Recent Advances in the Diagnosis of Classical Swine Fever and Future Perspectives
Abstract
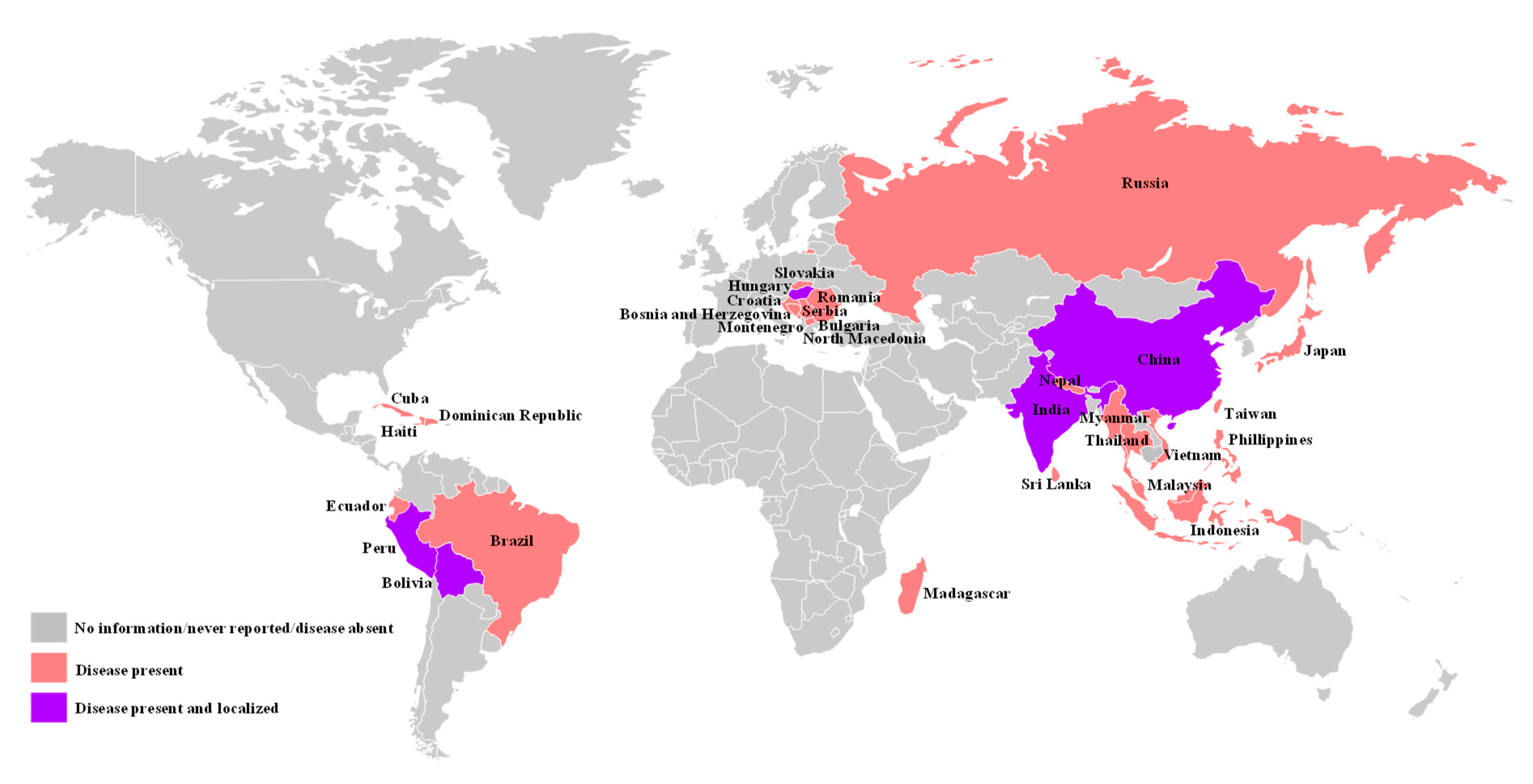
1. Introduction
2. Antigen Detection
2.1. Virus Isolation
2.2. Fluorescence Antibody Test (FAT)
2.3. Antigen-Capture ELISA
2.4. Real-Time Reverse Transcription Polymerase Chain Reaction (Real-Time RT-PCR)
2.5. Next Generation Sequencing (NGS)
3. Antibody Detection
3.1. Virus Neutralization Test (VNT)
3.2. Antibody ELISA
4. Differentiation of Infected from Vaccinated Animals (DIVA) Diagnostic Methods
4.1. Genetic DIVA
4.2. Serological DIVA
5. Point-of-Care (POC) Diagnostics
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical swine fever-an updated review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef]
- Brown, V.R.; Bevins, S.N. A review of classical swine fever virus and routes of introduction into the United States and the potential for virus establishment. Front. Vet. Sci. 2018, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- CABI. Invasive Species Compendium; CAB International: Wallingford, UK, 2020; Available online: www.cabi.org/isc (accessed on 29 May 2020).
