Identification of Essential Oils Including Garlic Oil and Black Pepper Oil with High Activity against Babesia duncani
Abstract
1. Introduction
2. Results
2.1. In Vitro Screening of Essential Oils for Inhibitory Activity against B. duncani
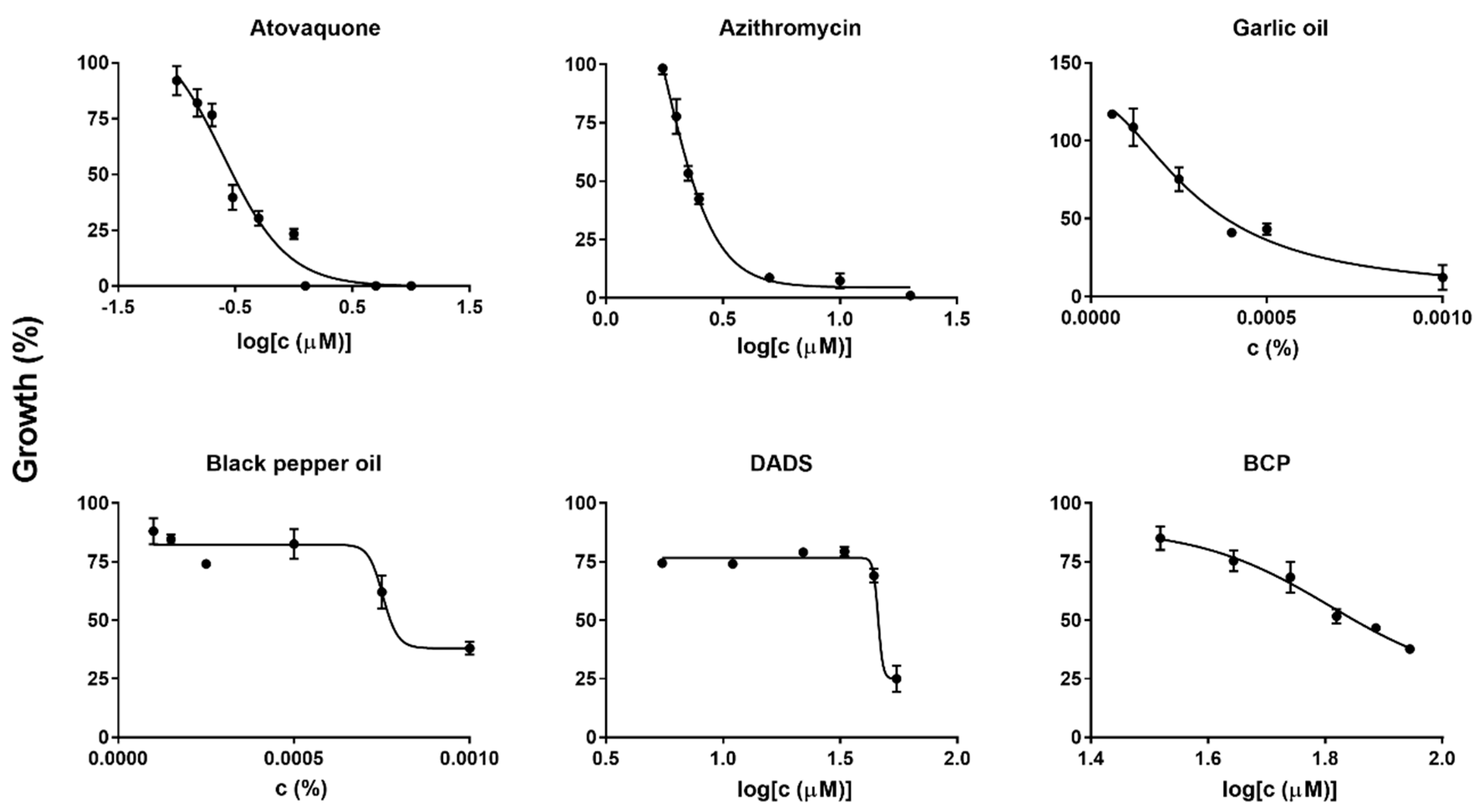
2.2. Diallyl Disulfide (DADS) and Β-Caryophyllene (BCP) as Highly Potent Active Ingredients of Garlic Oil and Black Pepper Oil against B. duncani Growth In Vitro
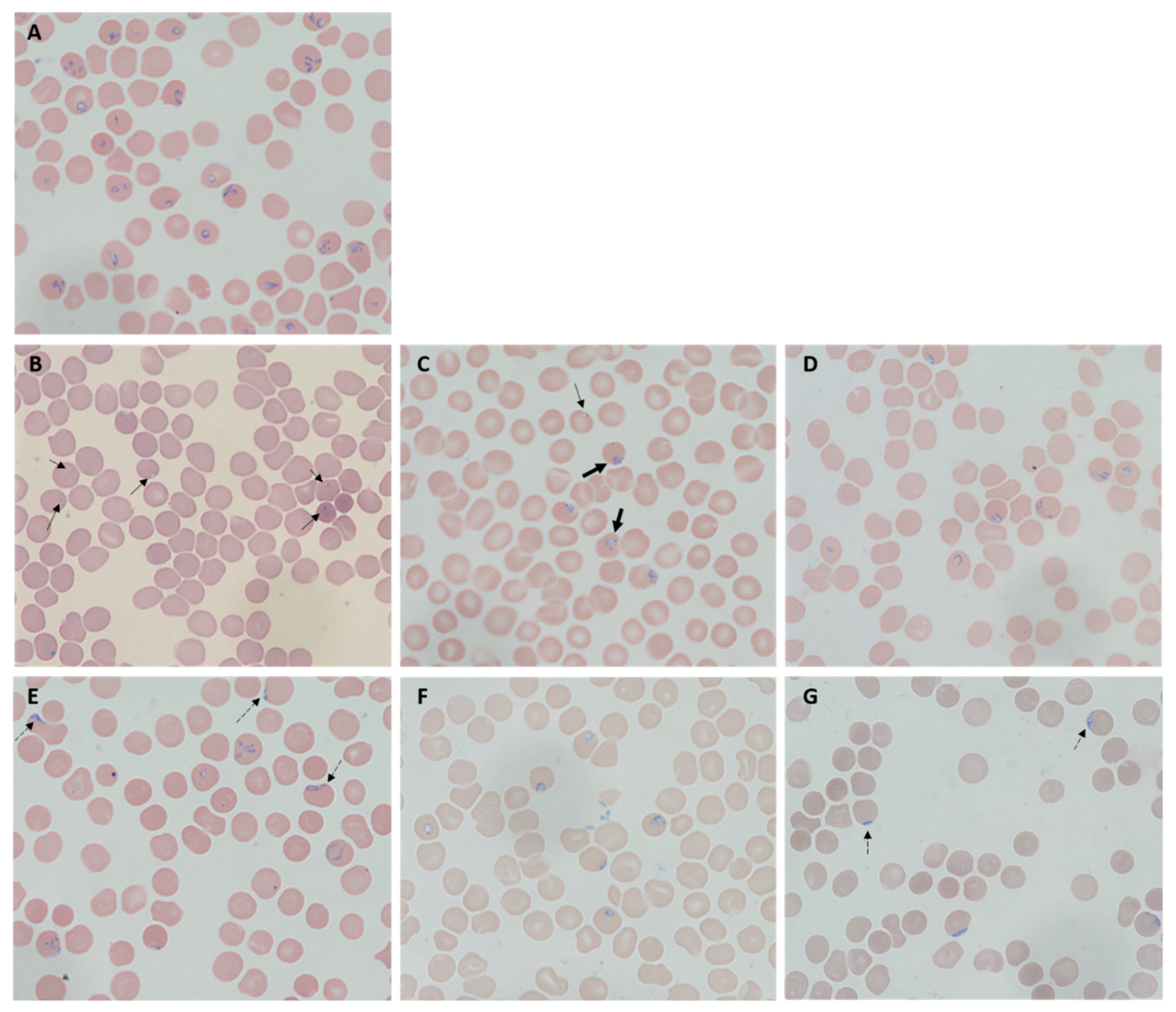
2.3. Morphological Changes of Treated B. duncani
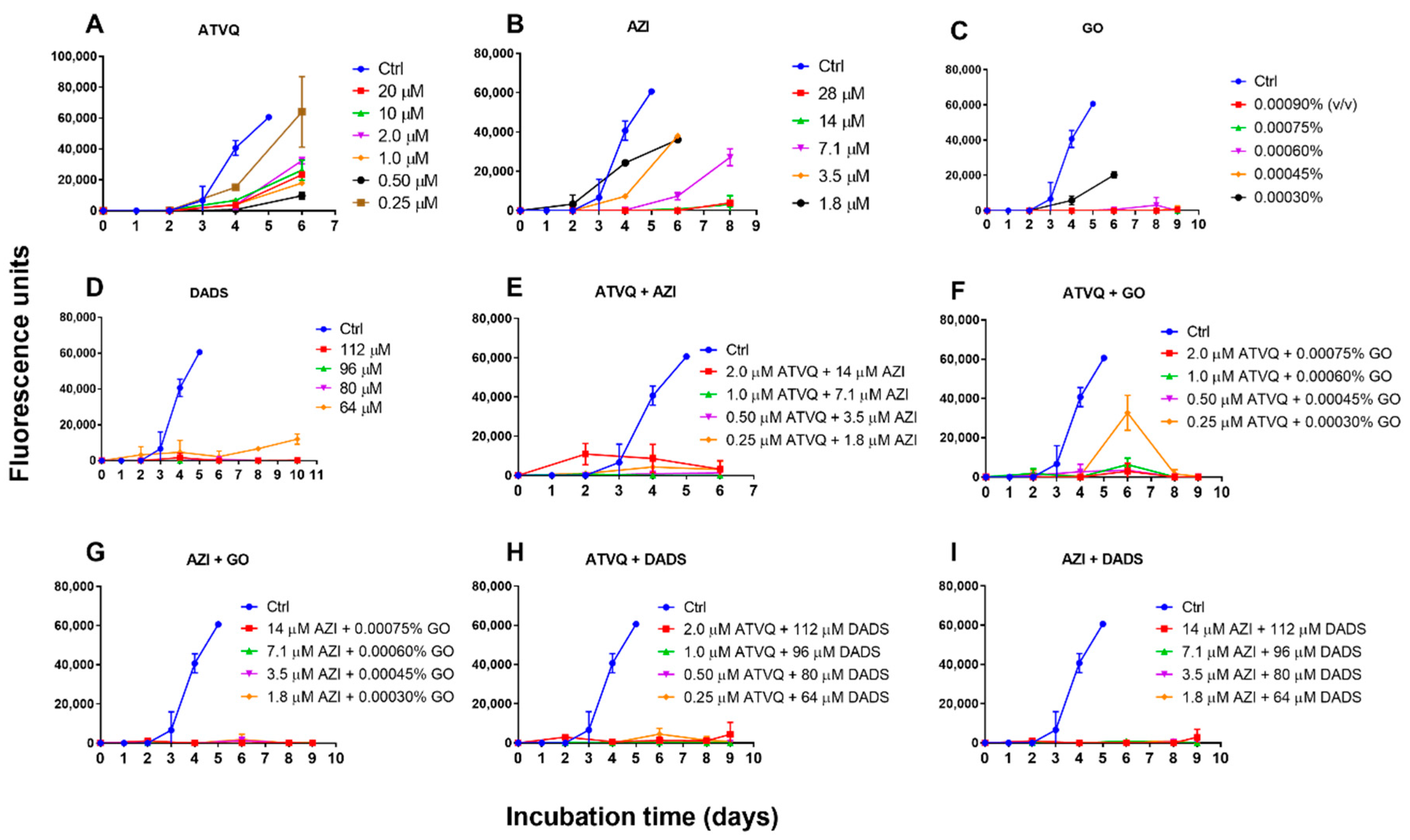
2.4. Subculture Studies to Evaluate the Viability of B. duncani Treated with Atovaquone, Azithromycin, Garlic Oil, DADS, and Their Combinations
3. Discussion
4. Materials and Methods
