Natural Compounds from the Marine Brown Alga Caulocystis cephalornithos with Potent In Vitro-Activity against the Parasitic Nematode Haemonchus contortus
Abstract
1. Introduction
2. Results
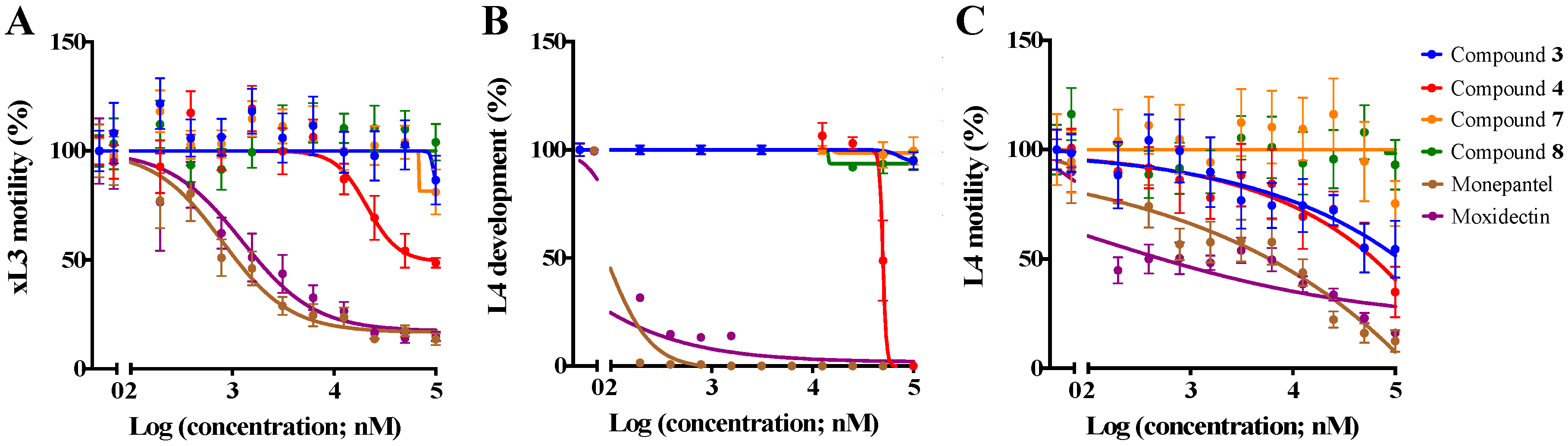
2.1. Reduction in Larval Motility and/or Development, and Phenotypic Alteration
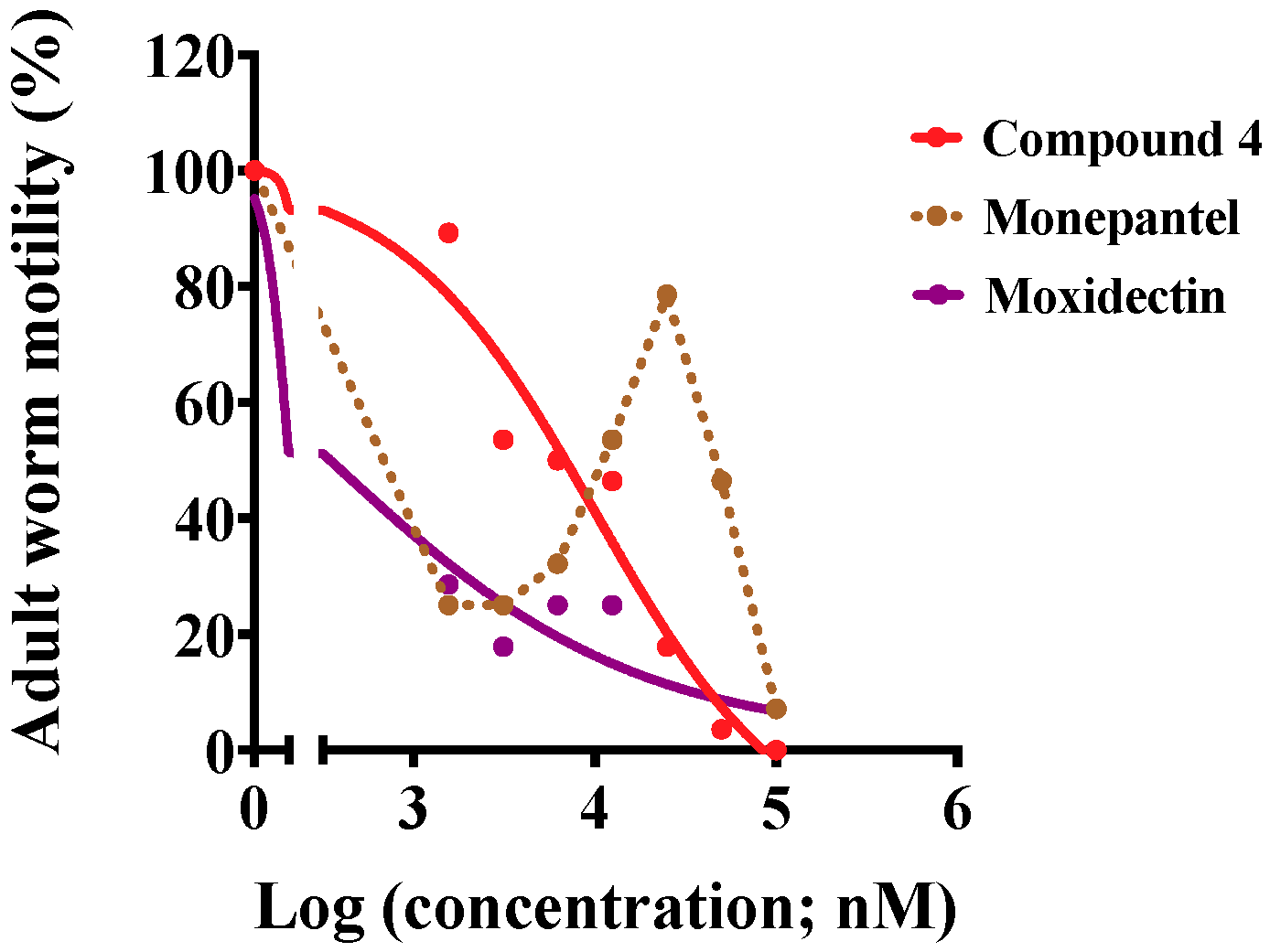
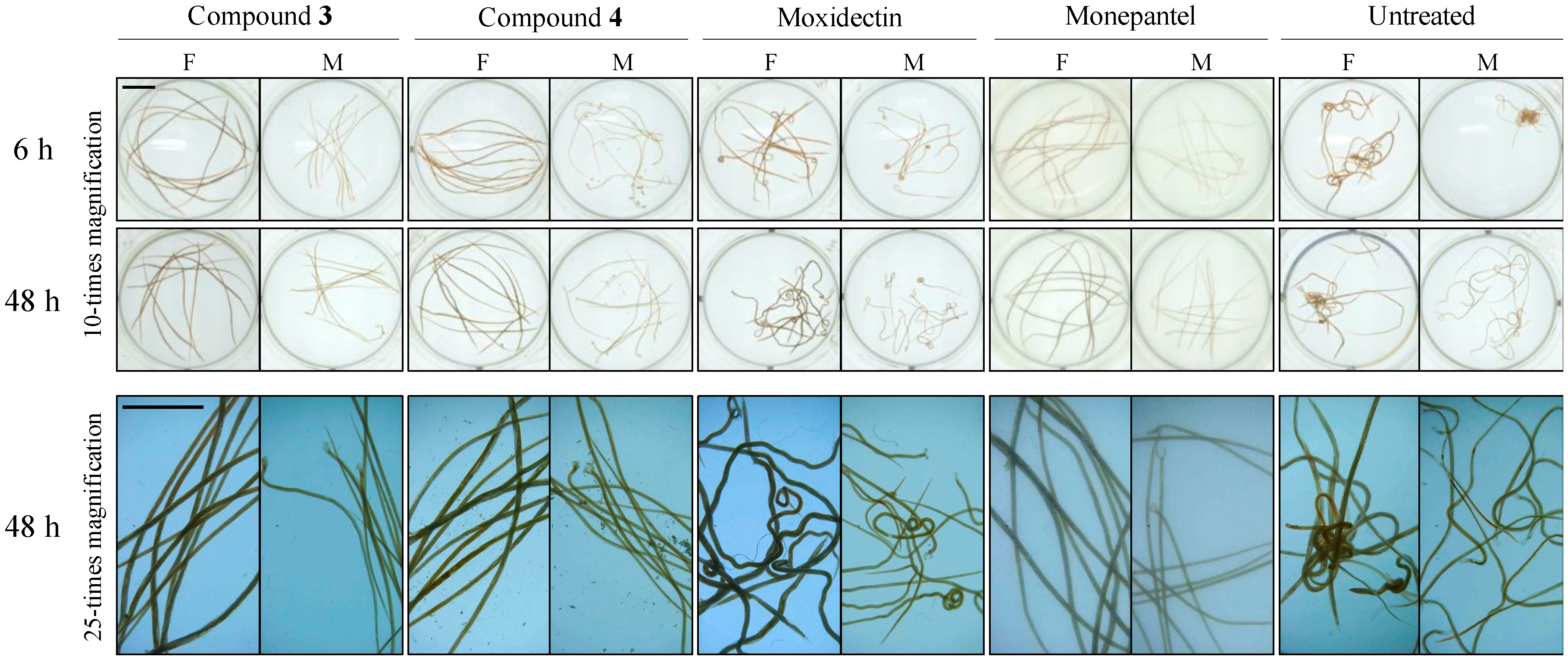