- Schirrmeier, H.; Strebelow, G.; Depner, K.; Hoffmann, B.; Beer, M. Genetic and antigenic characterization of an atypical pestivirus isolate, a putative member of a novel pestivirus species. J. Gen. Virol. 2004, 85, 3647–3652. [Google Scholar] [CrossRef] [PubMed]
- Thiel, H.J.; Stark, R.; Weiland, E.; Rumenapf, T.; Meyers, G. Hog cholera virus: Molecular composition of virions from a pestivirus. J. Virol. 1991, 65, 4705–4712. [Google Scholar] [CrossRef] [PubMed]
- Meyers, G.; Thiel, H.J.; Rümenapf, T. Classical swine fever virus: Recovery of infectious viruses from cDNA constructs and generation of recombinant cytopathogenic defective interfering particles. J. Virol. 1996, 70, 1588–1595. [Google Scholar] [CrossRef]
- Lowings, P.; Ibata, G.; Needham, J.; Paton, D. Classical swine fever virus diversity and evolution. J. Gen. Virol. 1996, 77, 1311–1321. [Google Scholar] [CrossRef]
- Paton, D.J.; Mcgoldrick, A.; Greiser-Wilke, I.; Parchariyanon, S.; Song, J.Y.; Liou, P.P.; Stadejek, T.; Lowings, J.P.; Björklund, H.; Belák, S. Genetic typing of classical swine fever virus. Vet. Microbiol. 2000, 73, 137–157. [Google Scholar] [CrossRef]
- Deng, M.C.; Huang, C.C.; Huang, T.S.; Chang, C.Y.; Lin, Y.J.; Chien, M.S.; Jong, M.H. Phylogenetic analysis of classical swine fever virus isolated from Taiwan. Vet. Microbiol. 2005, 106, 187–193. [Google Scholar] [CrossRef]
- Postel, A.; Moennig, V.; Becher, P. Classical swine fever in Europe-the current situation. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 468–475. [Google Scholar]
- Postel, A.; Nishi, T.; Kameyama, K.I.; Meyer, D.; Suckstorff, O.; Fukai, K.; Becher, P. Reemergence of Classical Swine Fever, Japan, 2018. Emerg. Infect. Dis. 2019, 25, 1228–1231. [Google Scholar] [CrossRef]
- Gomez-Villamandos, J.C.; Carrasco, L.; Bautista, M.J.; Sierra, M.A.; Quezada, M.; Hervas, J.; Chacón, M.F.; Ruiz-Villamor, E.; Salguero, F.J.; Sónchez-Cordón, P.J.; et al. African swine fever and classical swine fever: A review of the pathogenesis. Dtsch. Tierarztl. Wochenschr. 2003, 110, 165–169. [Google Scholar] [PubMed]
- Paton, D.J.; Greiser-Wilke, I. Classical swine fever: An update. Res. Vet. Sci. 2003, 75, 169–178. [Google Scholar] [CrossRef]
- Zhou, B. Classical swine fever in China—An update Minireview. Front. Vet. Sci. 2019, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Greiser-Wilke, I.; Blome, S.; Moennig, V. Diagnostic methods for detection of Classical swine fever virus—Status quo and new developments. Vaccine 2007, 25, 5524–5530. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V.; Floegel-Niesmann, G.; Greiser-Wilke, I. Clinical signs and epidemiology of classical swine fever: A review of new knowledge. Vet. J. 2003, 165, 11–20. [Google Scholar] [CrossRef]
- OIE Terrestrial Manual 2019. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.08.03_CSF.pdf (accessed on 29 May 2020).
- Grummer, B.; Fischer, S.; Depner, K.; Riebe, R.; Blome, S.; Greiser-Wilke, I. Replication of classical swine fever virus strains and isolates in different porcine cell lines. Dtsch. Tierarztl. Wochenschr. 2006, 113, 138–142. [Google Scholar] [PubMed]
- Chander, V.; Nandi, S.; Ravishankar, C.; Upmanyu, V.; Verma, R. Classical swine fever in pigs: Recent developments and future perspectives. Anim. Health Res. Rev. 2014, 15, 87–101. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, L.; Zhang, Y.; Wang, T.; Zou, X.; Zhu, Y.; Zhao, C.; Chen, K.; Sun, Y.; Sun, J.; et al. A novel ViewRNA in situ hybridization method for the detection of the dynamic distribution of Classical Swine Fever Virus RNA in PK15 cells. Virol. J. 2017, 14, 81. [Google Scholar] [CrossRef]
- Shannon, A.D.; Morrissy, C.; Mackintosh, S.G.; Westbury, H.A. Detection of hog cholera virus antigens in experimentally-infected pigs using an antigen-capture ELISA. Vet. Microbiol. 1993, 34, 233–248. [Google Scholar] [CrossRef]
- Penrith, M.L.; Vosloo, W.; Mather, C. Classical swine fever (hog cholera): Review of aspects relevant to control. Transbound. Emerg. Dis. 2011, 58, 187–196. [Google Scholar] [CrossRef]