4.1. Hamster Donor Blood
4.2. Babesia duncani Culture In Vitro
4.3. In Vitro Evaluation of Essential Oils and Other Chemicals on Inhibition of B. duncani
4.4. Subculture Studies to Assess Viability of B. Duncani Treated with Garlic Oil, DADS, Atovaquone, Azithromycin, and Their Combinations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Leiby, D.A. Babesiosis and blood transfusion: Flying under the radar. Vox Sang. 2006, 90, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Lux, J.Z.; Weiss, D.; Linden, J.V.; Kessler, D.; Herwaldt, B.L.; Wong, S.J.; Keithly, J.; Della-Latta, P.; Scully, B.E. Transfusion-associated babesiosis after heart transplant. Emerg. Infect. Dis. 2003, 9, 116–119. [Google Scholar] [CrossRef] [PubMed]
- CDC - Babesiosis - Data & Statistics Data. Available online: https://www.cdc.gov/parasites/babesiosis/data-statistics/index.html (accessed on 1 June 2020).
- Quick, R.E.; Herwaldt, B.L.; Thomford, J.W.; Garnett, M.E.; Eberhard, M.L.; Wilson, M.; Spach, D.H.; Dickerson, J.W.; Telford, S.R., 3rd; Steingart, K.R.; et al. Babesiosis in Washington State: A new species of Babesia? Ann. Intern. Med. 1993, 119, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Scott, C.M. Human Babesiosis Caused by Babesia duncani Has Widespread Distribution across Canada. Healthc. Basel 2018, 6, 49. [Google Scholar] [CrossRef]
- Conrad, P.A.; Kjemtrup, A.M.; Carreno, R.A.; Thomford, J.; Wainwright, K.; Eberhard, M.; Quick, R.; Telford, S.R., 3rd; Herwaldt, B.L. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int. J. Parasitol. 2006, 36, 779–789. [Google Scholar] [CrossRef]
- Virji, A.Z.; Thekkiniath, J.; Ma, W.X.; Lawres, L.; Knight, J.; Swei, A.; Le Roch, K.; Ben Mamoun, C. Insights into the evolution and drug susceptibility of Babesia duncani from the sequence of its mitochondrial and apicoplast genomes. Int. J. Parasitol. 2019, 49, 105–113. [Google Scholar] [CrossRef]
- Kjemtrup, A.M.; Conrad, P.A. Human babesiosis: An emerging tick-borne disease. Int. J. Parasitol. 2000, 30, 1323–1337. [Google Scholar] [CrossRef]
- CDC - Babesiosis - Resources for Health Professionals. Available online: https://www.cdc.gov/parasites/babesiosis/health_professionals/index.html#tx (accessed on 1 June 2020).