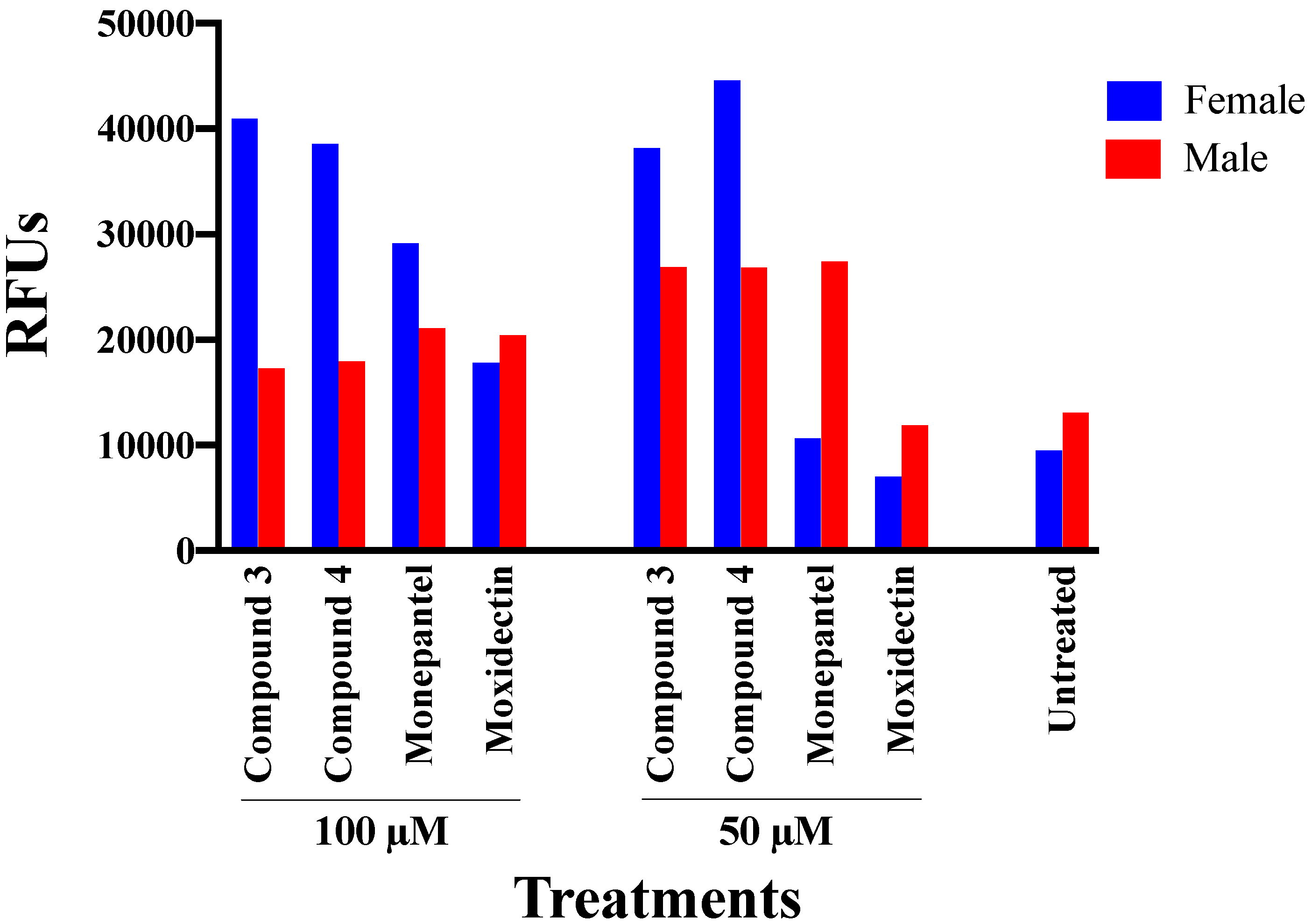
2.2. Acute Nematocidal Activity on Adults
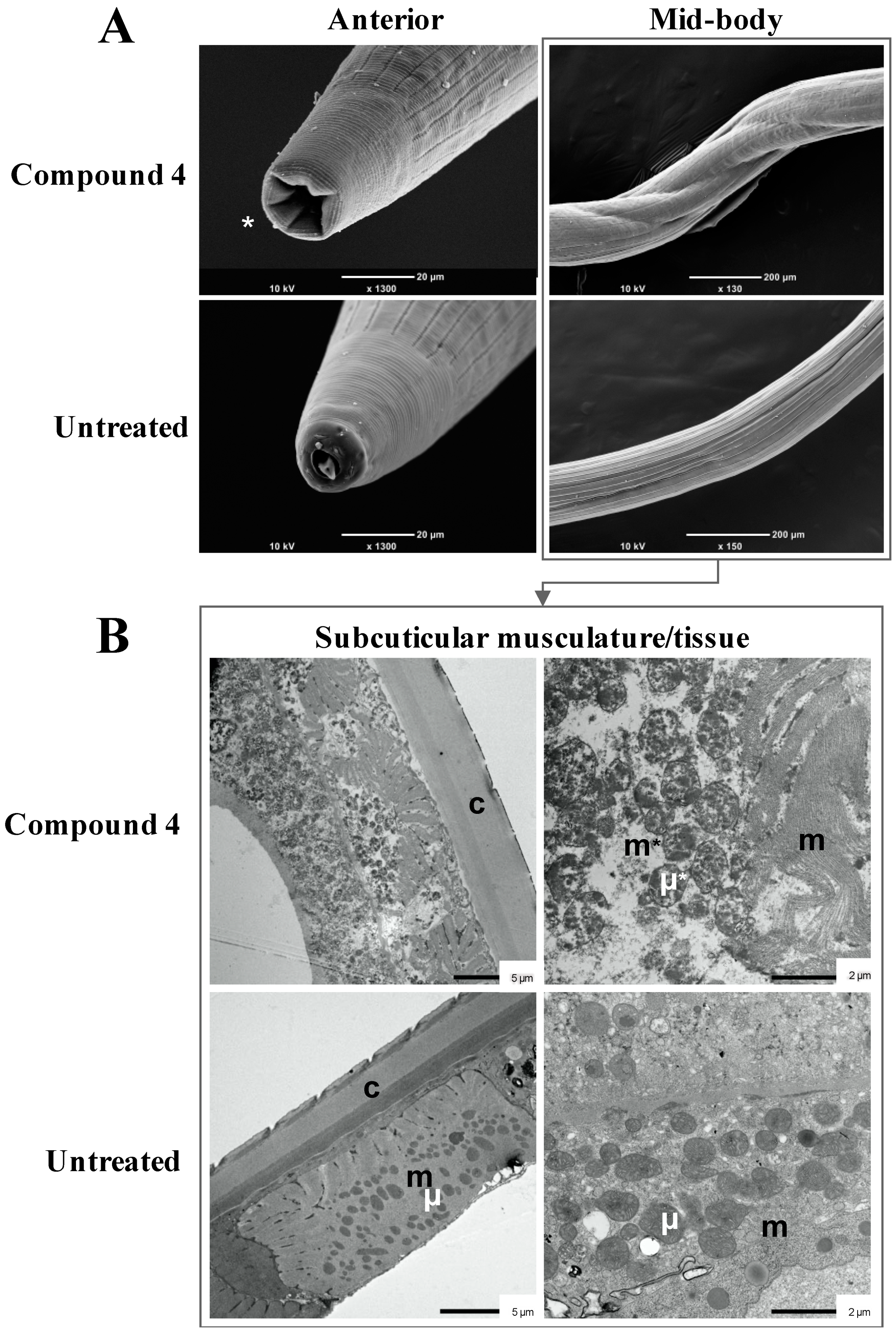
2.3. Subcuticular Tissue Damage in Adult Worms
3. Discussion
4. Materials and Methods
4.1. Compounds and Preparation
4.2. Procurement of Larval and Adult Stages of H. contortus
4.3. Screening of Compounds on xL3s of H. contortus
4.4. Dose-Response Assessments of Active Compounds on xL3s and L4s of H. contortus
4.5. Assessment of the Activity of Selected Compounds on Adult H. contortus
4.6. Scanning Electron Microscopy (SEM)
4.7. Transmission Electron Microscopy (TEM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Soil-Transmitted Helminth Infections. Available online: www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections. (accessed on 1 May 2020).
