Long-Term Determinants of Tuberculosis in the Ungulate Host Community of Doñana National Park
Abstract
1. Introduction
2. Results
2.1. General
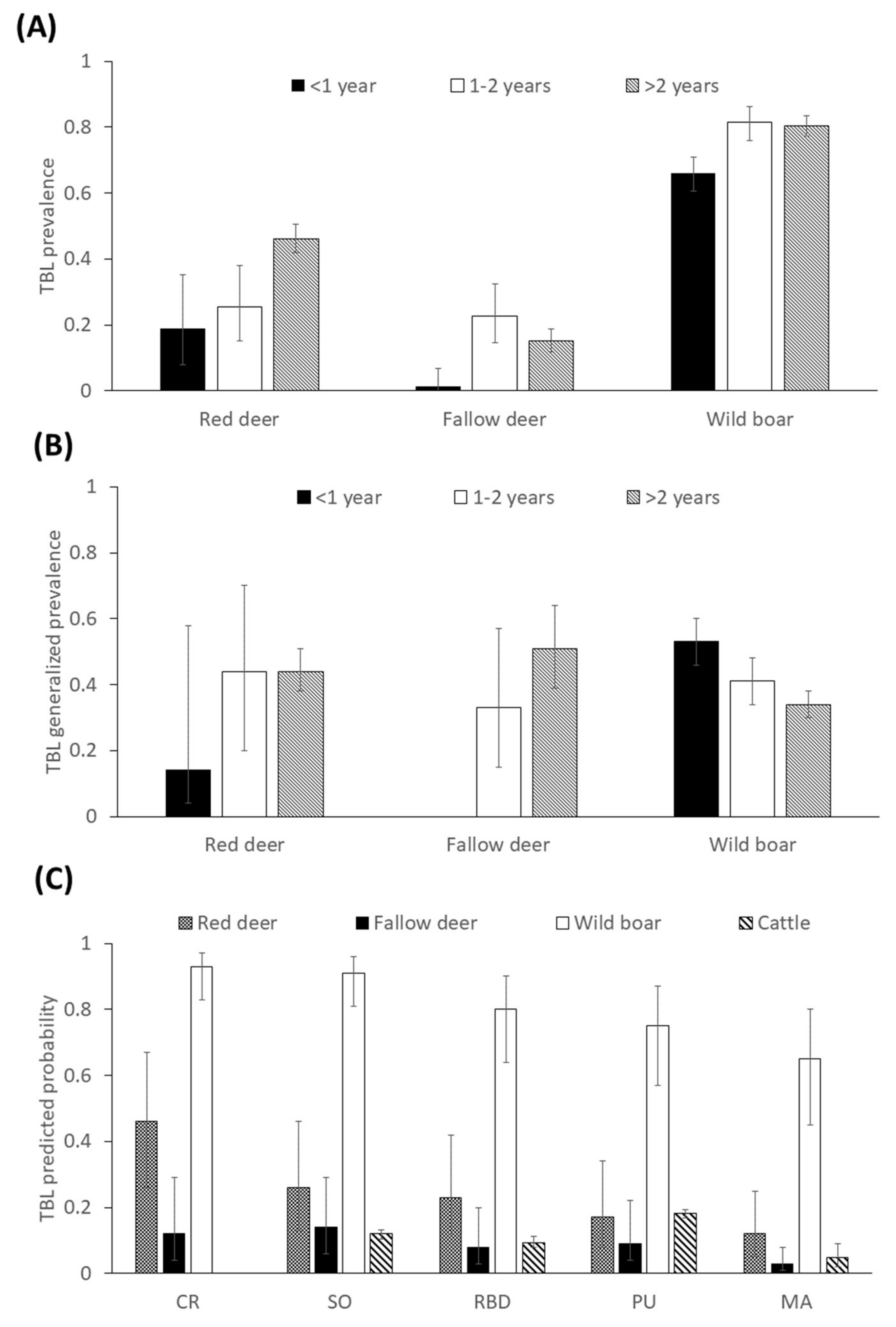
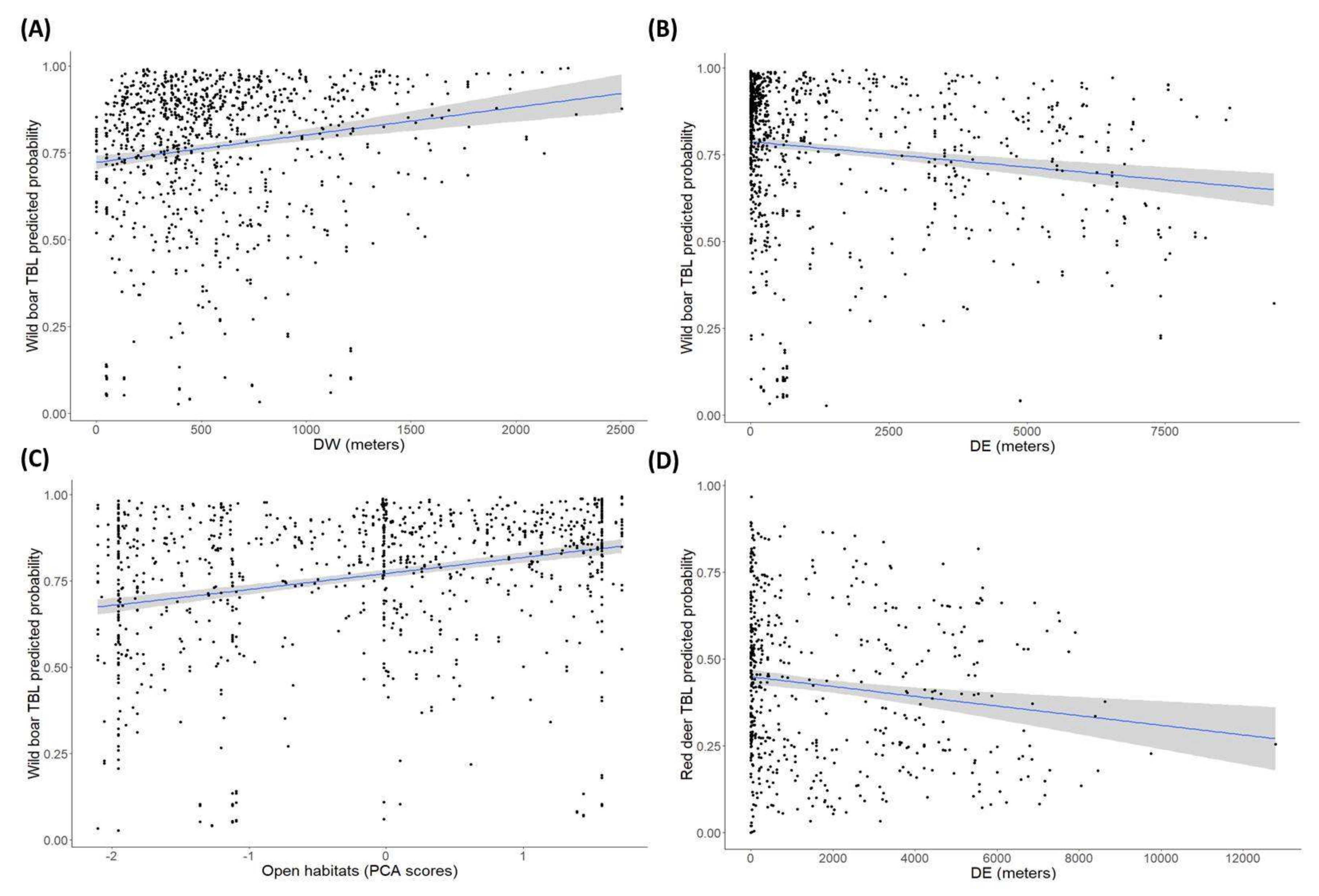
2.2. Factors Determining the Presence of