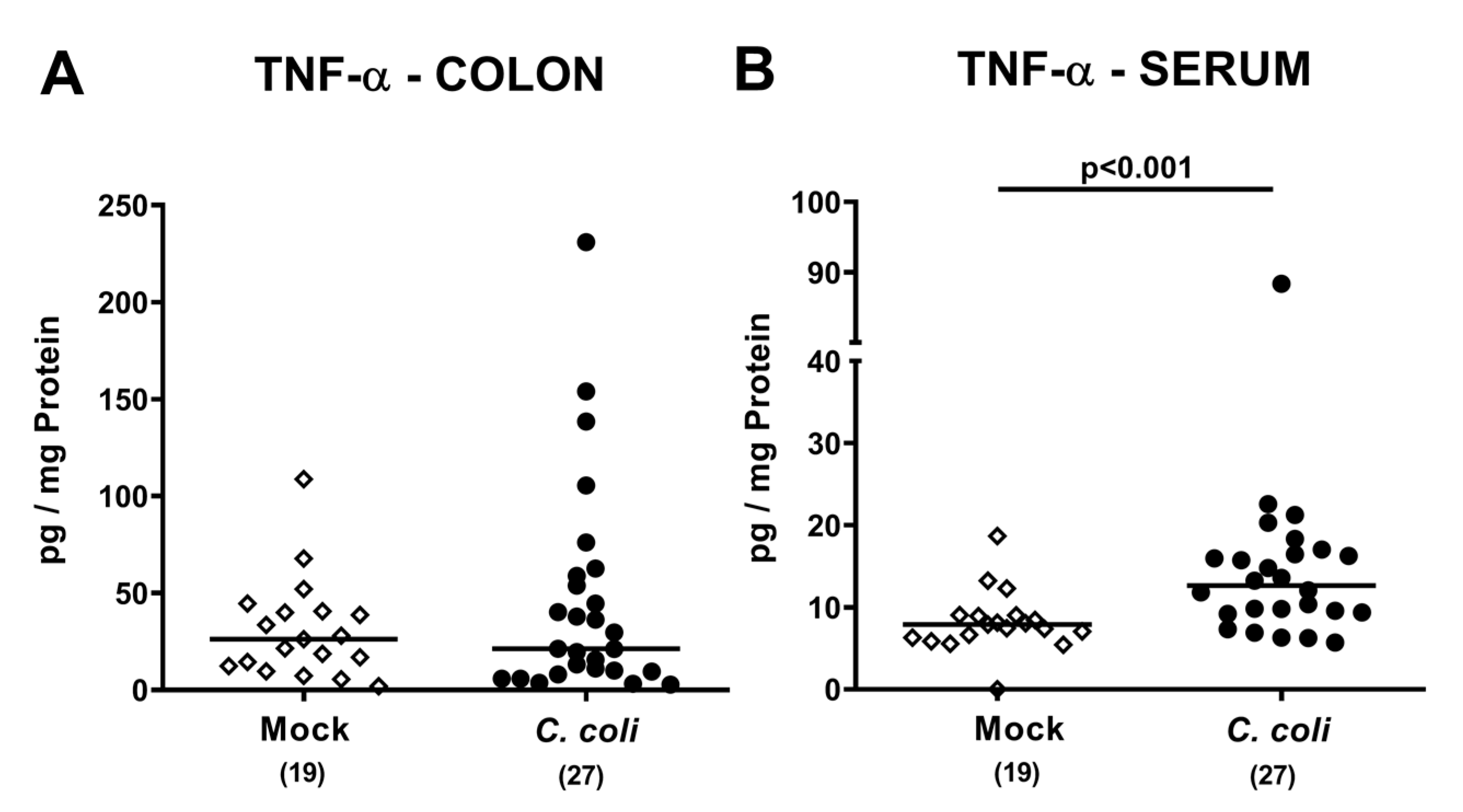
2.6. Colonic and Systemic TNF-α Secretion Following Peroral C. coli Infection of Aged Conventional IL-10-/- Mice with Chronic Colitis
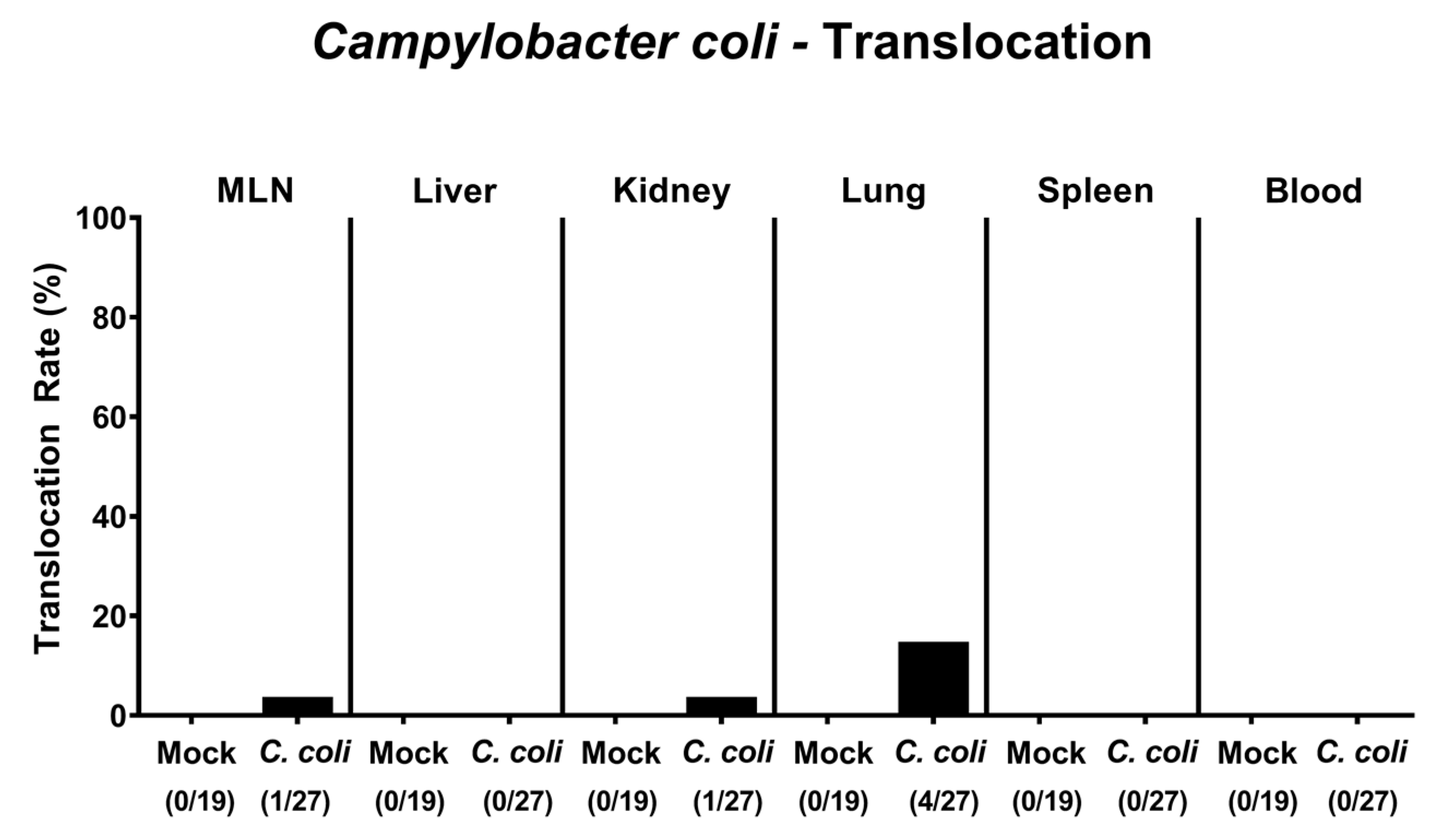
2.7. Bacterial Translocation Following Peroral C. coli Infection of Aged Conventional IL-10-/- Mice with Chronic Colitis
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Mice
4.3. C. coli Infection, Colonisation and Translocation
4.4. Analyses of the Gut Microbiota Composition
4.5. Clinical Conditions
4.6. Sampling Process
4.7. Histopathological Scoring
4.8. Immunohistochemistry
4.9. Pro-Inflammatory Cytokine Measurements in Large Intestinal and Systemic Compartments
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CBA | Cytometric Bead Array |
| CFU | colony-forming units |
| d | day |
| H&E | hematoxylin and eosin |
| HPF | High power fields |
| IBD | inflammatory bowel disease |
| IL | interleukin |
| LOS | lipooligosaccharide |
| LPS | lipopolysaccharide |
| MLN | mesenteric lymph nodes |
| n.s. | not significant |
| PBS | phosphate buffered saline |
| p-value | probability value |
| p.i. | post-infection |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| TNF | tumor necrosis factor |
| TLR-4 | Toll-like receptor-4 |
References
- World Health Organization (WHO). Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 6 April 2020).