- Mavrot, F.; Hertzberg, H.; Torgerson, P. Effect of gastro-intestinal nematode infection on sheep performance: A systematic review and meta-analysis. Parasit. Vectors 2015, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Gasser, R.B.; von Samson-Himmelstjerna, G. Haemonchus contortus and Haemonchosis—Past, Present and Future Trends. Adv. Parasit. Academic Press: London, England, 2016; Volume 93, ISBN 978-0-128-10395-1. [Google Scholar]
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. Diagnosis, treatment and management of Haemonchus contortus in small ruminants. Adv. Parasitol. 2016, 93, 181–238. [Google Scholar] [PubMed]
- Roeber, F.; Jex, A.R.; Gasser, R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance—an Australian perspective. Parasit. Vectors 2013, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Alvarado, M.; Basáñez, M.-G.; Bolliger, I.; Bourne, R.; Boussinesq, M.; Brooker, S.J.; Brown, A.S.; Buckle, G.; Budke, C.M.; et al. The global burden of disease study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014, 8, e2865. [Google Scholar] [CrossRef] [PubMed]
- Kotze, A.C.; Prichard, R.K. Anthelmintic resistance in Haemonchus contortus: History, mechanisms and diagnosis. Adv. Parasitol. 2016, 93, 397–428. [Google Scholar]
- Jiao, Y.; Preston, S.; Hofmann, A.; Taki, A.; Baell, J.; Chang, B.C.H.; Jabbar, A.; Gasser, R.B. A perspective on the discovery of selected compounds with anthelmintic activity against the barber’s pole worm—where to from here? Adv. Parasitol. 2020, 108, 1–45. [Google Scholar]
- Mederos, A.E.; Ramos, Z.; Banchero, G.E. First report of monepantel Haemonchus contortus resistance on sheep farms in Uruguay. Parasit. Vectors 2014, 7, 598. [Google Scholar] [CrossRef]
- Van den Brom, R.; Moll, L.; Kappert, C.; Vellema, P. Haemonchus contortus resistance to monepantel in sheep. Vet. Parasitol. 2015, 209, 278–280. [Google Scholar] [CrossRef]
- Sales, N.; Love, S. Resistance of Haemonchus sp. to monepantel and reduced efficacy of a derquantel/abamectin combination confirmed in sheep in NSW, Australia. Vet. Parasitol. 2016, 228, 193–196. [Google Scholar] [CrossRef]
- Geary, T.G.; Conder, G.A.; Bishop, B. The changing landscape of antiparasitic drug discovery for veterinary medicine. Trends Parasitol. 2004, 20, 449–455. [Google Scholar] [CrossRef]
- Zajíčková, M.; Nguyen, L.T.; Skálová, L.; Stuchlíková, L.R.; Matoušková, P. Anthelmintics in the future: Current trends in the discovery and development of new drugs against gastrointestinal nematodes. Drug Discov. Today 2019, 25, 430–437. [Google Scholar] [CrossRef]
- Taman, A.; Azab, M. Present-day anthelmintics and perspectives on future new targets. Parasitol. Res. 2014, 113, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Kumarasingha, R.; Preston, S.; Yeo, T.-C.; Lim, D.S.L.; Tu, C.-L.; Palombo, E.A.; Shaw, J.M.; Gasser, R.B.; Boag, P.R. Anthelmintic activity of selected ethno-medicinal plant extracts on parasitic stages of Haemonchus contortus. Parasit. Vectors 2016, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, G.; Hoste, H.; Karonen, M.; Salminen, J.-P.; Hendriks, W.H.; Pellikaan, W.F. The in vitro anthelmintic properties of browse plant species against Haemonchus contortus is determined by the polyphenol content and composition. Vet. Parasitol. 2017, 237, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.; Korhonen, P.K.; Mouchiroud, L.; Cornaglia, M.; McGee, S.L.; Young, N.D.; Davis, R.A.; Crawford, S.; Nowell, C.; Ansell, B.R.E.; et al. Deguelin exerts potent nematocidal activity via the mitochondrial respiratory chain. FASEB J. 2017, 31, 4515–4532. [Google Scholar] [CrossRef]