- Moennig, V.; Becher, P. Pestivirus control programs: How far have we come and where are we going? Anim. Health Res. Rev. 2015, 16, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.; Koenen, F.; Mintiens, K.; Denis, P.; Ribbens, S.; de Kruif, A. Analytical performance of several classical swine fever laboratory diagnostic techniques on live animals for detection of infection. J. Virol. Methods 2004, 119, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Depner, K.; Hoffmann, B.; Beer, M. Evaluation of real-time RT-PCR assay for the routine intra vitam diagnosis of classical swine fever. Vet. Microbiol. 2007, 121, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Austermann-Busch, S.; Petrov, A.; Moennig, V.; Becher, P. Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transbound. Emerg. Dis. 2018, 65, 248–261. [Google Scholar] [CrossRef]
- Espy, M.J.; Uhl, J.R.; Sloan, L.M.; Buckwalter, S.P.; Jones, M.F.; Vetter, E.A.; Yao, J.D.; Wengenack, N.L.; Rosenblatt, J.E.; Cockerill, F.R., 3rd; et al. Real-time PCR in clinical microbiology: Applications for routine laboratory testing. Clin. Microbiol. Rev. 2006, 19, 165–256. [Google Scholar] [CrossRef] [PubMed]
- Lung, O.; Pasick, J.; Fisher, M.; Buchanan, C.; Erickson, A.; Ambagala, A. Insulated isothermal reverse transcriptase PCR (iiRT-PCR) for rapid and sensitive detection of classical swine fever virus. Transbound. Emerg. Dis. 2016, 63, e395–e402. [Google Scholar] [CrossRef]
- Lung, O.; Fisher, M.; Erickson, A.; Nfon, C.; Ambagala, A. Fully automated and integrated multiplex detection of high consequence livestock viral genomes on a microfluidic platform. Transbound. Emerg. Dis. 2019, 66, 144–155. [Google Scholar] [CrossRef]
- Liu, L.; Xia, H.; Belák, S.; Widén, F. Development of a primer-probe energy transfer real-time PCR assay for improved detection of classical swine fever virus. J. Virol. Methods 2009, 160, 69–73. [Google Scholar] [CrossRef]
- Zhang, X.J.; Xia, H.; Everett, H.; Sosan, O.; Crooke, H.; Belák, S.; Widén, F.; Qiu, H.J.; Liu, L. Evaluation of a primer-probe energy transfer real-time PCR assay for detection of classical swine fever virus. J. Virol. Methods 2010, 168, 259–261. [Google Scholar] [CrossRef]
- Chen, H.T.; Zhang, J.; Ma, L.N.; Ma, Y.P.; Ding, Y.Z.; Liu, X.T.; Chen, L.; Ma, L.Q.; Zhang, Y.G.; Liu, Y.S. Rapid pre-clinical detection of classical swine fever by reverse transcription loop-mediated isothermal amplification. Mol. Cell. Probes 2009, 23, 71–74. [Google Scholar] [CrossRef]
- Yin, S.; Shang, Y.; Zhou, G.; Tian, H.; Liu, Y.; Cai, X.; Liu, X. Development and evaluation of rapid detection of classical swine fever virus by reverse transcription loop-mediated isothermal amplification (RT-LAMP). J. Biotechnol. 2010, 146, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Sun, Y.; Liu, L.; Belák, S.; Qiu, H.J. Validation of a loop-mediated isothermal amplification assay for visualised detection of wild-type classical swine fever virus. J. Virol. Methods 2010, 167, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Wu, Z.; Li, X.; Zhou, Y.; Hu, A.; Gong, X.; He, J.; Xia, Y.; Guo, K.; Zhang, R.; et al. Development of functionalized gold nanoparticles as nanoflare probes for rapid detection of classical swine fever virus. Colloids Surf. B Biointerfaces 2018, 171, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Pang, V.F.; Pan, C.H.; Chen, T.H.; Jong, M.H.; Huang, T.S.; Jeng, C.R. Development of a reverse transcription multiplex real-time PCR for the detection and genotyping of classical swine fever virus. J. Virol. Methods 2009, 160, 111–118. [Google Scholar] [CrossRef]
- Zheng, H.H.; Zhang, S.J.; Cui, J.T.; Zhang, J.; Wang, L.; Liu, F.; Chen, H.Y. Simultaneous detection of classical swine fever virus and porcine circovirus 3 by SYBR green I-based duplex real-time fluorescence quantitative PCR. Mol. Cell. Probes 2020, 50, 101524. [Google Scholar] [CrossRef]
- Díaz de Arce, H.; Pérez, L.J.; Frías, M.T.; Rosell, R.; Tarradas, J.; Núñez, J.I.; Ganges, L. A multiplex RT-PCR assay for the rapid and differential diagnosis of classical swine fever and other pestivirus infections. Vet. Microbiol. 2009, 139, 245–252. [Google Scholar] [CrossRef]
- Haines, F.J.; Hofmann, M.A.; King, D.P.; Drew, T.W.; Crooke, H.R. Development and validation of a multiplex, real-time RT PCR assay for the simultaneous detection of classical and African swine fever viruses. PLoS ONE 2013, 8, e71019. [Google Scholar] [CrossRef]
