Classical Swine Fever Virus Biology, Clinicopathology, Diagnosis, Vaccines and a Meta-Analysis of Prevalence: A Review from the Indian Perspective
Abstract
1. Introduction
2. CSFV Genome and Classification
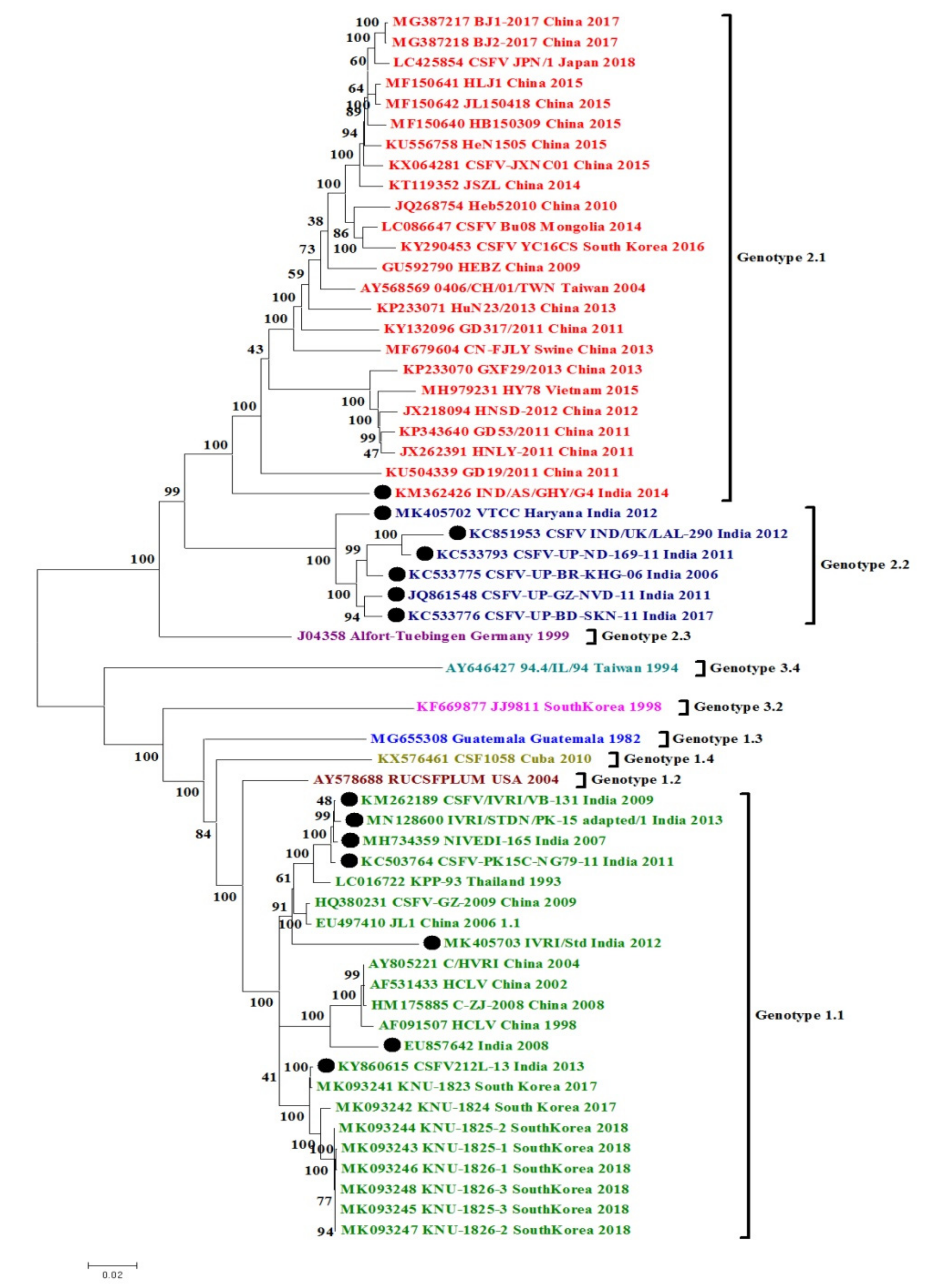
3. Phylogenetic and Sequence Analysis of Indian CSFV Isolates
3.1. CSFV Complete Genome Based Phylogenetic Analysis and Percent Similarity
3.2. Sequence Percent Homology Similarity Index of Other CSFV Gene Targets
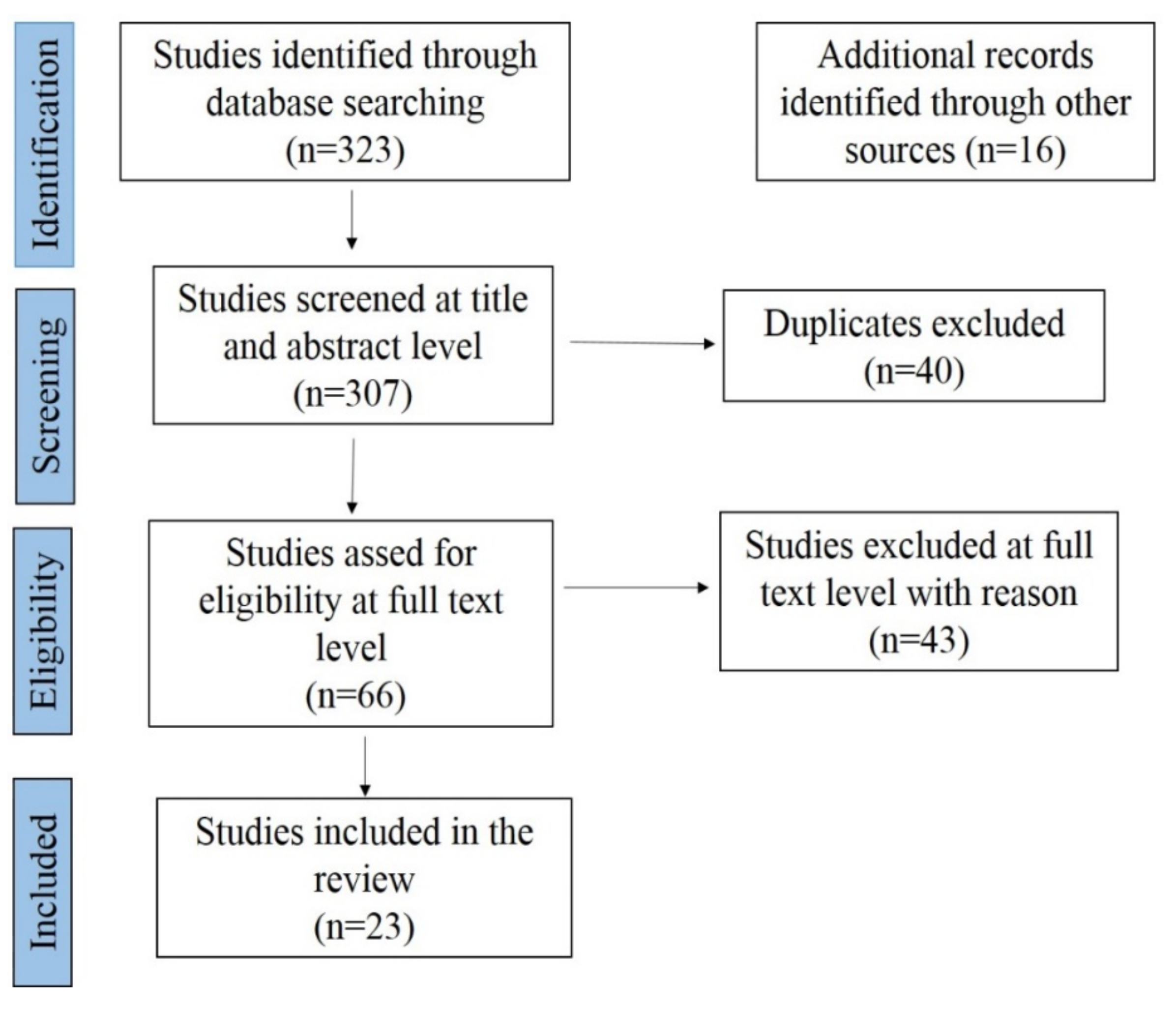
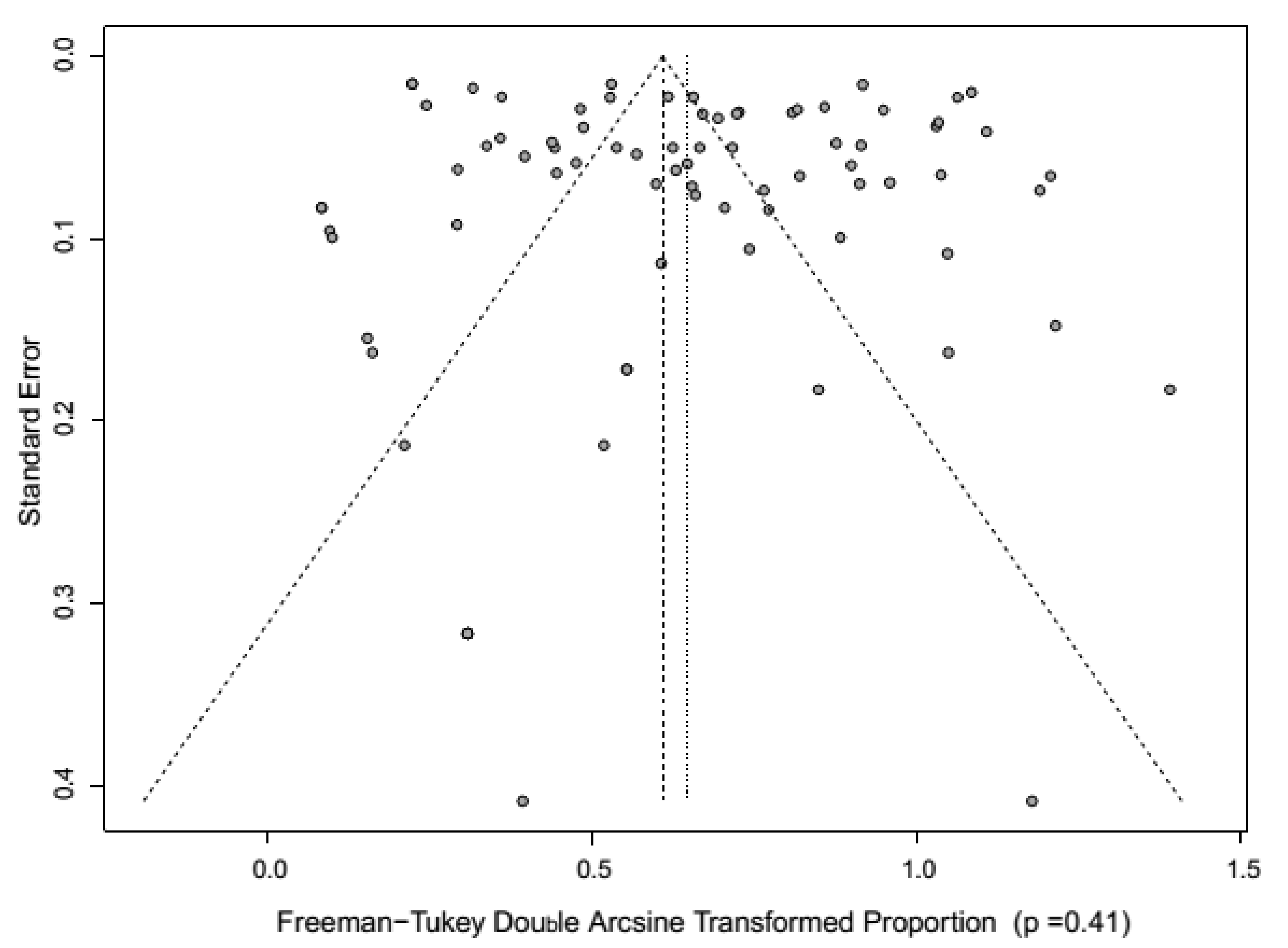
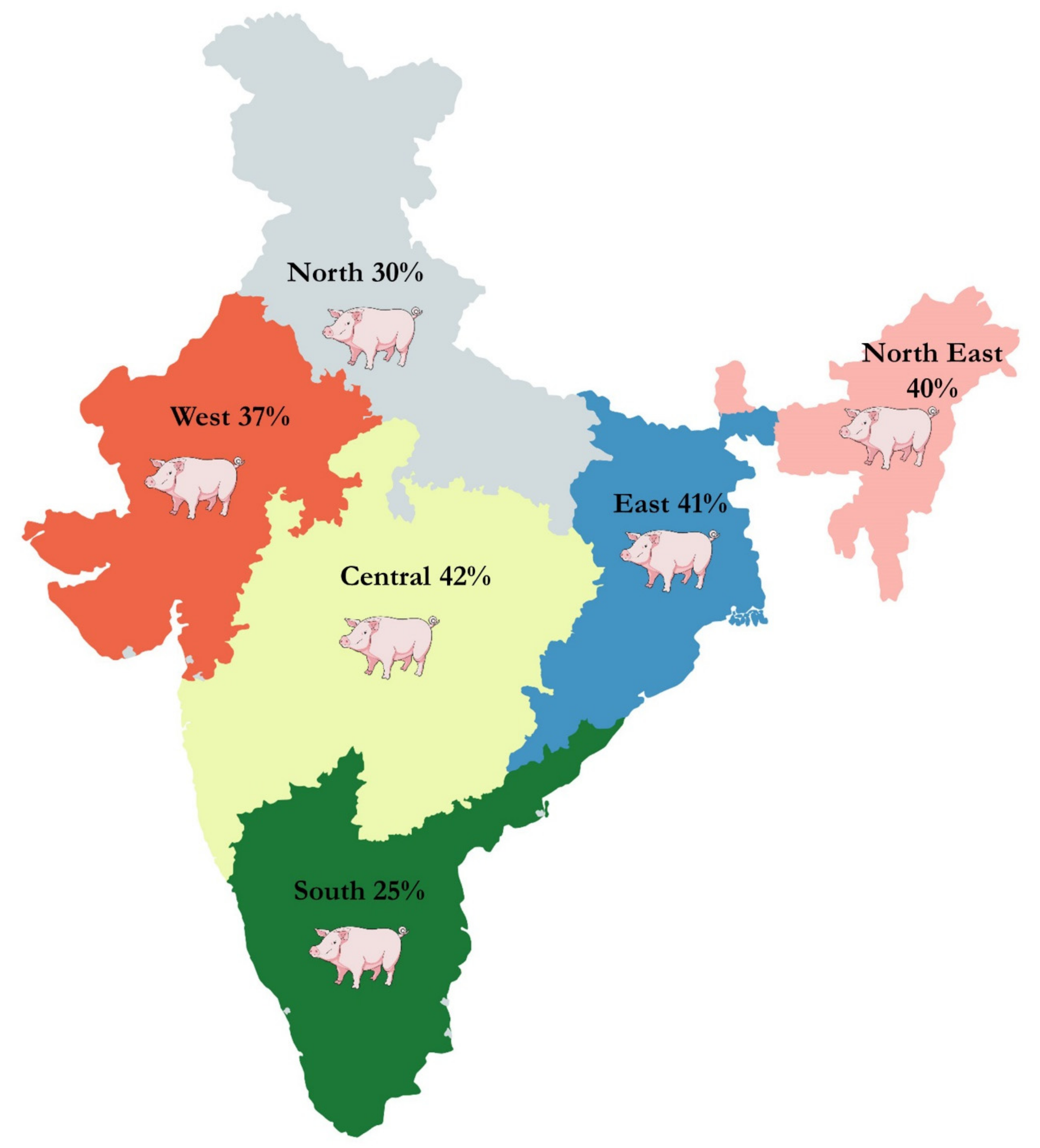
4. Meta-Analysis of CSF Prevalence in India