- Herath, H.M.P.D.; Preston, S.; Jabbar, A.; Garcia-Bustos, J.; Taki, A.C.; Addison, R.S.; Hayes, S.; Beattie, K.D.; McGee, S.L.; Martin, S.D.; et al. Identification of Fromiamycalin and Halaminol A from Australian Marine Sponge Extracts with Anthelmintic Activity against Haemonchus contortus. Mar. Drugs 2019, 17, 598. [Google Scholar] [CrossRef]
- Mravčáková, D.; Váradyová, Z.; Kopčáková, A.; Čobanová, K.; Grešáková, Ľ.; Kišidayová, S.; Babják, M.; Dolinská, M.U.; Dvorožňáková, E.; Königová, A.; et al. Natural chemotherapeutic alternatives for controlling of haemonchosis in sheep. BMC Vet. Res. 2019, 15, 302. [Google Scholar] [CrossRef]
- Chassagne, F.; Cabanac, G.; Hubert, G.; David, B.; Marti, G. The landscape of natural product diversity and their pharmacological relevance from a focus on the Dictionary of Natural Products®. Phytochem. Rev. 2019, 18, 601–622. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Lughadha, E.N.; Govaerts, R.; Belyaeva, I.; Black, N.; Lindon, H.; Allkin, R.; Magill, R.E.; Nicolson, N. Counting counts: Revised estimates of numbers of accepted species of flowering plants, seed plants, vascular plants and land plants with a review of other recent estimates. Phytotaxa 2016, 272, 82–88. [Google Scholar] [CrossRef]
- Simpson, B.S.; Bulone, V.; Semple, S.J.; Booker, G.W.; McKinnon, R.A.; Weinstein, P. Arid awakening: New opportunities for Australian plant natural product research. Rangeland J. 2016, 38, 467. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Biva, I.J.; Ndi, C.P.; Griesser, H.J.; Semple, S.J. Antibacterial constituents of Eremophila alternifolia: An Australian aboriginal traditional medicinal plant. J. Ethnopharmacol. 2016, 182, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Gunawardena, D.; Ahktar, M.; Low, M.; Reddell, P.; Münch, G. Anti-inflammatory chemical profiling of the Australian rainforest tree Alphitonia petriei (Rhamnaceae). Molecules 2016, 21, 1521. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Wibowo, M.; Wang, Q.; Holst, J.; Davis, R.A. Dihydro-β-agarofurans from the Australian rainforest plant Denhamia celastroides that inhibit leucine transport in prostate cancer cells. Magn. Reson. Chem. 2018, 57, 101–109. [Google Scholar] [CrossRef]
- Kumar, R.; Duffy, S.; Avery, V.M.; Carroll, A.R.; Davis, R.A. Microthecaline A, a quinoline serrulatane alkaloid from the roots of the Australian desert plant Eremophila microtheca. J. Nat. Prod. 2018, 81, 1079–1083. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S. Application of HPLC-NMR for the rapid chemical profiling of a southern Australian sponge, Dactylospongia sp. J. Sep. Sci. 2009, 32, 542–548. [Google Scholar] [CrossRef]
- Brkljača, R.; Gӧker, E.; Urban, S. Dereplication and chemotaxonomical studies of marine algae of the Ochrophyta and Rhodophyta phyla. Mar. Drugs 2015, 13, 2714–2731. [Google Scholar] [CrossRef]
- Brkljača, R.; Dahse, H.-M.; Voigt, K.; Urban, S. Antimicrobial evaluation of the constituents isolated from Macropidia fuliginosa (Hook.) Druce. Nat. Prod. Commun. 2019, 14, 1934578X1988441. [Google Scholar] [CrossRef]
- Lever, J.; Brkljača, R.; Kraft, G.; Urban, S. Natural products of marine macroalgae from south eastern Australia, with emphasis on the Port Phillip Bay and Heads regions of Victoria. Mar. Drugs 2020, 18, 142. [Google Scholar] [CrossRef]
- Buckle, P.J.; Baldo, B.A.; Taylor, K.M. The anti-inflammatory activity of marine natural products—6-n-tridecylsalicylic acid, flexibilide and dendalone 3-hydroxybutyrate. Agents Actions 1980, 10, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Narkowicz, C.K.; Blackman, A.J. Further acetogenins from Tasmanian collections of Caulocystis cephalornithos demonstrating chemical variability. Biochem. Syst. Ecol. 2006, 34, 635–641. [Google Scholar] [CrossRef]