- Shi, X.; Liu, X.; Wang, Q.; Das, A.; Ma, G.; Xu, L.; Sun, Q.; Peddireddi, L.; Jia, W.; Liu, Y.; et al. A multiplex real-time PCR panel assay for simultaneous detection and differentiation of 12 common swine viruses. J. Virol. Methods 2016, 236, 258–265. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Li, Q.; Wu, M.; Lei, L.; Pan, Z. A multiplex RT-PCR assay for rapid and simultaneous detection of four RNA viruses in swine. J. Virol. Methods 2019, 269, 38–42. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Kang, R.; Wu, X.; Lin, H.; Ye, Y.; Yu, J.; Ye, J.; Xie, J.; Cao, Y.; et al. Development and application of a novel Bio-Plex suspension array system for high-throughput multiplexed nucleic acid detection of seven respiratory and reproductive pathogens in swine. J. Virol. Methods 2018, 261, 104–111. [Google Scholar] [CrossRef]
- Deregt, D.; Gilbert, S.A.; Dudas, S.; Pasick, J.; Baxi, S.; Burton, K.M.; Baxi, M.K. A multiplex DNA suspension microarray for simultaneous detection and differentiation of classical swine fever virus and other pestiviruses. J. Virol. Methods 2006, 136, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.; Fisher, M.; Furukawa-Stoffer, T.; Ambagala, A.; Hodko, D.; Pasick, J.; King, D.P.; Nfon, C.; Ortega Polo, R.; Lung, O. A multiplex reverse transcription PCR and automated electronic microarray assay for detection and differentiation of seven viruses affecting swine. Transbound. Emerg. Dis. 2018, 65, e272–e283. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gao, S.; Sang, S.; Jian, A.; Duan, Q.; Ji, J.; Zhang, W. Detection system based on magnetoelastic sensor for classical swine fever virus. Biosens. Bioelectron. 2016, 82, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, W.; Dai, B.; Zheng, L.; Zhang, Z.; Qi, D.; Cheng, X.; Zhang, D.; Zhuang, D. Diagnosis of mixed infections with swine viruses using an integrated microfluidic platform. Sens. Actuators B Chem. 2020, 312, 128005. [Google Scholar] [CrossRef]
- Van Borm, S.; Belák, S.; Freimanis, G.; Fusaro, A.; Granberg, F.; Höper, D.; King, D.P.; Monne, I.; Orton, R.; Rosseel, T. Next-generation sequencing in veterinary medicine: How can the massive amount of information arising from high-throughput technologies improve diagnosis, control, and management of infectious diseases? Methods Mol. Biol. 2015, 1247, 415–436. [Google Scholar]
- Kumar, D.; Rao, P.P.; Hegde, N.R. Next-generation sequencing as diagnostic tool in veterinary research. J. Anim. Res. 2019, 9, 797–806. [Google Scholar] [CrossRef]
- Leifer, I.; Hoffmann, B.; Höper, D.; Bruun Rasmussen, T.; Blome, S.; Strebelow, G.; Höreth-Böntgen, D.; Staubach, C.; Beer, M. Molecular epidemiology of current classical swine fever virus isolates of wild boar in Germany. J. Gen. Virol. 2010, 91, 2687–2697. [Google Scholar] [CrossRef]
- Töpfer, A.; Höper, D.; Blome, S.; Beer, M.; Beerenwinkel, N.; Ruggli, N.; Leifer, I. Sequencing approach to analyze the role of quasispecies for classical swine fever. Virology 2013, 438, 14–19. [Google Scholar] [CrossRef]
- Fahnøe, U.; Pedersen, A.G.; Johnston, C.M.; Orton, R.J.; Höper, D.; Beer, M.; Bukh, J.; Belsham, G.J.; Rasmussen, T.B. Virus adaptation and selection following challenge of animals vaccinated against classical swine fever virus. Viruses 2019, 11, 932. [Google Scholar] [CrossRef]
- Malik, Y.S.; Bhat, S.; Kumar, O.R.V.; Yadav, A.K.; Sircar, S.; Ansari, M.I.; Sarma, D.K.; Rajkhowa, T.K.; Ghosh, S.; Dhama, K. Classical swine fever virus biology, clinicopathology, diagnosis, vaccines and a meta-analysis of prevalence: A review from the Indian Perspective. Pathogens 2020, 9, 500. [Google Scholar] [CrossRef]
- Madera, R.; Gong, W.; Wang, L.; Burakova, Y.; Lleellish, K.; Galliher-Beckley, A.; Nietfeld, J.; Henningson, J.; Jia, K.; Li, P.; et al. Pigs immunized with a novel E2 subunit vaccine are protected from heterologous classical swine fever virus challenge. BMC Vet. Res. 2016, 12, 197. [Google Scholar] [CrossRef]