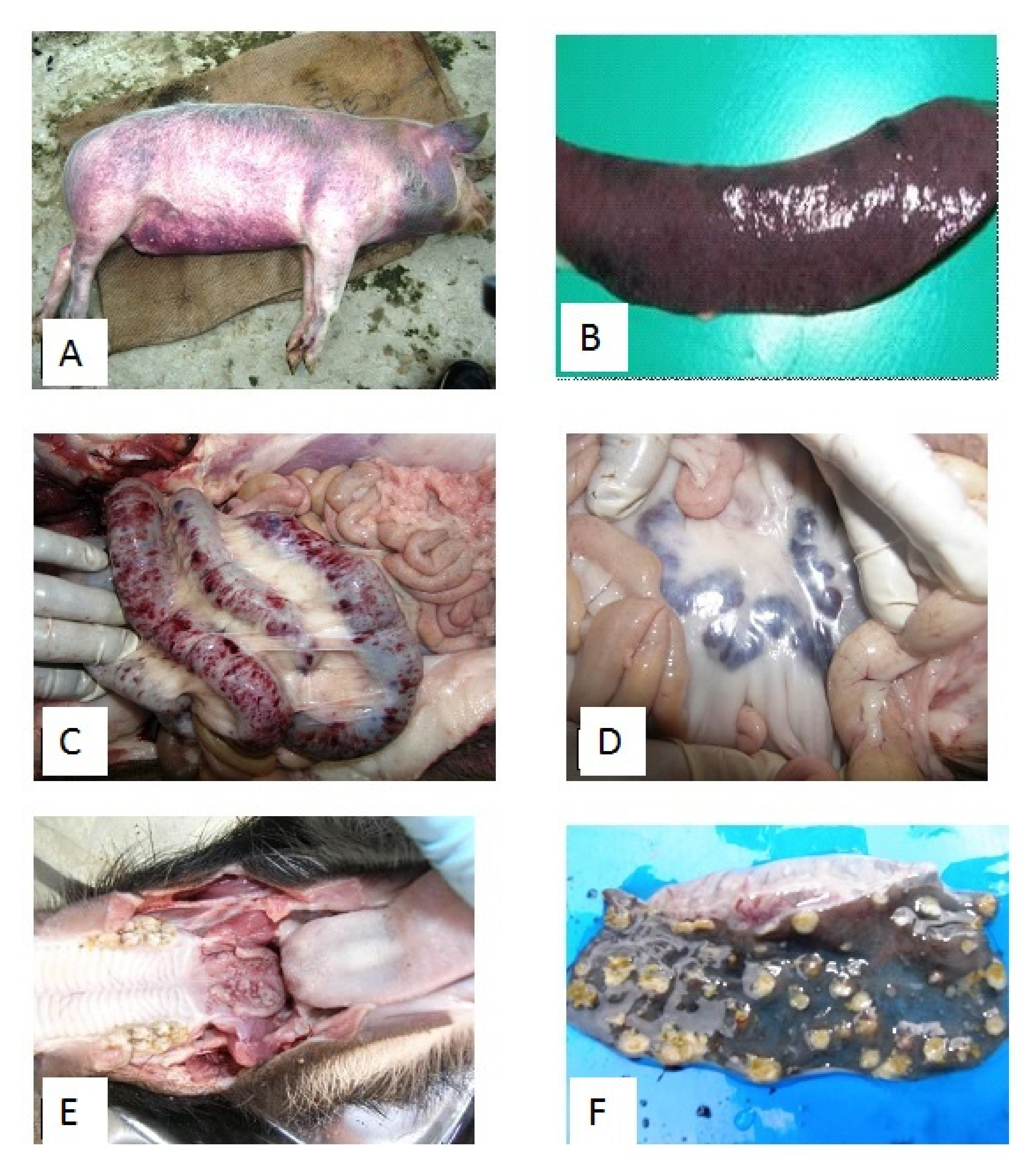
5. Clinical Disease and Pathology on the CSF Outbreaks in India
6. CSF Laboratory Diagnosis in India
6.1. Serological Methods of Diagnosis of CSF
6.2. Molecular Methods of Diagnosis of CSF
6.3. Compelete Genome Sequencing of Indian CSFV Isolates
7. CSF Virus Vaccines in India
8. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Everett, H.; Crooke, H.; Gurrala, R.; Dwarka, R.; Kim, J.; Botha, B.; Lubisi, A.; Pardini, A.; Gers, S.; Vosloo, W.; et al. Experimental infection of common warthogs (Phacochoerus africanus) and bushpigs (Potamochoerus larvatus) with classical swine fever virus. I: Susceptibility and transmission. Transbound. Emerg. Dis. 2011, 58, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.N.; Chen, Y.H. Marker vaccine strategies and candidate CSFV marker vaccines. Vaccine 2007, 25, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.; Laevens, H.; Koenen, F.; Mintiens, K.; De Kruif, A. An experimental infection with classical swine fever virus in pregnant sows: Transmission of the virus, course of the disease, antibody response and effect on gestation. J. Vet. Med. B. 2001, 48, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.F.; Wang, Y.; Gao, H.; Li, N.; Sun, Y.; Liang, B.B.; Qiu, H.J. Immune responses induced by a BacMam virus expressing the E2 protein of ckoopokkokkokjjjlassical swine fever virus in mice. Immunol. Lett. 2009, 125, 145–150. [Google Scholar] [CrossRef]
- Edward, S.; Fukusho, A.; Lefevre, P.C.; Lipowski, A.; Pejsak, Z.; Roehe, P.; Westergaard, J. Classical swine fever: The global situation. Vet. Microbiol. 2000, 73, 103–119. [Google Scholar] [CrossRef]
- Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2016. Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.08.03_CSF.pdf (accessed on 1 February 2017).