- Cho, H.-I.; Park, J.-H.; Choi, H.-S.; Kwak, J.H.; Lee, D.-U.; Lee, S.K.; Lee, S.-M. Protective mechanisms of acacetin against D-galactosamine and lipopolysaccharide-induced fulminant hepatic failure in mice. J. Nat. Prod. 2014, 77, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Brkljača, R.; Urban, S. HPLC-NMR and HPLC-MS profiling and bioassay-guided identification of secondary metabolites from the Australian plant Haemodorum spicatum. J. Nat. Prod. 2015, 78, 1486–1494. [Google Scholar] [CrossRef]
- Brkljača, R.; White, J.M.; Urban, S. Phytochemical investigation of the constituents derived from the Australian plant Macropidia fuliginosa. J. Nat. Prod. 2015, 78, 1600–1608. [Google Scholar] [CrossRef]
- Preston, S.; Jabbar, A.; Nowell, C.; Joachim, A.; Ruttkowski, B.; Baell, J.; Cardno, T.; Korhonen, P.K.; Piedrafita, D.; Ansell, B.R.E.; et al. Low cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 2015, 45, 333–343. [Google Scholar] [CrossRef]
- Tritten, L.; Silbereisen, A.; Keiser, J. In vitro and in vivo efficacy of Monepantel (AAD 1566) against laboratory models of human intestinal nematode infections. PLoS Negl. Trop. Dis. 2011, 5, e1457. [Google Scholar] [CrossRef]
- Veglia, F. The anatomy and life history of Haemonchus contortus (Rud.). J. Comp. Pathol. Ther. 1916, 29, 265–277. [Google Scholar]
- Weise, R.W. A light and electron microscopic study of the dorsal buccal lancet of Haemonchus contortus. J. Parasitol. 1977, 63, 854–857. [Google Scholar] [CrossRef]
- Sommerville, R.I. The development of Haemonchus contortus to the fourth stage in vitro. J. Parasitol. 1966, 52, 127–136. [Google Scholar] [CrossRef]
- Jiao, Y.; Preston, S.; Garcia-Bustos, J.F.; Baell, J.B.; Ventura, S.; Le, T.; McNamara, N.; Nguyen, N.; Botteon, A.; Skinner, C.; et al. Tetrahydroquinoxalines induce a lethal evisceration phenotype in Haemonchus contortus in vitro. Int. J. Parasitol. Drugs Drug Resist. 2018, 9, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ortiz-de-Montellano, C.; Torres-Acosta, J.F.d.J.; Fourquaux, I.; Sandoval-Castro, C.A.; Hoste, H. Ultrastructural study of adult Haemonchus contortus exposed to polyphenol-rich materials under in vivo conditions in goats. Parasite 2019, 26, 65. [Google Scholar]
- Martínez-Ortíz-de-Montellano, C.; Arroyo-López, C.; Fourquaux, I.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Hoste, H. Scanning electron microscopy of Haemonchus contortus exposed to tannin-rich plants under in vivo and in vitro conditions. Exp. Parasitol. 2013, 133, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Barone, C.D.; Zajac, A.M.; Manzi-Smith, L.A.; Howell, A.B.; Reed, J.D.; Krueger, C.G.; Petersson, K.H. Anthelmintic efficacy of cranberry vine extracts on ovine Haemonchus contortus. Vet. Parasitol. 2018, 253, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, F.; Shan, X.; Pan, J.; Yu, P.; Mao, Z. Effects of the molluscicidal agent GA-C13:0, a natural occurring ginkgolic acid, on snail mitochondria. Pestic. Biochem. Phys. 2012, 103, 115–120. [Google Scholar] [CrossRef]
- Jex, A.R.; Gasser, R.B.; Schwarz, E.M. Transcriptomic resources for parasitic nematodes of veterinary importance. Trends Parasitol. 2018, 35, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Kumarasingha, R.; Young, N.D.; Yeo, T.-C.; Lim, D.S.L.; Tu, C.-L.; Palombo, E.A.; Shaw, J.M.; Gasser, R.B.; Boag, P.R. Transcriptional alterations in Caenorhabditis elegans following exposure to an anthelmintic fraction of the plant Picria fel-terrae Lour. Parasit. Vectors 2019, 12, 181. [Google Scholar] [CrossRef]