- Madera, R.; Wang, L.; Gong, W.; Burakova, Y.; Buist, S.; Nietfeld, J.; Henningson, J.; Ozuna, A.G.C.; Tu, C.; Shi, J. Towards the development of a one-dose classical swine fever subunit vaccine: Antigen titration, onset and duration of immunity. J. Vet. Sci. 2018, 19, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, R.C.; Madera, R.; Peres, Y.; Berquist, B.R.; Wang, L.; Buist, S.; Burakova, Y.; Palle, S.; Chung, C.J.; Rasmussen, M.V.; et al. Plant-made E2 glycoprotein single-dose vaccine protects pigs against classical swine fever. Plant. Biotechnol. J. 2019, 17, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mi, S.; Madera, R.; Ganges, L.; Borca, M.V.; Ren, J.; Cunningham, C.; Cino-Ozuna, A.G.; Li, H.; Tu, C.; et al. A neutralizing monoclonal antibody-based competitive ELISA for classical swine fever C-strain post-vaccination monitoring. BMC Vet. Res. 2020, 16, 14. [Google Scholar] [CrossRef]
- Tetsuo, M.; Matsuno, K.; Tamura, T.; Fukuhara, T.; Kim, T.; Okamatsu, M.; Tautz, N.; Matsuura, Y.; Sakoda, Y. Development of a high-throughput serum neutralization test using recombinant pestiviruses possessing a small reporter tag. Pathogens 2020, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Ruggli, N.; Tratschin, J.D.; Hofmann, M.A. Detection of antibodies against classical swine fever virus in swine sera by indirect ELISA using recombinant envelope glycoprotein E2. Vet. Microbiol. 1996, 51, 41. [Google Scholar] [CrossRef]
- Cheng, T.C.; Pan, C.H.; Chen, C.S.; Chuang, K.H.; Chuang, C.H.; Huang, C.C.; Chu, Y.Y.; Yang, Y.C.; Chu, P.Y.; Kao, C.H.; et al. Direct coating of culture medium from cells secreting classical swine fever virus E2 antigen on ELISA plates for detection of E2-specific antibodies. Vet. J. 2015, 205, 107–109. [Google Scholar] [CrossRef]
- Clavijo, A.; Lin, M.; Riva, J.; Mallory, M.; Lin, F.; Zhou, E.M. Development of a competitive ELISA using a truncated E2 recombinant protein as antigen for detection of antibodies to classical swine fever virus. Res. Vet. Sci. 2001, 70, 1–7. [Google Scholar] [CrossRef]
- Kumar, R.; Barman, N.N.; Khatoon, E.; Kumar, S. Development of single dilution immunoassay to detect E2 protein specific classical swine fever virus antibody. Vet. Immunol. Immunopathol. 2016, 172, 50–54. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.J.; Li, N.; Shi, Z.; Cheng, D.; Zhu, Q.H.; Tu, C.; Tong, G.Z.; Qiu, H.J. A multiplex nested RT-PCR for the detection and differentiation of wild-type viruses from C-strain vaccine of classical swine fever virus. J. Virol. Methods 2007, 143, 16–22. [Google Scholar] [CrossRef]
- Zhao, J.J.; Cheng, D.; Li, N.; Sun, Y.; Shi, Z.; Zhu, Q.H.; Tu, C.; Tong, G.Z.; Qiu, H.J. Evaluation of a multiplex real-time RT-PCR for quantitative and differential detection of wild-type viruses and C-strain vaccine of Classical swine fever virus. Vet. Microbiol. 2008, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leifer, I.; Depner, K.; Blome, S.; Le Potier, M.F.; Le Dimna, M.; Beer, M.; Hoffmann, B. Differentiation of C-strain “Riems” or CP7_E2alf vaccinated animals from animals infected by classical swine fever virus field strains using real-time RT-PCR. J. Virol. Methods 2009, 158, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, H.; Everett, H.; Sosan, O.; Crooke, H.; Meindl-Böhmer, A.; Qiu, H.; Moennig, V.; Belák, S.; Widén, F. A generic real-time TaqMan assay for specific detection of lapinized Chinese vaccines against classical swine fever. J. Virol. Methods 2011, 175, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Han, Q.Y.; Sun, Y.; Zhang, X.; Qiu, H.J. Development of a triplex TaqMan real-time RT-PCR assay for differential detection of wild-type and HCLV vaccine strains of classical swine fever virus and bovine viral diarrhea virus 1. Res. Vet. Sci. 2012, 92, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Everett, H.E.; Crudgington, B.S.; Sosan-Soulé, O.; Crooke, H.R. Differential detection of classical swine fever virus challenge strains in C-strain vaccinated pigs. BMC Vet. Res. 2014, 10, 281. [Google Scholar] [CrossRef][Green Version]
- Widén, F.; Everett, H.; Blome, S.; Fernandez Pinero, J.; Uttenthal, A.; Cortey, M.; von Rosen, T.; Tignon, M.; Liu, L. Comparison of two real-time RT-PCR assays for differentiation of C-strain vaccinated from classical swine fever infected pigs and wild boars. Res. Vet. Sci. 2014, 97, 455–457. [Google Scholar] [CrossRef]
- Cho, H.S.; Park, S.J.; Park, N.Y. Development of a reverse-transcription polymerase chain reaction assay with fluorogenic probes to discriminate Korean wild-type and vaccine isolates of Classical swine fever virus. Can. J. Vet. Res. 2006, 70, 226–229. [Google Scholar]