Tuberculosis-Like Lesions (TBL)
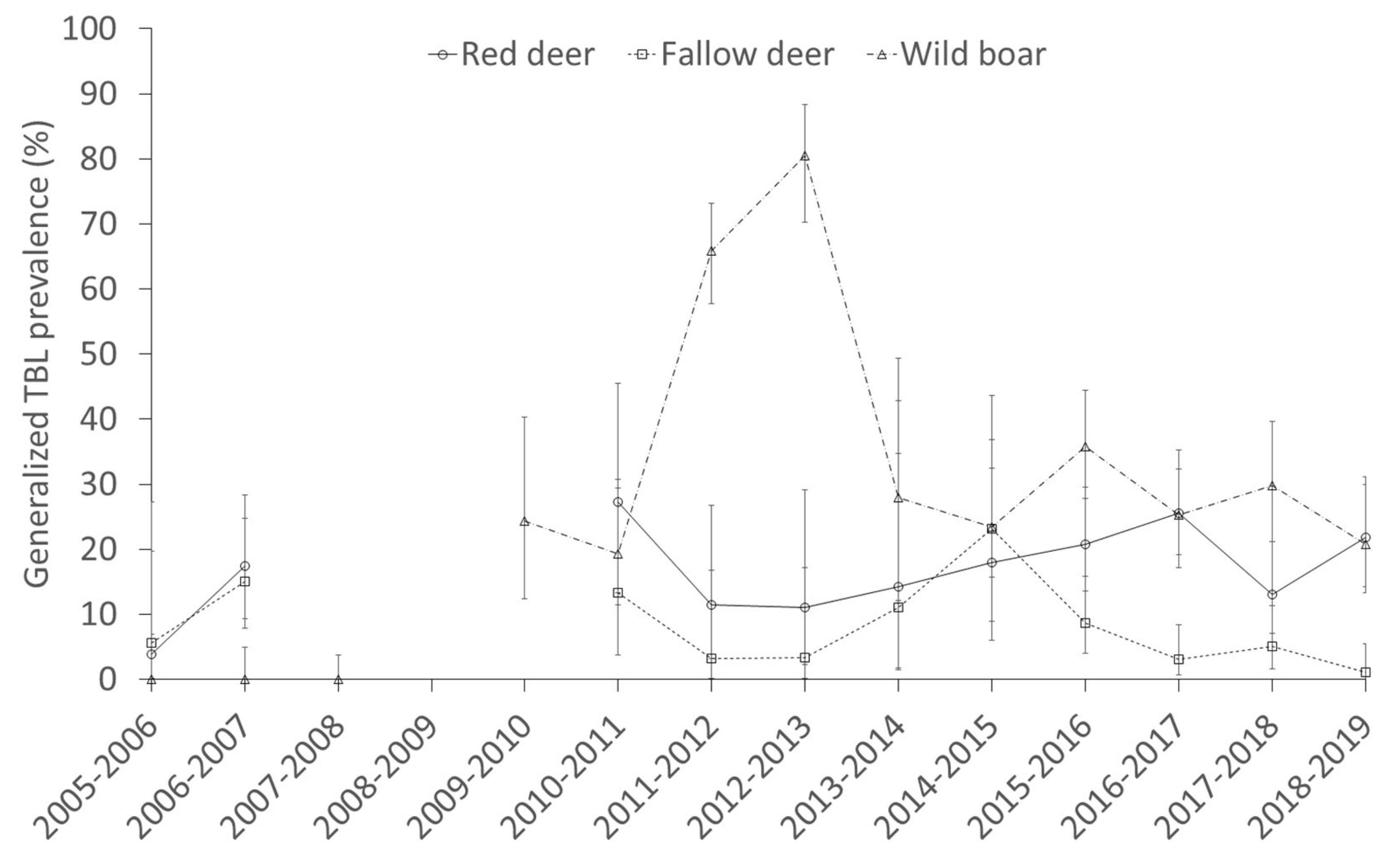
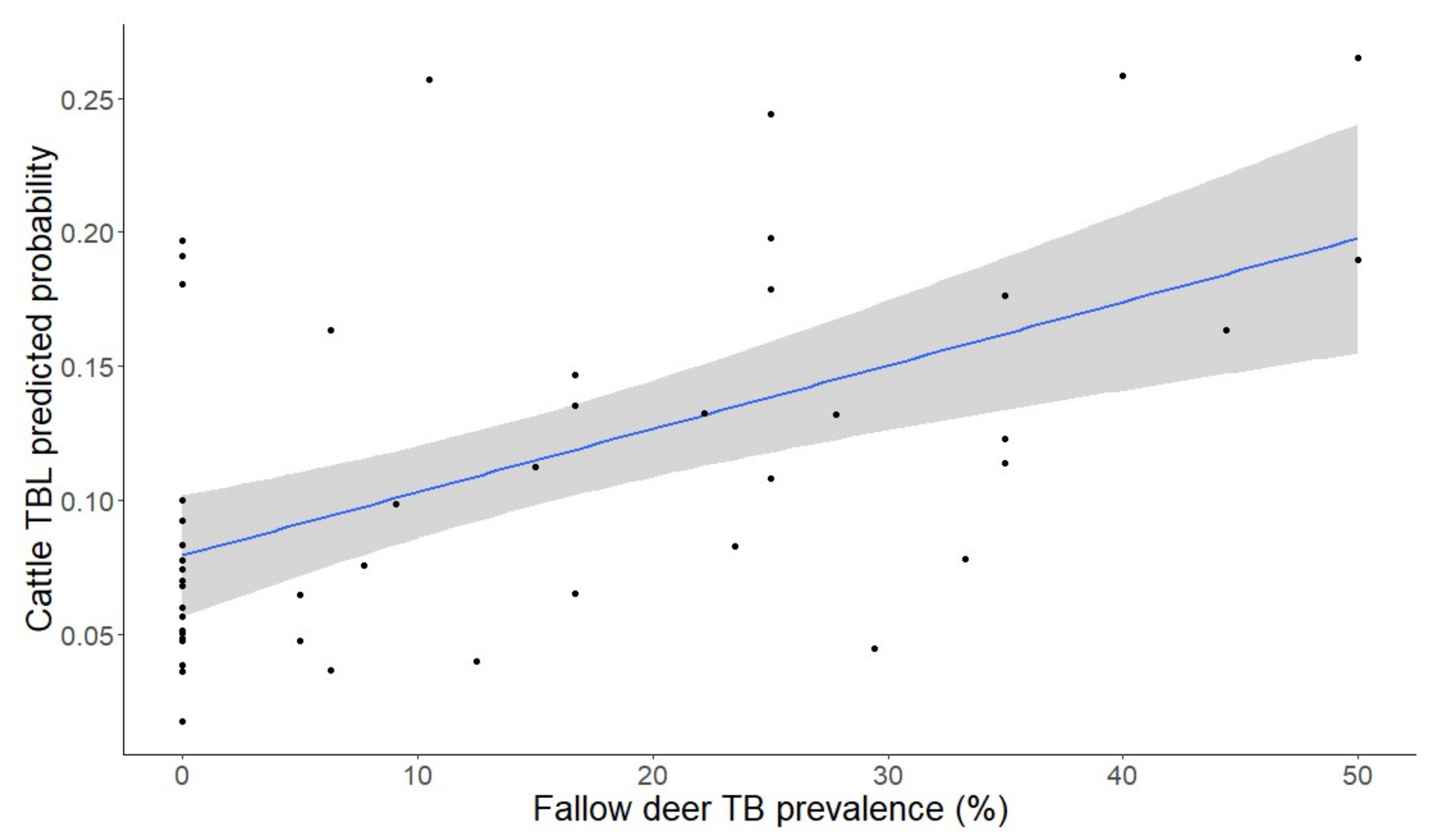