- Wang, T.; Ma, G.; Ang, C.-S.; Korhonen, P.K.; Xu, R.; Nie, S.; Koehler, A.V.; Simpson, R.J.; Greening, D.W.; Reid, G.E.; et al. Somatic proteome of Haemonchus contortus. Int. J. Parasitol. 2019, 49, 311–320. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Mulder, J.; Murphy, P.; Wells, R. New metabolites from the brown alga Caulocystis cephalornithos. Aust. J. Chem. 1980, 33, 2097–2101. [Google Scholar] [CrossRef]
- Kelm, J.M.; Lal-Nag, M.; Sittampalam, G.S.; Ferrer, M. Translational in vitro research: Integrating 3D drug discovery and development processes into the drug development pipeline. Drug Discov. Today 2018, 24, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, L.; Chadwick, A.E.; Bayliss, M.; French, N.S.; Monshouwer, M.; Snoeys, J.; Park, B.K. The utility of HepG2 cells to identify direct mitochondrial dysfunction in the absence of cell death. Toxicol. In Vitro 2015, 29, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Zeng, S. Cytotoxicity of ginkgolic acid in HepG2 cells and primary rat hepatocytes. Toxicol. Lett. 2009, 187, 131–136. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Du, W.; Feng, Y.; Kong, X.; Li, Y.; Xiao, L.; Zhang, P. Antitumor effects of ginkgolic Acid in human cancer cell occur via cell cycle arrest and decrease the Bcl-2/Bax ratio to induce apoptosis. Chemotherapy 2010, 56, 393–402. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, X.G.; Shen, Y.J.; Lu, Z.X.; Tu, G.H.; Fu, X.L.; Chen, S.X.; Cao, J.P. Efficacy of ginkgolic acids against Cryptosporidium andersoni in cell culture. Parasitol. Res. 2011, 109, 1475–1479. [Google Scholar] [CrossRef]
- Ugwu, C.E.; Jiang, Y.Y.; Wu, L.; Xu, Y.X.; Yin, J.H.; Duan, L.P.; Chen, S.X.; Liu, H.; Pan, W.; Quan, H.; et al. In vitro Screening of ginkgolic acids for antiparasitic activity against Cryptosporidium andersoni. Biomed. Environ. Sci. 2019, 32, 300–303. [Google Scholar] [PubMed]
- Lü, J.-M.; Yan, S.; Jamaluddin, S.; Weakley, S.M.; Liang, Z.; Siwak, E.B.; Yao, Q.; Chen, C. Ginkgolic acid inhibits HIV protease activity and HIV infection in vitro. Medical Sci. Monit. Int. Medical J. Exp. Clin. Res. 2012, 18, BR293–BR298. [Google Scholar] [CrossRef]
- Zhang, Z.-B.; Ruan, C.-C.; Chen, D.-R.; Zhang, K.; Yan, C.; Gao, P.-J. Activating transcription factor 3 SUMOylation is involved in angiotensin II-induced endothelial cell inflammation and dysfunction. J. Mol. Cell Cardiol. 2016, 92, 149–157. [Google Scholar] [CrossRef]
- Qiu, F.; Dong, C.; Yanxin, L.; Shao, X.; Huang, D.; Han, Y.; Wang, B.; Liu, Y.; Huo, R.; Paulo, P.; et al. Pharmacological inhibition of SUMO-1 with ginkgolic acid alleviates cardiac fibrosis induced by myocardial infarction in mice. Toxicol. Appl. Pharm. 2018, 345, 1–9. [Google Scholar] [CrossRef]
- Baek, S.; Lee, J.; Kim, C.; Ko, J.-H.; Ryu, S.-H.; Lee, S.-G.; Yang, W.; Um, J.-Y.; Chinnathambi, A.; Alharbi, S.; et al. Ginkgolic acid C 17:1, derived from Ginkgo biloba leaves, suppresses constitutive and inducible STAT3 activation through induction of PTEN and SHP-1 tyrosine phosphatase. Molecules 2017, 22, 276. [Google Scholar] [CrossRef]
- Kleijnen, J.; Knipschild, P. Ginkgo biloba . Lancet 1992, 340, 1136–1139. [Google Scholar] [CrossRef]
- Chassagne, F.; Huang, X.; Lyles, J.T.; Quave, C.L. Validation of a 16th century traditional Chinese medicine use of Ginkgo biloba as a topical antimicrobial. Front. Microbiol. 2019, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharmacokinet. 2013, 52, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, M.; Guo, H. An updated review of randomized clinical trials testing the improvement of cognitive function of Ginkgo biloba extract in healthy people and Alzheimer’s patients. Front. Pharmacol. 2020, 10, 1688. [Google Scholar] [CrossRef]