- Pan, C.H.; Jong, M.H.; Huang, Y.L.; Huang, T.S.; Chao, P.H.; Lai, S.S. Rapid detection and differentiation of wild-type and three attenuated lapinized vaccine strains of classical swine fever virus by reverse transcription polymerase chain reaction. J. Vet. Diagn. Investig. 2008, 20, 448–456. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Staubach, C.; Leifer, I.; Strebelow, G.; Beer, M. Genetic differentiation of infected from vaccinated animals after implementation of an emergency vaccination strategy against classical swine fever in wild boar. Vet. Microbiol. 2011, 153, 373–376. [Google Scholar] [CrossRef]
- Schroeder, S.; von Rosen, T.; Blome, S.; Loeffen, W.; Haegeman, A.; Koenen, F.; Uttenthal, A. Evaluation of classical swine fever virus antibody detection assays with an emphasis on the differentiation of infected from vaccinated animals. Rev. Sci. Tech. 2012, 31, 997–1010. [Google Scholar] [CrossRef]
- Lin, M.; Trottier, E.; Pasick, J. Antibody responses of pigs to defined Erns fragments after infection with classical swine fever virus. Clin. Diagn. Lab. Immunol. 2005, 12, 180–186. [Google Scholar] [CrossRef]
- Moormann, R.J.; Bouma, A.; Kramps, J.A.; Terpstra, C.; De Smit, H.J. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet. Microbiol. 2000, 73, 209–219. [Google Scholar] [CrossRef]
- Langedijk, J.P.; Middel, W.G.; Meloen, R.H.; Kramps, J.A.; de Smit, J.A. Enzyme-linked immunosorbent assay using a virus type-specific peptide based on a subdomain of envelope protein Erns for serologic diagnosis of pestivirus infections in swine. J. Clin. Microbiol. 2001, 39, 906–912. [Google Scholar] [CrossRef] [PubMed]
- de Smit, A.J. Laboratory diagnosis, epizootiology, and efficacy of marker vaccines in classical swine fever: A review. Vet. Q. 2000, 22, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Pannhorst, K.; Fröhlich, A.; Staubach, C.; Meyer, D.; Blome, S.; Becher, P. Evaluation of an Erns-based enzyme-linked immunosorbent assay to distinguish Classical swine fever virus-infected pigs from pigs vaccinated with CP7_E2alf. J. Vet. Diagn. Investig. 2015, 27, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Fritsche, S.; Luo, Y.; Engemann, C.; Blome, S.; Beyerbach, M.; Chang, C.Y.; Qiu, H.J.; Becher, P.; Postel, A. The double-antigen ELISA concept for early detection of Erns -specific classical swine fever virus antibodies and application as an accompanying test for differentiation of infected from marker vaccinated animals. Transbound. Emerg. Dis. 2017, 64, 2013–2022. [Google Scholar] [CrossRef]
- Xia, H.; Harimoorthy, R.; Vijayaraghavan, B.; Blome, S.; Widén, F.; Beer, M.; Belák, S.; Liu, L. Differentiation of classical swine fever virus infection from CP7_E2alf marker vaccination by a multiplex microsphere immunoassay. Clin. Vaccine Immunol. 2015, 22, 65–71. [Google Scholar] [CrossRef]
- Bruderer, U.; van de Velde, J.; Frantzen, I.; De Bortoli, F. Discrimination within epitope specific antibody populations against classical swine fever virus is a new means of differentiating infection from vaccination. J. Immunol. Methods 2015, 420, 18–23. [Google Scholar] [CrossRef]
- Luo, Y.; Li, L.; Austermann-Busch, S.; Dong, M.; Xu, J.; Shao, L.; Lei, J.; Li, N.; He, W.R.; Zhao, B.; et al. Enhanced expression of the Erns protein of classical swine fever virus in yeast and its application in an indirect enzyme-linked immunosorbent assay for antibody differentiation of infected from vaccinated animals. J. Virol. Methods 2015, 222, 22–27. [Google Scholar] [CrossRef]
- Manessis, G.; Gelasakis, A.I.; Bossis, I. The challenge of introducing point of care diagnostics in farm animal health management. Biomed. J. Sci. Tech. Res. 2019, 14, 002601. [Google Scholar]
- Chowdry, V.K.; Luo, Y.; Widén, F.; Qiu, H.J.; Shan, H.; Belák, S.; Liu, L. Development of a loop-mediated isothermal amplification assay combined with a lateral flow dipstick for rapid and simple detection of classical swine fever virus in the field. J. Virol. Methods 2014, 197, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Shi, X.; Zhao, D.; Yang, J.; Yang, S.; Zhang, G. Development of an immunochromatographic strip for rapid detection of antibodies against classical swine fever virus. J. Virol. Methods 2012, 180, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Sastre, P.; Pérez, T.; Costa, S.; Yang, X.; Räber, A.; Blome, S.; Goller, K.V.; Gallardo, C.; Tapia, I.; García, J.; et al. Development of a duplex lateral flow assay for simultaneous detection of antibodies against African and Classical swine fever viruses. J. Vet. Diagn. Investig. 2016, 28, 543–549. [Google Scholar] [CrossRef]