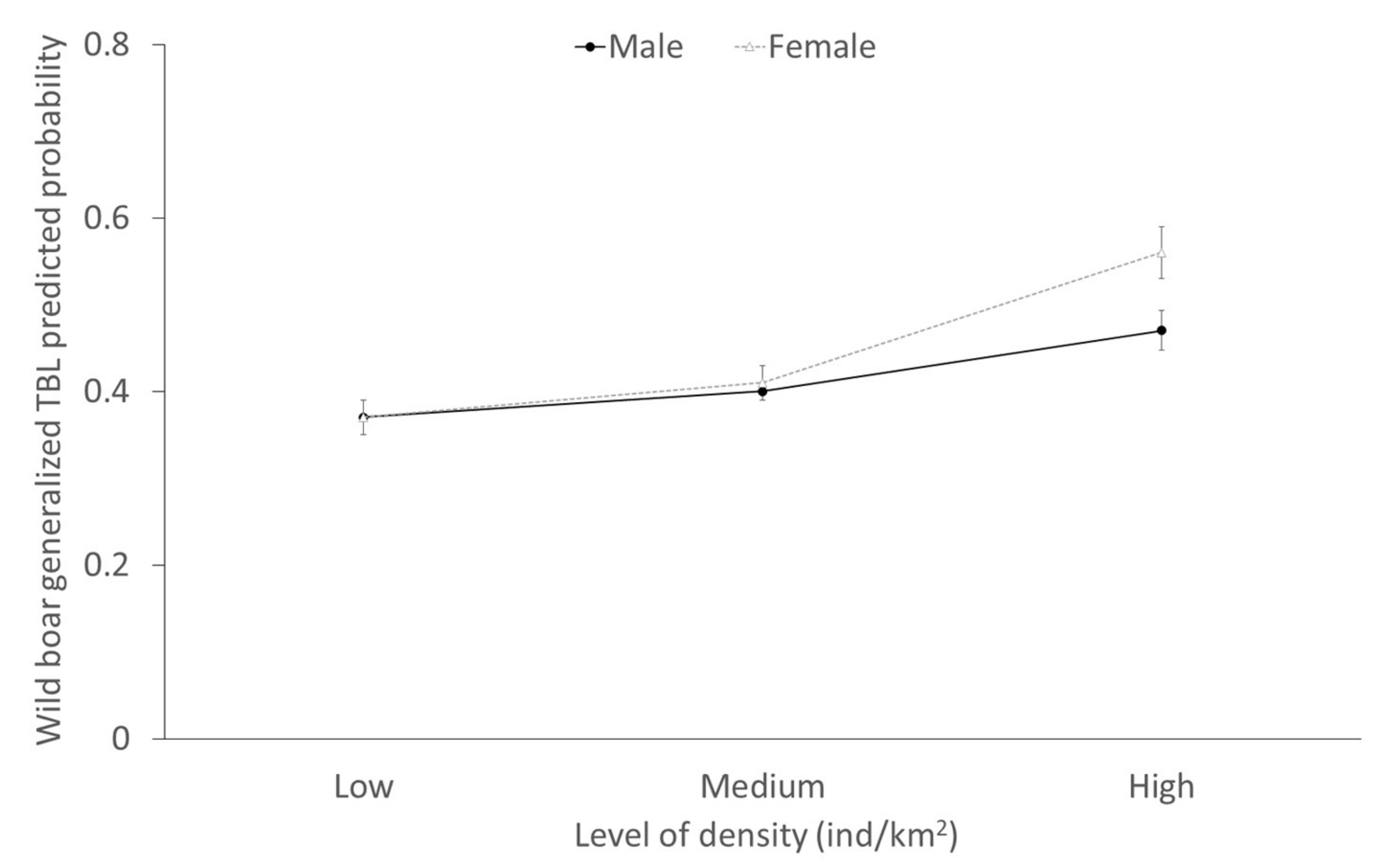
2.3. Factors Determining the Presence of Generalized TBL
3. Discussion
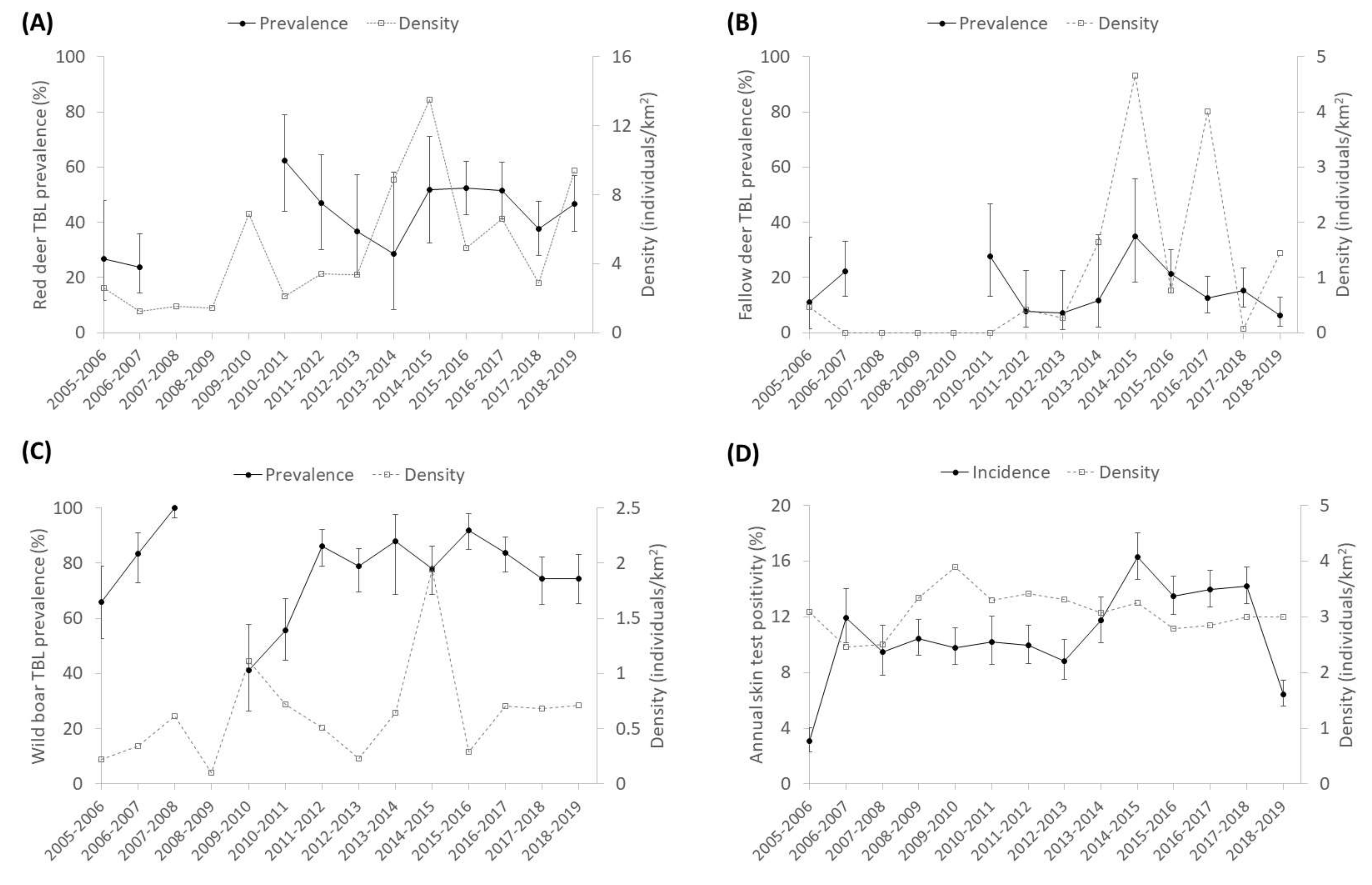