- Xia, H.; Wahlberg, N.; Qiu, H.J.; Widén, F.; Belák, S.; Liu, L. Lack of phylogenetic evidence that the Shimen strain is the parental strain of the lapinized Chinese strain (C-strain) vaccine against classical swine fever. Arch. Virol. 2011, 156, 1041–1044. [Google Scholar] [CrossRef]
- Krishnamurthy, D.; Adlakha, S.C. A preliminary report on the swine fever epidemic in Uttar Pradesh. Indian Vet. J. 1962, 39, 406–419. [Google Scholar]
- Sapre, S.N.; Moghe, R.G.; Bhagwat, S.V.; Choudhary, P.G.; Purohit, B.L. Observations on swine fever in Maharashtra. Indian Vet. J. 1962, 39, 527–529. [Google Scholar]
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Muerhoff, A.S.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P.; et al. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef]
- Rümenapf, T.; Unger, G.; Strauss, J.H.; Thiel, H.J. Processing of the envelope glycoproteins of pestiviruses. J. Virol. 1993, 67, 3288–3294. [Google Scholar] [CrossRef]
- Lowings, P.; Ibata, G.; Needham, J.; Paton, D. Classical swine fever virus diversity and evolution. J. Gen. Virol. 1996, 77, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.H.; Cao, H.W.; Cui, Y.D.; Hua, Z. Phylogenetic analysis of E2 genes of classical swine fever virus in China. Israel J. Vet. Med. 2010, 65, 151–155. [Google Scholar]
- Paton, D.J.; McGoldrick, A.; Greiser-Wilke, I.; Parchariyanon, S.; Song, J.Y.; Liou, P.P.; Belak, S. Genetic typing of classical swine fever virus. Vet. Microbiol. 2000, 73, 137–157. [Google Scholar] [CrossRef]
- Chander, V.; Nandi, S.; Ravishankar, C.; Upmanyu, V.; Verma, R. Classical swine fever in pigs: Recent developments and future perspectives. Anim. Health. Res. Rev. 2014, 15, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B. Classical swine fever in China-an update Minireview. Front. Vet. Sci. 2019, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.C.; Huang, C.C.; Huang, T.S.; Chang, C.Y.; Lin, Y.J.; Chien, M.S.; Jong, M.H. Phylogenetic analysis of classical swine fever virus isolated from Taiwan. Vet. Microbiol. 2005, 106, 187–193. [Google Scholar] [CrossRef]
- Gong, W.; Wu, J.; Lu, Z.; Zhang, L.; Qin, S.; Chen, F.; Peng, Z.; Wang, Q.; Ma, L.; Bai, A.; et al. Genetic diversity of subgenotype 2.1 isolates of classical swine fever virus. Infect. Genet. Evol. 2016, 41, 218–226. [Google Scholar] [CrossRef]
- Sarma, D.K.; Mishra, N.; Vilcek, S.; Rajukumar, K.; Behera, S.P.; Nema, R.K.; Dubey, P.; Dubey, S.C. Phylogenetic analysis of recent classical swine fever virus (CSFV) isolates from Assam, India. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 11–15. [Google Scholar] [CrossRef]
- Singh, V.K.; Rajak, K.K.; Kumar, A.; Yadav, S.K. Classical swine fever in India: Current status and future perspective. Trop. Anim. Health Prod. 2018, 50, 1181–1191. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 2, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Lu, Z.; Li, H.; Yu, X.; Liu, X.; Li, Y.; Zang, H.; Yin, Z. Phylogenetic comparison of classical swine fever virus in China. Virus Res. 2011, 81, 29–37. [Google Scholar] [CrossRef]
- Patil, S.S.; Hemadri, D.; Shankar, B.P.; Raghavendra, A.G.; Veeresh, H.; Sindhoora, B.; Chandan, S.; Gagendragad, M.R.; Sreekala, K.; Prabhudas, K. Genetic typing of recent classical swine fever isolates from India. Vet. Microbiol. 2010, 141, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Shivaraj, D.B.; Patil, S.S.; Rathnamma, D.; Hemadri, D.; Isloor, S.; Geetha, S.; Manjunathareddy, G.B.; Gajendragad, M.R.; Rahman, H. Genetic clustering of recent classical swine fever virus isolates from Karnataka, India revealed the emergence of subtype 2.2 replacing subtype 1.1. VirusDisease 2015, 26, 170–179. [Google Scholar] [CrossRef][Green Version]
- Patil, S.S.; Suresh, K.P.; Saha, S.; Prajapati, A.; Hemadri, D.; Roy, P. Meta-analysis of classical swine fever prevalence in pigs in India: A 5-year study. Vet. World 2018, 11, 297–303. [Google Scholar] [CrossRef]