- Krause, P.J.; Spielman, A.; Telford, S.R., 3rd; Sikand, V.K.; McKay, K.; Christianson, D.; Pollack, R.J.; Brassard, P.; Magera, J.; Ryan, R.; et al. Persistent parasitemia after acute babesiosis. N. Engl. J. Med. 1998, 339, 160–165. [Google Scholar] [CrossRef]
- Raffalli, J.; Wormser, G.P. Persistence of babesiosis for >2 years in a patient on rituximab for rheumatoid arthritis. Diagn. Microbiol. Infect. Dis. 2016, 85, 231–232. [Google Scholar] [CrossRef]
- Thomford, J.W.; Conrad, P.A.; Telford, S.R., 3rd; Mathiesen, D.; Bowman, B.H.; Spielman, A.; Eberhard, M.L.; Herwaldt, B.L.; Quick, R.E.; Persing, D.H. Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. J. Infect. Dis. 1994, 169, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Brasov, I.; Thekkiniath, J.; Kilian, N.; Lawres, L.; Gao, R.; DeBus, K.; He, L.; Yu, X.; Zhu, G.; et al. Establishment of a continuous in vitro culture of Babesia duncani in human erythrocytes reveals unusually high tolerance to recommended therapies. J. Biol. Chem. 2018, 293, 19974–19981. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kapoor, I.P.; Pandey, S.K.; Singh, U.K.; Singh, R.K. Studies on essential oils: Part 10; antibacterial activity of volatile oils of some spices. Phytother. Res. 2002, 16, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Gousia, P.; Economou, V.; Sakkas, V.; Petsios, S.; Papadopoulou, C. In vitro antimicrobial activity of five essential oils on multidrug resistant Gram-negative clinical isolates. J. Intercult. Ethnopharmacol. 2016, 5, 212–218. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, S.; Shi, W.T.; Zubcevik, N.; Miklossy, J.; Zhang, Y. Selective Essential Oils from Spice or Culinary Herbs Have High Activity against Stationary Phase and Biofilm Borrelia burgdorferi. Front. Med. Lausanne 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shi, W.; Zhang, Y. Essential Oils with High Activity against Stationary Phase Bartonella henselae. Antibiotics (Basel) 2019, 8, 246. [Google Scholar] [CrossRef]
- Menon, A.N.; Padmakumari, K.P.; Jayalekshmy, A. Essential oil composition of four major cultivars of black pepper (Piper nigrum L.) III. J. Essent. Oil Res. 2003, 15, 155–157. [Google Scholar] [CrossRef]
- Gray, E.B.; Herwaldt, B.L. Babesiosis Surveillance—United States, 2011–2015. MMWR Surveill. Summ. 2019, 68, 1–11. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; Linden, J.V.; Bosserman, E.; Young, C.; Olkowska, D.; Wilson, M. Transfusion-associated babesiosis in the United States: A description of cases. Ann. Intern. Med. 2011, 155, 509–519. [Google Scholar] [CrossRef]
- Ross, Z.M.; O’Gara, E.A.; Hill, D.J.; Sleightholme, H.V.; Maslin, D.J. Antimicrobial properties of garlic oil against human enteric bacteria: Evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl. Environ. Microbiol. 2001, 67, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S-flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Rees, L.P.; Minney, S.F.; Plummer, N.T.; Slater, J.H.; Skyrme, D.A. A quantitative assessment of the antimicrobial activity of garlic (Allium sativum). World J. Microbiol. Biotechnol. 1993, 9, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.C.; Cottrell, S.L.; Plummer, S.; Lloyd, D. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol. 2001, 57, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Adetumbi, M.; Javor, G.T.; Lau, B.H. Allium sativum (garlic) inhibits lipid synthesis by Candida albicans. Antimicrob. Agents Chemother. 1986, 30, 499–501. [Google Scholar] [CrossRef]