- Nannucci, L.; Barattini, P.; Bossis, I.; Woźniakowski, G.; Balka, G.; Pugliese, C. Point-of-service diagnostic technology for detection of swine viral diseases. J. Vet. Res. 2020, 64, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef]
- Arshad Ali, S.; Baloch, M.; Ahmed, N.; Arshad Ali, A.; Iqbal, A. The outbreak of coronavirus disease 2019 (COVID-19)-An emerging global health threat. J. Infect. Public Health 2020, 13, 644–646. [Google Scholar] [CrossRef]
| Method | Application | Advantages | Disadvantages |
|---|---|---|---|
| Virus Isolation | Confirmation of clinical cases; Making virus collections; May be used for individual animal freedom from infection prior to movement | “reference standard"; Very sensitive; Indicates active infection | Work intensive and time consuming; Requires specialized microscope and expertise |
| FAT 1 | Confirmation of clinical cases | Quick and direct visualization of antigens in tissue | Requires specialized equipment, expertise, and confirmatory test |
| Antigen-capture ELISA 2 | Population infection-free status; May be used for confirmation of clinical cases | Fast, does not require specialized equipment and suitable for herd screening | Low sensitivity; Cross-reactivity with other Pestiviruses |
| RT-PCR 3 | Confirmation of clinical cases; Prevalence of infection surveillance; May be used for population or individual animal freedom from infection prior to movement | Fast, sensitive, and specific | Specialized equipment; Possibility for false negative results due to sample degradation |
| VNT 4 | Individual animal infection-free status prior to movement; Prevalence of infection-surveillance; Immune status in individual animals or populations post-vaccination; Confirmation of clinical cases; May be used for population freedom from infection | Gold standard for sensitivity and specificity | Work intensive and time consuming; Requires specialized microscope and expertise |
| Antibody ELISA | Population freedom from infection; Individual animal freedom from infection prior to movement; Prevalence of infection-surveillance; Immune status in individual animals or populations post-vaccination | Fast, does not require specialized equipment and suitable for herd screening | Cross-reactivity with other Pestiviruses |
| Name | Producer | Test Principle | Suitable Sample Materials | DIVA Potential | Web Site |
|---|---|---|---|---|---|
| IDEXX CSFV Ag Serum Plus | IDEXX Laboratories, Inc. | DAS ELISA test Erns | Serum, plasma, tissue | Yes | https://www.idexx.com/en/livestock/livestock-tests/swine-tests/idexx-csfv-ag-serum-plus-test/ |
| PrioCHECK™ CSFV Antigen ELISA kit | Thermo Fisher Scientific, Inc. | DAS ELISA test E2 | Serum, blood, plasma, leukocyte concentrate, tissue extract | No | https://www.thermofisher.com/order/catalog/product/7610047?SID=srch-srp-7610047#/7610047?SID=srch-srp-7610047 |
| VDPro® CSFV AG ELISA | MEDIAN Diagnostics Inc. | DAS ELISA test E2 | Cell cultures, leukocyte concentrate, tissue extract | No | http://www.mediandiagnostics.com/eng/es-csf-02.php |
| Name | Producer | Test Principle | Suitable Sample Materials | Web Site |
|---|---|---|---|---|
| ADIAVET™ CSF REAL TIME | BioMérieux | Real-time RT-PCR test CSFV RNA | Serum, blood, viral culture, tissue | https://www.biomerieux-nordic.com/csfv-classical-swine-fever |
| CSFV dtec-RT- qPCR Test | Genetic PCR solutions™ | Real-time RT-PCR test CSFV RNA | Serum, plasma, blood, viral culture, tissue | http://www.geneticpcr.com/index.php/en/pathogen-r-d-qpcr/classical-swine-fever-virus |
| CSFV genesig® Kits | PrimerdesignTM Ltd | Real-time RT-PCR test CSFV RNA | Serum, plasma, blood, viral culture, tissue | https://www.genesig.com/products/9770-classical-swine-fever-virus |
| CSFV Real Time RT-PCR Kit | Creative Biogene | Real-time RT-PCR test CSFV RNA | Serum, plasma, tissue | https://www.creative-biogene.com/Classical-Swine-Fever-Virus-CSFV-Real-Time-RT-PCR-Kit-PDAS-AR002-1290596-88.html |