- Barman, N.N.; Patil, S.S.; Kurli, R.; Deka, P.; Bora, D.P.; Deka, G.; Ranjitha, K.M.; Shivaranjini, C.; Roy, P.; Suresh, K.P. Meta-analysis of the prevalence of livestock diseases in North Eastern Region of India. Vet. World 2020, 13, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Baujat, B.; Mahe, C.; Pignon, J.P.; Hill, C. A Graphical Method for Exploring Heterogeneity in Meta-Analyses: Application to a Meta-Analysis of 65 Trials. Stat. Med. 2002, 21, 2641–2652. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Controll. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Harris, R.J.; Deeks, J.J.; Altman, D.G.; Bradburn, M.J.; Harbord, R.M.; Sterne, J.A. Metan: Fixed-and random-effects meta-analysis. Stata J. 2008, 8, 3–28. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneide, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; Wiley: Hoboken, New Jersey, USA, 2008; pp. 243–296. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.; Koenen, F.; Mintiens, K.; Denis, P.; Ribbens, S.; de Kruif, A. Analytical performance of several classical swine fever laboratory diagnostic techniques on live animals for detection of infection. J. Virol. Meth 2004, 119, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Gabriel, C.; Staubach, C.; Leifer, I.; Strebelow, G.; Beer, M. Genetic differentiation of infected from vaccinated animals after implementation of an emergency vaccination strategy against classical swine fever in wild boar. Vet. Microbiol. 2011, 153, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V.; Floegel, N.G.; Greiser-Wilke, I. Clinical signs and epidemiology of classical swine fever: A review of new knowledge. Vet. J. 2003, 165, 11–20. [Google Scholar] [CrossRef]
- Dahle, J.; Liess, B. A review on classical swine fever infections in pigs: Epizootiology, clinical disease and pathology. Comp. Immunol. Microbiol. Infect Dis. 1992, 15, 203–211. [Google Scholar] [CrossRef]
- Muñoz-González, S.; Ruggli, N.; Rosell, R.; Pérez, L.J.; Frías-Leuporeau, M.T.; Fraile, L.; Montoya, M.; Cordoba, L.; Domingo, M.; Ehrensperger, F.; et al. Postnatal persistent infection with classical swine fever virus and its immunological implications. PLoS ONE 2015, 10, e0125692. [Google Scholar] [CrossRef]
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever—An Updated Review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef]
- Rout, M.; Saikumar, G. Virus load in pigs affected with different clinical forms of classical swine fever. Transbound. Emerg. Dis. 2012, 59, 128–133. [Google Scholar] [CrossRef]
- Rajkhowa, T.K.; Hauhnar, L.; Lalrohlua, I.; Jagan, M.G. Emergence of 2.1 subgenotype of classical swine fever virus in pig population of India in 2011. Vet. Q. 2014, 34, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Teifke, J.P.; Lange, E.; Klopfleisch, R.; Kaden, V. Nictitating membrane as a potentially useful postmortem diagnostic specimen for classical swine fever. J. Vet Diagn. Investig. 2005, 17, 341–345. [Google Scholar] [CrossRef]
- Van Oirschot, J. Hog cholera. In Infectious Diseases of Livestock, 2nd ed.; Coetzer, J.A.W., Tustin, R.C., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 975–986. [Google Scholar]
- Sarma, D.K.; Sarma, P.C. ELISA for detection of hog cholera virus antigen. Ind. J. Anim. Sci. 1995, 65, 650–651. [Google Scholar]
- Sarma, D.K.; Meshram, D.J. Comparison of sandwich and dot ELISA for detection of CSF virus antigen in pigs. Indian Vet. J. 2008, 85, 915–918. [Google Scholar]
- Barman, N.N.; Gupt, R.S.; Singh, N.K.; Tiwari, A.K.; Singh, R.K.; Das, S.K. Comparative evaluation of molecular and antibody based technique for detection of classical swine fever virus infecting pigs of NE region, India. Indian J. Anim. Sci. 2009, 79, 974–977. [Google Scholar]
- Kumar, R.; Kumar, V.; Kekungu, P.; Barman, N.N.; Kumar, S. Evaluation of surface glycoproteins of classical swine fever virus as immunogens and reagents for serological diagnosis of infections in pigs: A recombinant Newcastle disease virus approach. Arch. Virol. 2019, 164, 3007–3017. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Saini, M.; Bisht, D.; Rana, M.; Bachan, R.; Gogoi, S.M.; Buragohain, B.M.; Barman, N.N.; Gupta, P.K. Lentiviral-mediated delivery of classical swine fever virus Erns gene into porcine kidney-15 cells for production of recombinant ELISA diagnostic antigen. Mol. Biol. Rep. 2019, 46, 3865–3876. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.K.; Karam, A.; Mukherjee, P.; Barkalita, L.; Borah, P.; Das, S.; Sanjukta, R.; Puro, K.; Ghatak, S.; Shakuntala, I.; et al. Detection of classical swine fever virus E2 gene in cattle serum samples from cattle herds of Meghalaya. VirusDisease 2018, 29, 89–95. [Google Scholar] [CrossRef]