- Alter, T.; Bereswill, S.; Glünder, G.; Haag, L.-M.; Hänel, I.; Heimesaat, M.; Lugert, R.; Rautenschlein, S.; Weber, R.; Zautner, A.; et al. Die Campylobacteriose des Menschen. Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz 2011, 54, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Pielsticker, C.; Glünder, G.; Rautenschlein, S. Colonization properties of Campylobacter jejuni in chickens. Eur. J. Microbiol. Immunol. 2012, 2, 61–65. [Google Scholar] [CrossRef]
- Walker, R.I.; Caldwell, M.B.; Lee, E.C.; Guerry, P.; Trust, T.J.; Ruiz-Palacios, G.M. Pathophysiology of Campylobacter enteritis. Microbiol. Rev. 1986, 50, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Kist, M.; Bereswill, S. Campylobacter jejuni. Contrib. Microbiol. 2001, 8, 150–165. [Google Scholar] [PubMed]
- Backert, S.; Tegtmeyer, N.; Cróinín, T.Ó.; Boehm, M.; Heimesaat, M.M. Human campylobacteriosis. In Campylobacter; Klein, Ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 1; pp. 1–25. [Google Scholar] [CrossRef]
- Van Spreeuwel, J.P.; Duursma, G.C.; Meijer, C.J.; Bax, R.; Rosekrans, P.C.; Lindeman, J. Campylobacter colitis: Histological immunohistochemical and ultrastructural findings. Gut 1985, 26, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.; Krogfelt, K.; Cawthraw, S.A.; Van Pelt, W.; Wagenaar, J.A.; Owen, R.J. Host-Pathogen Interactions in Campylobacter Infections: The Host Perspective. Clin. Microbiol. Rev. 2008, 21, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.K.; Maiden, M.C. The Evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harb. Perspect. Boil. 2015, 7, a018119. [Google Scholar] [CrossRef] [PubMed]
- Allos, B.M. Association between Campylobacter infection and Guillain-Barré syndrome. J. Infect. Dis. 1997, 176, S125–S128. [Google Scholar] [CrossRef]
- Stallmach, A.; Carstens, O. Role of Infections in the Manifestation or Reactivation of Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2002, 8, 213–218. [Google Scholar] [CrossRef]
- Rodríguez, L.A.G.; Ruigómez, A.; Panés, J. Acute Gastroenteritis Is Followed by an Increased Risk of Inflammatory Bowel Disease. Gastroenterology 2006, 130, 1588–1594. [Google Scholar] [CrossRef]
- Gradel, K.O.; Nielsen, H.L.; Schønheyder, H.C.; Ejlertsen, T.; Kristensen, B.; Nielsen, H. Increased Short- and Long-Term Risk of Inflammatory Bowel Disease after Salmonella or Campylobacter Gastroenteritis. Gastroenterology 2009, 137, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kalischuk, L.D.; Buret, A.G. A role for Campylobacter jejuni-induced enteritis in inflammatory bowel disease? Am. J. Physiol. Liver Physiol. 2010, 298, G1–G9. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.A.; Saeed, S.A. Gastrointestinal infection as a trigger for inflammatory bowel disease. Curr. Opin. Gastroenterol. 2012, 28, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Lambert, J. Campylobacter Jejuni Causing Flare-up in Inflammatory Bowel Disease. Lancet 1980, 316, 919. [Google Scholar] [CrossRef]
- Boyanova, L.; Gergova, G.; Spassova, Z.; Koumanova, R.; Yaneva, P.; Mitov, I.; Derejian, S.; Krastev, Z. Campylobacter infection in 682 bulgarian patients with acute enterocolitis, inflammatory bowel disease, and other chronic intestinal diseases. Diagn. Microbiol. Infect. Dis. 2004, 49, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Llavat, M.; Domènech, E.; Bernal, I.; Sánchez-Delgado, J.; Manterola, J.M.; García-Planella, E.; Mañosa, M.; Cabre, E.; Gassull, M.A. Prospective, Observational, Cross-Sectional Study of Intestinal Infections among Acutely Active Inflammatory Bowel Disease Patients. Digestion 2009, 80, 25–29. [Google Scholar] [CrossRef]
- Arora, Z.; Mukewar, S.; Wu, X.; Shen, B. Risk factors and clinical implication of superimposed Campylobacter jejuni infection in patients with underlying ulcerative colitis. Gastroenterol. Rep. 2015, 4, 287–292. [Google Scholar] [CrossRef]