- Weber, N.D.; Andersen, D.O.; North, J.A.; Murray, B.K.; Lawson, L.D.; Hughes, B.G. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992, 58, 417–423. [Google Scholar] [CrossRef]
- Viswanathan, V.; Phadatare, A.G.; Mukne, A. Antimycobacterial and Antibacterial Activity of Allium sativum Bulbs. Indian J. Pharm. Sci. 2014, 76, 256–261. [Google Scholar] [PubMed]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Mandrell, R.; Friedman, M. Antibacterial effects of allspice, garlic, and oregano essential oils in tomato films determined by overlay and vapor-phase methods. J. Food Sci. 2009, 74, M390–M397. [Google Scholar] [CrossRef]
- Coppi, A.; Cabinian, M.; Mirelman, D.; Sinnis, P. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob. Agents Chemother. 2006, 50, 1731–1737. [Google Scholar] [CrossRef]
- Mirelman, D.; Monheit, D.; Varon, S. Inhibition of growth of Entamoeba histolytica by allicin, the active principle of garlic extract (Allium sativum). J. Infect. Dis. 1987, 156, 243–244. [Google Scholar] [CrossRef]
- Salama, A.A.; AbouLaila, M.; Terkawi, M.A.; Mousa, A.; El-Sify, A.; Allaam, M.; Zaghawa, A.; Yokoyama, N.; Igarashi, I. Inhibitory effect of allicin on the growth of Babesia and Theileria equi parasites. Parasitol. Res. 2014, 113, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecien, I.; Wlodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, K.P.; Zhang, X.; Pan, D.D.; Sun, Y.Y.; Cao, J.X. Antibacterial Activity and Mechanism of Action of Black Pepper Essential Oil on Meat-Borne Escherichia coli. Front. Microbiol. 2016, 7, 2094. [Google Scholar] [CrossRef] [PubMed]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, J.; Abbas, Z.; Beg, M.A.; Naz, S.; Awan, S.; Hamid, S.; Jafri, W. In vitro sensitivity of Blastocystis hominis to garlic, ginger, white cumin, and black pepper used in diet. Parasitol. Res. 2011, 109, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lawres, L.A.; Garg, A.; Kumar, V.; Bruzual, I.; Forquer, I.P.; Renard, I.; Virji, A.Z.; Boulard, P.; Rodriguez, E.X.; Allen, A.J.; et al. Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J. Exp. Med. 2016, 213, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.S.; Moreira, C.K.; Elsworth, B.; Allred, D.R.; Duraisingh, M.T. Extensive Shared Chemosensitivity between Malaria and Babesiosis Blood-Stage Parasites. Antimicrob. Agents Chemother. 2016, 60, 5059–5063. [Google Scholar] [CrossRef]
- Bigger, J.W. Treatment of staphylococcal infections with penicillin—By intermittent sterilisation. Lancet 1944, 2, 497–500. [Google Scholar] [CrossRef]
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan persister-like cells and drug treatment failure. Nat. Rev. Microbiol. 2019, 17, 607–620. [Google Scholar] [CrossRef]
- Delarze, E.; Sanglard, D. Defining the frontiers between antifungal resistance, tolerance and the concept of persistence. Drug Resist. Update 2015, 23, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Gewurz, B.E.; Hill, D.; Marty, F.M.; Vannier, E.; Foppa, I.M.; Furman, R.R.; Neuhaus, E.; Skowron, G.; Gupta, S.; et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin. Infect. Dis. 2008, 46, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Teuscher, F.; Chen, N.; Kyle, D.E.; Gatton, M.L.; Cheng, Q. Phenotypic changes in artemisinin-resistant Plasmodium falciparum lines in vitro: Evidence for decreased sensitivity to dormancy and growth inhibition. Antimicrob. Agents Chemother. 2012, 56, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T.; Seki, T. Antithrombotic and anticancer effects of garlic-derived sulfur compounds: A review. Biofactors 2006, 26, 93–103. [Google Scholar] [CrossRef]