- Schwarz, E.M.; Korhonen, P.K.; Campbell, B.E.; Young, N.D.; Jex, A.R.; Jabbar, A.; Hall, R.S.; Mondal, A.; Howe, A.C.; Pell, J.; et al. The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biol. 2013, 14, R89. [Google Scholar] [CrossRef]
- Kaminsky, R.; Ducray, P.; Jung, M.; Clover, R.; Rufener, L.; Bouvier, J.; Weber, S.S.; Wenger, A.; Wieland-Berghausen, S.; Goebel, T.; et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature 2008, 452, 176–180. [Google Scholar] [CrossRef]
- Rufener, L.; Bedoni, N.; Baur, R.; Rey, S.; Glauser, D.A.; Bouvier, J.; Beech, R.; Sigel, E.; Puoti, A. acr-23 encodes a monepantel-sensitive channel in Caenorhabditis elegans. PLoS Pathog. 2013, 9, e1003524. [Google Scholar] [CrossRef]
- Prichard, R.K.; Geary, T.G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 69–83. [Google Scholar] [CrossRef]

| Worm Sex | Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound 3 | Compound 4 | Monepantel | Moxidectin | Untreated | |||||
| 50 μM | 100 μM | 50 μM | 100 μM | 50 μM | 100 μM | 50 μM | 100 μM | (1% DMSO) | |
| Female | 1; 10% 0; 90% | 0; 100% | 1; 10% 0; 90% | 0; 100% | 3; 80% 2; 20% | 2; 20% 0; 80% | 2; 30% 1; 30% 0; 60% | 1; 10% 0; 90% | 3; 90% 2; 10% |
| Male | 0; 100% | 0; 100% | 0; 100% | 0; 100% | 2; 20% 0; 80% | 1; 10% 0: 90% | 2; 20% 1; 10% 0; 70% | 0; 100% | 3; 100% |
| Compound Code | Compound | Molecular Weight | Origin (Common Name) |
|---|---|---|---|
| 1 | pentaprenylated p-quiniol | 450.4 | Dactylospongia sp. (Mustard sponge) |
| 2 | furospinosulin-1 | 354.3 | Dactylospongia sp. (Mustard sponge) |
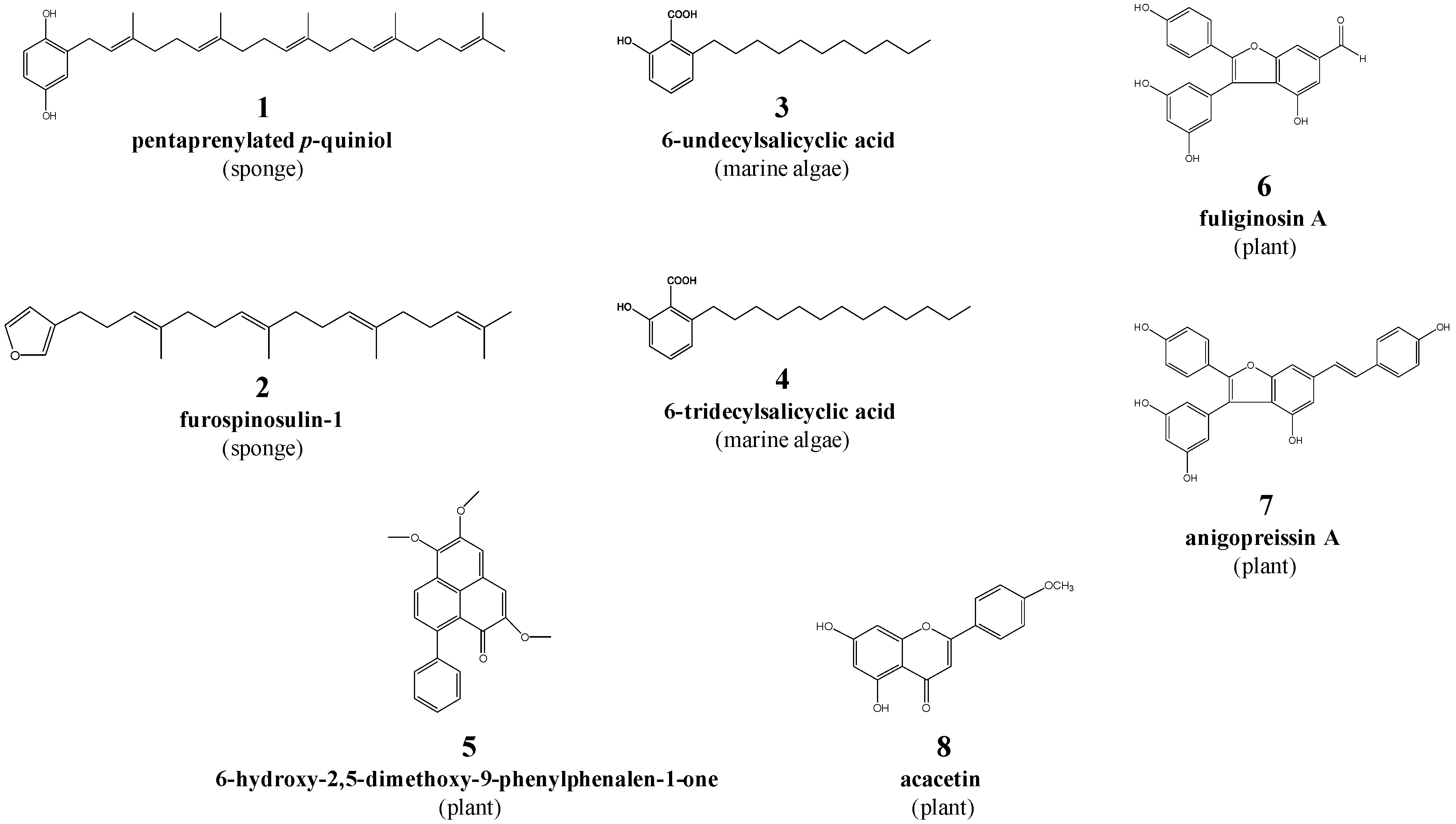
| 3 | 6-undecylsalicyclic acid | 292.2 | Caulocystis cephalornithos (Marine brown alga) |
| 4 | 6-tridecylsalicylic acid | 320.2 | Caulocystis cephalornithos (Marine brown alga) |
| 5 | 6-hydroxy-2,5-dimethoxy-9-phenylphenalen-1-one | 346.1 | Haemodorum spicatum (Bloodroot; plant) |
| 6 | fuliginosin A | 362.1 | Macropidia fuliginosa (Black kangaroo paw; plant) |
| 7 | anigopreissin A | 452.1 | Macropidia fuliginosa (Black kangaroo paw; plant) |
| 8 | acacetin | 284.1 | Agastache rugosa (Korean mint; plant) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taki, A.C.; Brkljača, R.; Wang, T.; Koehler, A.V.; Ma, G.; Danne, J.; Ellis, S.; Hofmann, A.; Chang, B.C.H.; Jabbar, A.; et al. Natural Compounds from the Marine Brown Alga Caulocystis cephalornithos with Potent In Vitro-Activity against the Parasitic Nematode Haemonchus contortus. Pathogens 2020, 9, 550. https://doi.org/10.3390/pathogens9070550
Taki AC, Brkljača R, Wang T, Koehler AV, Ma G, Danne J, Ellis S, Hofmann A, Chang BCH, Jabbar A, et al. Natural Compounds from the Marine Brown Alga Caulocystis cephalornithos with Potent In Vitro-Activity against the Parasitic Nematode Haemonchus contortus. Pathogens. 2020; 9(7):550. https://doi.org/10.3390/pathogens9070550
Chicago/Turabian StyleTaki, Aya C., Robert Brkljača, Tao Wang, Anson V. Koehler, Guangxu Ma, Jill Danne, Sarah Ellis, Andreas Hofmann, Bill C. H. Chang, Abdul Jabbar, and et al. 2020. "Natural Compounds from the Marine Brown Alga Caulocystis cephalornithos with Potent In Vitro-Activity against the Parasitic Nematode Haemonchus contortus" Pathogens 9, no. 7: 550. https://doi.org/10.3390/pathogens9070550
APA StyleTaki, A. C., Brkljača, R., Wang, T., Koehler, A. V., Ma, G., Danne, J., Ellis, S., Hofmann, A., Chang, B. C. H., Jabbar, A., Urban, S., & Gasser, R. B. (2020). Natural Compounds from the Marine Brown Alga Caulocystis cephalornithos with Potent In Vitro-Activity against the Parasitic Nematode Haemonchus contortus. Pathogens, 9(7), 550. https://doi.org/10.3390/pathogens9070550