- Wohlgemuth, S.; Keller, S.; Kertscher, R.; Stadion, M.; Kisling, S.; Loh, G.; Haller, D.; Jahreis, G.; Blaut, M. Intestinal steroid profiles and microbiota composition in colitic mice. Gut Microbes 2011, 2, 159–166. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Alutis, M.; Grundmann, U.; Fischer, A.; Tegtmeyer, N.; Böhm, M.; Kühl, A.A.; Göbel, U.B.; Backert, S.; Bereswill, S. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front. Microbiol. 2014, 4. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Fuchs, D.; Struck, D.; Niebergall, J.; Jahn, H.-K.; Dunay, I.R.; Moter, A.; Gescher, D.M.; et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006, 177, 8785–8795. [Google Scholar] [CrossRef]
- Bereswill, S.; Fischer, A.; Plickert, R.; Haag, L.-M.; Otto, B.; Kühl, A.A.; Dashti, J.I.; Zautner, A.E.; Muñoz, M.; Loddenkemper, C.; et al. Novel Murine Infection Models Provide Deep Insights into the “Ménage à Trois” of Campylobacter jejuni, Microbiota and Host Innate Immunity. PLoS ONE 2011, 6, e20953. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar] [PubMed]
- Weber, P.; Koch, M.; Heizmann, W.R.; Scheurlen, M.; Jenss, H.; Hartmann, F. Microbic Superinfection in Relapse of Inflammatory Bowel Disease. J. Clin. Gastroenterol. 1992, 14, 302–308. [Google Scholar] [CrossRef]
- Mylonaki, M.; Langmead, L.; Pantes, A.; Johnson, F.; Rampton, D.S. Enteric infection in relapse of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2004, 16, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, E.; Baldoni, M.; Giovenali, P.; Villanacci, V.; Essatari, M.; Bassotti, G. Intestinal superinfections in patients with inflammatory bowel diseases. J. Crohns. Coliti. 2012, 6, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Genger, C.; Kløve, S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. The conundrum of colonization resistance against Campylobacter reloaded: The gut microbota composition in conventional mice does not prevent from Campylobacter coli infection. Eur. J. Microbiol. Immunol. 2020. [Google Scholar] [CrossRef]
- Backert, S.; Hofreuter, D. Molecular methods to investigate adhesion, transmigration, invasion and intracellular survival of the foodborne pathogen Campylobacter jejuni. J. Microbiol. Methods 2013, 95, 8–23. [Google Scholar] [CrossRef]
- Burnham, P.M.; Hendrixson, D.R. Campylobacter jejuni: Collective components promoting a successful enteric lifestyle. Nat. Rev. Genet. 2018, 16, 551–565. [Google Scholar] [CrossRef]
- Kløve, S.; Genger, C.; Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Toll-Like Receptor-4 Dependent Intestinal and Systemic Sequelae Following Peroral Campylobacter coli Infection of IL10 Deficient Mice Harboring a Human Gut Microbiota. Pathog. 2020, 9, 386. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Fischer, A.; Jahn, H.-K.; Niebergall, J.; Freudenberg, M.; Blaut, M.; Liesenfeld, O.; Schumann, R.R.; Gobel, U.B.; Bereswill, S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut 2007, 56, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Fischer, A.; Siegmund, B.; Kupz, A.; Niebergall, J.; Fuchs, D.; Jahn, H.-K.; Freudenberg, M.; Loddenkemper, C.; Batra, A.; et al. Shift Towards Pro-inflammatory Intestinal Bacteria Aggravates Acute Murine Colitis via Toll-like Receptors 2 and 4. PLoS ONE 2007, 2, e662. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C.; Duncan, S.H.; Bereswill, S.; Heimesaat, M.M. The Induction of Colitis and Ileitis in Mice Is Associated with Marked Increases in Intestinal Concentrations of Stimulants of TLRs 2, 4, and 5. PLoS ONE 2010, 5, e9125. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Nogai, A.; Bereswill, S.; Plickert, R.; Fischer, A.; Loddenkemper, C.; Steinhoff, U.; Tchaptchet, S.; Thiel, E.; Freudenberg, M.A.; et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 2010, 59, 1079–1087. [Google Scholar] [CrossRef]