- Moennig, V.; Becher, P. Pestivirus control programs: How far have we come and where are we going? Anim. Health Res. Rev. 2015, 16, 83–87. [Google Scholar] [CrossRef]
- Depner, K.; Hoffmann, B.; Beer, M. Evaluation of real-time RT-PCR assay for the routine intra vitam diagnosis of classical swine fever. Vet. Microbiol. 2007, 121, 338–343. [Google Scholar] [CrossRef]
- Le Dimna, M.; Vrancken, R.; Koenen, F.; Bougeard, S.; Mesplede, A.; Hutet, E.; Kuntz-Simon, G.; Le Potier, M.F. Validation of two commercial real-time RT-PCR kits for rapid and specific diagnosis of classical swine fever virus. J. Virol. Methods 2008, 147, 136–142. [Google Scholar] [CrossRef]
- Nagarajan, K.; Saikumar, G. Fluorescent in-situ hybridization technique for the detection and localization of classical swine fever virus in infected tissues. Veterinarski Arhiv 2012, 82, 495–504. [Google Scholar]
- Singh, V.K.; Kumar, G.S.; Paliwal, O.P. Detection of classical swine fever virus in archival formalin-fixed tissues by reverse transcription-polymerase chain reaction. Res. Vet. Sci. 2005, 79, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, Y.; Zheng, H.; Fang, Y.; Fande, K.; Shen, H.; He, Q.; Huang, Y.; Wen, F. Study on colloidal gold strip in detecting classical swine fever virus. Fujian J. Anim. Husb. Vet. Med. 2007, 6, 3–5. [Google Scholar]
- Conlan, J.V.; Khounsy, S.; Blacksell, S.D.; Morrissy, C.J.; Wilks, C.R.; Gleeson, L.J. Development and evaluation of a rapid immunomagnetic bead assay for the detection of classical swine fever virus antigen. Trop. Anim. Health Prod. 2009, 41, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Pang, V.F.; Pan, C.H.; Chen, T.H.; Jong, M.H.; Huang, T.S.; Jeng, C.R. Development of a reverse transcription multiplex real time PCR for the detection and genotyping of classical swine fever virus. J. Virol. Methods 2013, 160, 111–118. [Google Scholar] [CrossRef]
- Haines, F.J.; Hofmann, M.A.; King, D.P.; Drew, T.W.; Crooke, H.R. Development and validation of a multiplex real time RT-PCR assay for the simultaneous detection of classical and African swine fever viruses. PLoS ONE 2013, 8, e71019. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Saini, M.; Dahiya, S.S.; Patel, C.L.; Sonwane, A.A.; Rai, D.V.; Pandey, K.D. Molecular characterization of lapinized classical Swine Fever vaccine strain by full-length genome sequencing and analysis. Anim. Biotechnol. 2011, 22, 111–117. [Google Scholar] [CrossRef]
- Kumar, R.; Rajak, K.K.; Chandra, T.; Thapliyal, A.; Muthuchelvan, D.; Sudhakar, S.B.; Sharma, K.; Saxena, A.; Raut, S.D.; Singh, V.K.; et al. Whole-genome sequence of a classical Swine Fever virus isolated from the uttarakhand state of India. Genome Announc. 2014, 2, e00371-14. [Google Scholar] [CrossRef]
- Kamboj, A.; Patel, C.L.; Chaturvedi, V.K.; Saini, M.; Gupta, P.K. Complete genome sequence of an Indian field isolate of classical Swine Fever virus belonging to subgenotype 1.1. Genome Announc. 2014, 2, e00886-14. [Google Scholar] [CrossRef]
- Ahuja, A.; Bhattacharjee, U.; Chakraborty, A.K.; Karam, A.; Ghatak, S.; Puro, K.; Das, S.; Shakuntala, I.; Srivastava, N.; Ngachan, S.V.; et al. Complete genome sequence of classical swine fever virus subgenogroup 2.1 from Assam, India. Genome Announc 2015, 3, e01437-14. [Google Scholar] [CrossRef]
- Tomar, N.; Sharma, V.; John, J.K.; Sethi, M.; Ray, P.K.; Arya, R.S.; Das, T.; Saikumar, G. Complete Genome Sequence of a Field Isolate of Classical Swine Fever Virus Belonging to Subgenotype 2.2 from India. Genome Announc. 2018, 6, e00288-18. [Google Scholar] [CrossRef]
- Tomar, N.; Gupta, A.; Arya, R.S.; Somvanshi, R.; Sharma, V.; Saikumar, G. Genome sequence of classical Swine Fever virus genotype 1.1 with a genetic marker of attenuation detected in a continuous porcine cell line. Genome Announc. 2015, 3, e00375-15. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, D.; Singh, R.K.; Dhar, P.; Kumar, S. Potential role of technology in increasing productivity and income at National level: A case of cell-culture vaccine against classical swine fever. Agric. Econ. Res. Rev. 2017, 30, 161–170. [Google Scholar] [CrossRef][Green Version]
- Nath, M.K.; Sarma, D.K.; Das, B.C.; Deka, P.; Kalita, D.; Dutta, J.B.; Mahato, G.; Sarma, S.; Roychoudhury, P. Evaluation of specific humoral immune response in pigs vaccinated with cell culture adapted classical swine fever vaccine. Vet. World 2016, 9, 308–312. [Google Scholar] [CrossRef] [PubMed][Green Version]