| Classical swine fever virus detection kits | Bioingentech Ltd | Real-time RT-PCR test CSFV RNA | Serum, blood, viral culture, tissue | https://www.kitpcr.com/pcr-kit/classical-swine-fever-virus-detection-kits/ |
| IDEXX RealPCR CSFV RNA Mix | IDEXX Laboratories, Inc. | Real-time RT-PCR test CSFV RNA | Serum, plasma, blood, viral culture, tissue | https://www.idexx.com/en/livestock/livestock-tests/swine-tests/realpcr-csfv/ |
| virotype® CSFV RT-PCR Kit | Indical Bioscience, GMBH | Real-time RT-PCR test CSFV RNA | Serum, plasma, blood, viral culture, tissue | https://www.indical.com/products/assays/ |
| Name | Producer | Test Principle | Suitable Sample Materials | DIVA Potential | Web Site |
|---|---|---|---|---|---|
| BioChek CSFV E2 Antibody ELISA | Biocheck | Indirect ELISA test E2 antibodies | Serum | No | https://www.biochek.com/swine-elisa/classical-swine-fever-antibody-test-kit/ |
| Classical Swine Fever Virus Antibody(IgG) ELISA Kit | Cusabio Technology LLC | Indirect ELISA test CSFV antibodies | Serum | No | https://www.cusabio.com/ELISA-Kit/Classical-Swine-Fever-Virus-AntibodyIgG--ELISA-Kit-114911.html |
| IDEXX CSFV Ab | IDEXX Laboratories, Inc. | Blocking ELISA test CSFV antibodies | Serum, plasma | No | https://www.idexx.com/en/livestock/livestock-tests/swine-tests/idexx-csfv-ab-test/ |
| ID Screen©Classical Swine Fever E2 Competition | ID VET | Competitive ELISA test E2 antibodies | Serum, plasma | No | https://www.id-vet.com/produit/id-screen-classical-swine-fever-e2-competition/ |
| LiliF™ Classical Swine Fever virus Ab rapid test kit | iNtRON Biotechnology, Inc. | Lateral flow immuno-chromatographic assay test CSFV antibodies | Blood | No | https://intronbio.com:6001/intronbioen/product/product_view.php?PRDT_ID=1891&page=1&Scate1=2&Scate2=2&Scate3=4&Scate4=16&Scate5=1&Scate6=-91-&Sword= |
| Pigtype CSFV Erns ELISA | Indical Bioscience, GMBH | Double-antigen ELISA test Erns antibodies | Serum, plasma | Yes | https://www.indical.com/products/assays/ |
| PrioCHECK™ Porcine CSFV Ab 2.0 strip kit | Thermo Fisher Scientific, Inc. | Blocking ELISA (E2 antibodies) | Serum, plasma | No | https://www.thermofisher.com/order/catalog/product/7610600?SID=srch-srp-7610600#/7610600?SID=srch-srp-7610600 |
| PrioCHECK™ CSFV Antibody ELISA kit | Thermo Fisher Scientific, Inc. | Blocking ELISA test E2 antibodies | Serum, plasma | No | https://www.thermofisher.com/order/catalog/product/7610046#/7610046 |
| PrioCHECK™ CSFV Erns Antibody ELISA Kit | Thermo Fisher Scientific, Inc. | Blocking ELISA test Erns antibodies | Serum | Yes | https://www.thermofisher.com/order/catalog/product/7610370#/7610370 |
| SVANOVIR®CSFV-Ab | Boehringer Ingelheim | Indirect ELISA test E2 antibodies | Serum | No | https://www.svanova.com/products/porcine/pp031.html |
| VDPro® CSFV AB C-ELISA | Median Diagnostics Inc. | Blocking ELISA test E2 antibodies | Serum | No | http://www.mediandiagnostics.com/eng/es-csf-02.php |
| VDPro® CSFV Erns Ab b-ELISA | Median Diagnostics Inc. | Blocking ELISA test Erns antibodies | Serum | Yes | http://www.mediandiagnostics.com/eng/es-csf-02.php |
| Classical Swine Fever Virus Antibodies Rapid Test Kit | Antibodies-online Inc | Sandwich GICA test CSFV antibodies | Blood, serum | No | https://www.antibodies-online.com/kit/5708730/Classical+Swine+Fever+Virus+Antibodies+Rapid+Test+Kit/ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Madera, R.; Li, Y.; McVey, D.S.; Drolet, B.S.; Shi, J. Recent Advances in the Diagnosis of Classical Swine Fever and Future Perspectives. Pathogens 2020, 9, 658. https://doi.org/10.3390/pathogens9080658
Wang L, Madera R, Li Y, McVey DS, Drolet BS, Shi J. Recent Advances in the Diagnosis of Classical Swine Fever and Future Perspectives. Pathogens. 2020; 9(8):658. https://doi.org/10.3390/pathogens9080658
Chicago/Turabian StyleWang, Lihua, Rachel Madera, Yuzhen Li, David Scott McVey, Barbara S. Drolet, and Jishu Shi. 2020. "Recent Advances in the Diagnosis of Classical Swine Fever and Future Perspectives" Pathogens 9, no. 8: 658. https://doi.org/10.3390/pathogens9080658
APA StyleWang, L., Madera, R., Li, Y., McVey, D. S., Drolet, B. S., & Shi, J. (2020). Recent Advances in the Diagnosis of Classical Swine Fever and Future Perspectives. Pathogens, 9(8), 658. https://doi.org/10.3390/pathogens9080658