- Fiebiger, U.; Bereswill, S.; Heimesaat, M.M. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: Lessons learned from germfree and gnotobiotic animal models. Eur. J. Microbiol. Immunol. 2016, 6, 253–271. [Google Scholar] [CrossRef]
- Swidsinski, A.; Ladhoff, A.; Pernthaler, A.; Swidsinski, S.; Loening–Baucke, V.; Ortner, M.; Weber, J.; Hoffmann, U.; Schreiber, S.; Dietel, M.; et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002, 122, 44–54. [Google Scholar] [CrossRef]
- Swidsinski, A.; Weber, J.; Loening-Baucke, V.; Hale, L.P.; Lochs, H. Spatial Organization and Composition of the Mucosal Flora in Patients with Inflammatory Bowel Disease. J. Clin. Microbiol. 2005, 43, 3380–3389. [Google Scholar] [CrossRef]
- Rausch, S.; Held, J.; Fischer, A.; Heimesaat, M.M.; Kühl, A.A.; Bereswill, S.; Hartmann, S. Small Intestinal Nematode Infection of Mice Is Associated with Increased Enterobacterial Loads alongside the Intestinal Tract. PLoS ONE 2013, 8, e74026. [Google Scholar] [CrossRef]
- Ekmekciu, I.; Von Klitzing, E.; Fiebiger, U.; Escher, U.; Neumann, C.; Bacher, P.; Scheffold, A.; Kühl, A.A.; Bereswill, S.; Heimesaat, M.M. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front. Immunol. 2017, 8, 372. [Google Scholar] [CrossRef]
- Alutis, M.E.; Grundmann, U.; Fischer, A.; Hagen, U.; Kühl, A.A.; Göbel, U.B.; Bereswill, S.; Heimesaat, M.M. The role of gelatinases inCampylobacter jejuniinfection of gnotobiotic mice. Eur. J. Microbiol. Immunol. 2015, 5, 256–267. [Google Scholar] [CrossRef]
- Alutis, M.E.; Grundmann, U.; Hagen, U.; Fischer, A.; Kühl, A.A.; Göbel, U.B.; Bereswill, S.; Heimesaat, M.M. Matrix Metalloproteinase-2 Mediates Intestinal Immunopathogenesis inCampylobacter jejuni-infected infant mice. Eur. J. Microbiol. Immunol. 2015, 5, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Lugert, R.; Fischer, A.; Alutis, M.; Kühl, A.A.; Zautner, A.E.; Tareen, A.M.; Göbel, U.B.; Bereswill, S. Impact of Campylobacter jejuni cj0268c Knockout Mutation on Intestinal Colonization, Translocation, and Induction of Immunopathology in Gnotobiotic IL-10 Deficient Mice. PLoS ONE 2014, 9, e90148. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Gatea, F. Correlations between Microbiota Bioactivity and Bioavailability of Functional Compounds: A Mini-Review. Biomedicines 2020, 8, 39. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heimesaat, M.M.; Genger, C.; Biesemeier, N.; Klove, S.; Weschka, D.; Mousavi, S.; Bereswill, S. Inflammatory Immune Responses and Gut Microbiota Changes Following Campylobacter coli Infection of IL-10-/- Mice with Chronic Colitis. Pathogens 2020, 9, 560. https://doi.org/10.3390/pathogens9070560
Heimesaat MM, Genger C, Biesemeier N, Klove S, Weschka D, Mousavi S, Bereswill S. Inflammatory Immune Responses and Gut Microbiota Changes Following Campylobacter coli Infection of IL-10-/- Mice with Chronic Colitis. Pathogens. 2020; 9(7):560. https://doi.org/10.3390/pathogens9070560
Chicago/Turabian StyleHeimesaat, Markus M., Claudia Genger, Nina Biesemeier, Sigri Klove, Dennis Weschka, Soraya Mousavi, and Stefan Bereswill. 2020. "Inflammatory Immune Responses and Gut Microbiota Changes Following Campylobacter coli Infection of IL-10-/- Mice with Chronic Colitis" Pathogens 9, no. 7: 560. https://doi.org/10.3390/pathogens9070560
APA StyleHeimesaat, M. M., Genger, C., Biesemeier, N., Klove, S., Weschka, D., Mousavi, S., & Bereswill, S. (2020). Inflammatory Immune Responses and Gut Microbiota Changes Following Campylobacter coli Infection of IL-10-/- Mice with Chronic Colitis. Pathogens, 9(7), 560. https://doi.org/10.3390/pathogens9070560