| CSFV Genotype | Gene/Target | Country | Nt Similarity (%) India |
|---|---|---|---|
| CSFV 1.1 | Whole Genome | India | 92.07–96.38 |
| China | 94.8–98.16 | ||
| South Korea | 93.15–99.88 | ||
| E2 | India | 89.2–99.8 | |
| China | 90.1–97.5 | ||
| South Korea | 90.7–99.8 | ||
| NS5B | India | 92–99.8 | |
| China | 92.1–97.6 | ||
| South Korea | 93.1–99.1 | ||
| 5′ UTR | India | 93.9–98.2 | |
| China | 91–96.8 | ||
| South Korea | 91.6–98.4 | ||
| CSFV 2.2 | Whole Genome | India | 83.42–84.99 |
| E2 | India | 95.9–98.4 | |
| NS5B | India | 92.6–99.6 | |
| 5′ UTR | India | 91–97.4 | |
| CSFV 2.1 | Whole Genome | South Korea | 90.84 |
| China | 90.65–91.35 | ||
| Mongolia | 91.33 | ||
| Vietnam | 90.13 | ||
| Taiwan | 91.83 | ||
| Japan | 90.88 | ||
| E2 | South Korea | 84.2–85.4 | |
| China | 84.2–86.5 | ||
| Mongolia | 84.4–85.7 | ||
| Vietnam | 83.7––84.8 | ||
| Taiwan | 85.1–86.3 | ||
| Japan | 84.3–85.6 | ||
| NS5B | South Korea | 88.9–89.1 | |
| China | 88.5–89.7 | ||
| Mongolia | 89–89.3 | ||
| Vietnam | 87.9–88.1 | ||
| Taiwan | 89.3–89.9 | ||
| Japan | 88.7–89.2 | ||
| 5′ UTR | South Korea | 90–93.4 | |
| China | 88.7–95 | ||
| Mongolia | 89.4–92.6 | ||
| Vietnam | 89.4–91.8 | ||
| Taiwan | 90.8–94.2 | ||
| Japan | 62–63.1 |
| S. No | Variables | Samples Tested | Positive Samples | Pooled Estimate (RE) (95% CI) | Pooled Estimate (FE) (95% CI) | p-Value | I2 Value | Tau Square | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Geographic region | Northern India | 2569 | 272 | 30% (14%–50%) | 8% (7%–9%) | <0.01 | 99% | 0.05 |
| Western India | 332 | 145 | 37% (8%–73%) | 39% (34%–45%) | <0.01 | 98% | 0.22 | ||
| Central India | 593 | 302 | 42% (24%–61%) | 51% (47%–55%) | <0.01 | 94% | 0.04 | ||
| Southern India | 3661 | 883 | 25% (18%–33%) | 22% (21%–23%) | <0.01 | 95% | 0.03 | ||
| Eastern India | 54 | 28 | 41% (10%–75%) | 52% (37%–66%) | <0.01 | 80% | 0.09 | ||
| North Eastern India | 6064 | 2678 | 40% (29%–51%) | 43% (41%–44%) | <0.01 | 98% | 0.07 | ||
| India | 1207 | 786 | 72% (54%–87%) | 65% (63%–68%) | <0.01 | 95% | 0.01 | ||
| 2. | Serological test | ELISA | 9224 | 3200 | 30% (22%–38%) | 31% (30%–32%) | 0.00 | 98% | 0.08 |
| RT–PCR | 1988 | 423 | 33% (8%–64%) | 17% (15%–18%) | < 0.01 | 99% | 0.10 | ||
| S-ELISA | 357 | 27 | 52% (0%–100%) | 4% (2%–7%) | < 0.01 | 97% | 0.63 | ||
| AGID | 196 | 136 | 60% (18%–95%) | 71% (64%–77%) | <0.01 | 97% | 0.09 | ||
| I-ELISA | 2605 | 1253 | 61% (42%–78%) | 48% (46%–50%) | <0.01 | 99% | 0.07 | ||
| IIP | 110 | 65 | 59% (50%–68%) | 59% (50%–68%) | NA | NA | NA | ||
| 3. | Study period | 2011-15 | 9019 | 2356 | 36% (28%–43%) | 21% (20%–22%) | 0.00 | 98% | 0.07 |
| 2016-19 | 5461 | 2738 | 35% (24%–47%) | 50% (48%–51%) | <0.01 | 98% | 0.06 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, Y.S.; Bhat, S.; Kumar, O.R.V.; Yadav, A.K.; Sircar, S.; Ansari, M.I.; Sarma, D.K.; Rajkhowa, T.K.; Ghosh, S.; Dhama, K. Classical Swine Fever Virus Biology, Clinicopathology, Diagnosis, Vaccines and a Meta-Analysis of Prevalence: A Review from the Indian Perspective. Pathogens 2020, 9, 500. https://doi.org/10.3390/pathogens9060500
Malik YS, Bhat S, Kumar ORV, Yadav AK, Sircar S, Ansari MI, Sarma DK, Rajkhowa TK, Ghosh S, Dhama K. Classical Swine Fever Virus Biology, Clinicopathology, Diagnosis, Vaccines and a Meta-Analysis of Prevalence: A Review from the Indian Perspective. Pathogens. 2020; 9(6):500. https://doi.org/10.3390/pathogens9060500
Chicago/Turabian StyleMalik, Yashpal Singh, Sudipta Bhat, O. R. Vinodh Kumar, Ajay Kumar Yadav, Shubhankar Sircar, Mohd Ikram Ansari, Dilip Kumar Sarma, Tridib Kumar Rajkhowa, Souvik Ghosh, and Kuldeep Dhama. 2020. "Classical Swine Fever Virus Biology, Clinicopathology, Diagnosis, Vaccines and a Meta-Analysis of Prevalence: A Review from the Indian Perspective" Pathogens 9, no. 6: 500. https://doi.org/10.3390/pathogens9060500
APA StyleMalik, Y. S., Bhat, S., Kumar, O. R. V., Yadav, A. K., Sircar, S., Ansari, M. I., Sarma, D. K., Rajkhowa, T. K., Ghosh, S., & Dhama, K. (2020). Classical Swine Fever Virus Biology, Clinicopathology, Diagnosis, Vaccines and a Meta-Analysis of Prevalence: A Review from the Indian Perspective. Pathogens, 9(6), 500. https://doi.org/10.3390/pathogens9060500