- Dausch, J.G.; Nixon, D.W. Garlic: A review of its relationship to malignant disease. Prev. Med. 1990, 19, 346–361. [Google Scholar] [CrossRef]
- Nakagawa, S.; Masamoto, K.; Sumiyoshi, H.; Harada, H. [Acute toxicity test of garlic extract]. J. Toxicol. Sci. 1984, 9, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.; Ayyala, U. Differentiating and growth inhibitory effects of diallyl disulfide on cancer cells. Int. J. Oncol. 1997, 11, 181–185. [Google Scholar] [CrossRef]
- Caporaso, N.; Smith, S.M.; Eng, R.H. Antifungal activity in human urine and serum after ingestion of garlic (Allium sativum). Antimicrob. Agents Chemother. 1983, 23, 700–702. [Google Scholar] [CrossRef]
- Munday, R.; Munday, J.S.; Munday, C.M. Comparative effects of mono-, di-, tri-, and tetrasulfides derived from plants of the allium family: Redox cycling in vitro and hemolytic activity and phase 2 enzyme induction in vivo. Free Radic. Biol. Med. 2003, 34, 1200–1211. [Google Scholar] [CrossRef]
- Schmitt, D.; Levy, R.; Carroll, B. Toxicological Evaluation of beta-Caryophyllene Oil: Subchronic Toxicity in Rats. Int. J. Toxicol. 2016, 35, 558–567. [Google Scholar] [CrossRef]
- Liu, H.; Yang, G.; Tang, Y.; Cao, D.; Qi, T.; Qi, Y.; Fan, G. Physicochemical characterization and pharmacokinetics evaluation of beta-caryophyllene/beta-cyclodextrin inclusion complex. Int. J. Pharm. 2013, 450, 304–310. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.P.; Sun, B.G.; Wang, C.T. Physicochemical and release characterisation of garlic oil-beta-cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Levy, M.G.; Ristic, M. Babesia bovis: Continuous cultivation in a microaerophilous stationary phase culture. Science 1980, 207, 1218–1220. [Google Scholar] [CrossRef] [PubMed]
| Essential Oils and Control Drugs | Plants | Inhibition (%) |
|---|---|---|
| Garlic | Allium sativum | 87 |
| Black pepper | Piper nigrum | 64 |
| Tarragon | Artemisia dracunculus | 57 |
| Palo santo | Bursera graveolens | 56 |
| Coconut oil | Cocos nucifera | 55 |
| Pine oil | Pinus sylvestris L. | 53 |
| Meditation | Synergy blend of lavender, cedarwood atlas, tangerine, bergamot, palo santo, patchouli, vetiver, lemon, clove bud, ylang ylang, lime, Peru balsam, cedarwood Virginia, cistus, and chamomile | 53 |
| Cajeput | Melaleuca cajuputi | 53 |
| Moringa oil | Moringa oleifera | 52 |
| Stress relief | Synergy blend of essential oils of bergamot, patchouli, sweet orange, ylang ylang, pink grapefruit, and gurjum | 51 |
| Atovaquone | 100 | |
| Azithromycin | 88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Bai, C.; Shi, W.; Alvarez-Manzo, H.; Zhang, Y. Identification of Essential Oils Including Garlic Oil and Black Pepper Oil with High Activity against Babesia duncani. Pathogens 2020, 9, 466. https://doi.org/10.3390/pathogens9060466
Zhang Y, Bai C, Shi W, Alvarez-Manzo H, Zhang Y. Identification of Essential Oils Including Garlic Oil and Black Pepper Oil with High Activity against Babesia duncani. Pathogens. 2020; 9(6):466. https://doi.org/10.3390/pathogens9060466
Chicago/Turabian StyleZhang, Yumin, Chunxiang Bai, Wanliang Shi, Hector Alvarez-Manzo, and Ying Zhang. 2020. "Identification of Essential Oils Including Garlic Oil and Black Pepper Oil with High Activity against Babesia duncani" Pathogens 9, no. 6: 466. https://doi.org/10.3390/pathogens9060466
APA StyleZhang, Y., Bai, C., Shi, W., Alvarez-Manzo, H., & Zhang, Y. (2020). Identification of Essential Oils Including Garlic Oil and Black Pepper Oil with High Activity against Babesia duncani. Pathogens, 9(6), 466. https://doi.org/10.3390/pathogens9060466