3.1. General Patterns of the Presence of TBL
3.2. The Environmental Features
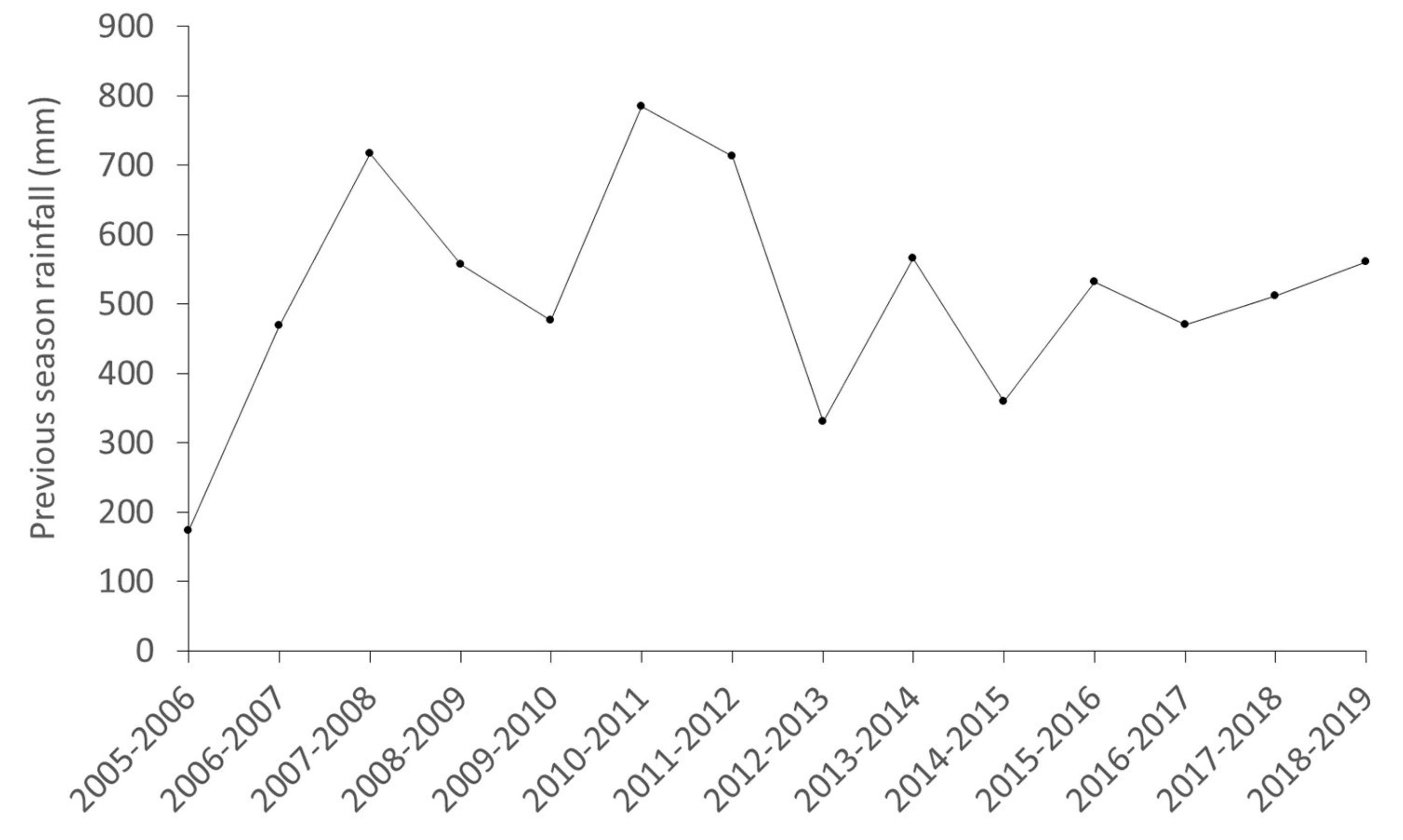
3.3. Stochasticity: Rainfall
3.4. Interspecific Relationships
4. Materials and Methods
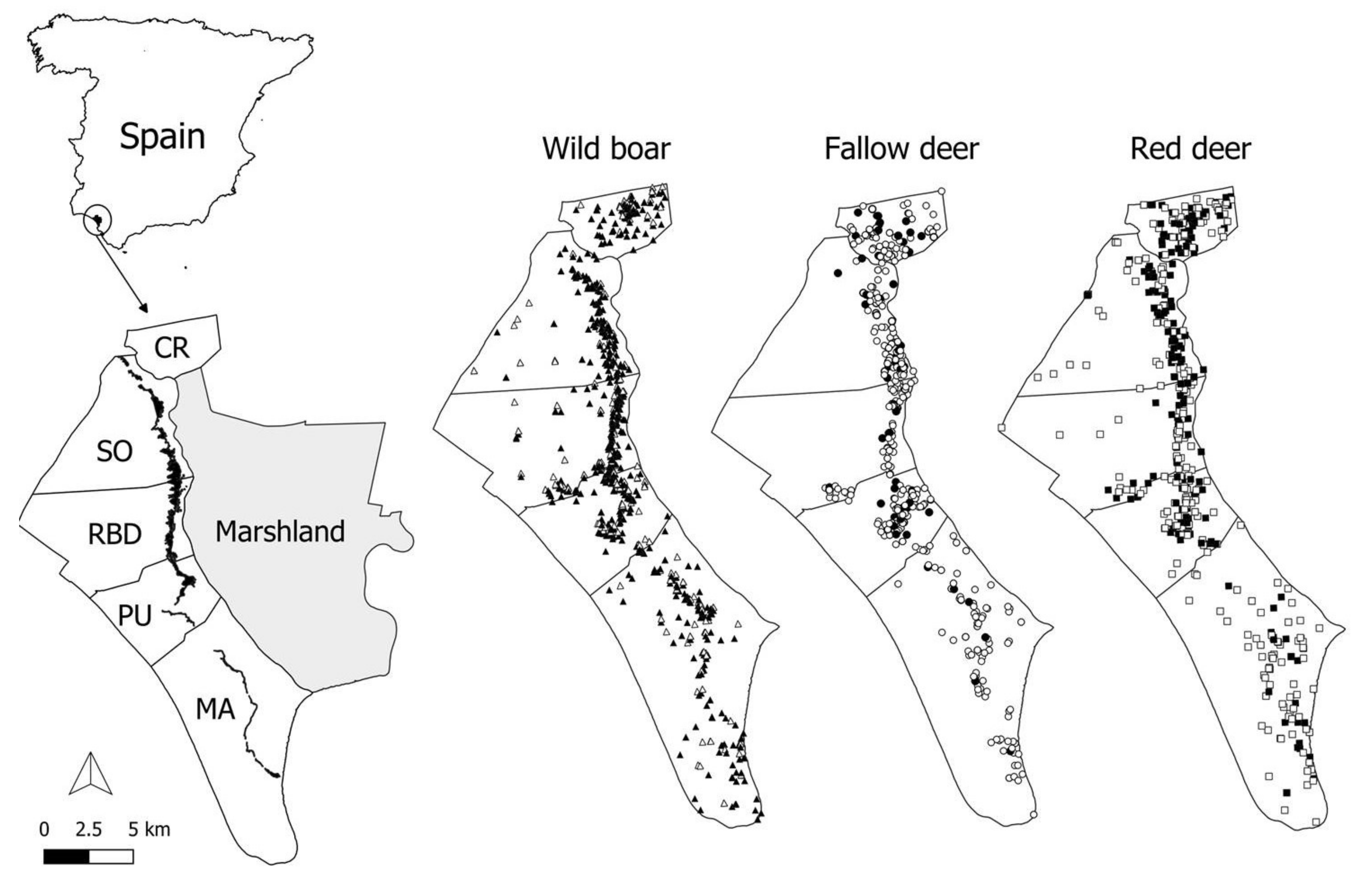
4.1. Study Area
4.2. Animal Sampling and Data Collection
4.3. Statistics
5. Conclusions
- The comprehensive monitoring carried out during the study period at the livestock–wildlife interface in DNP has provided evidence of relevant epidemiological processes which operate in the long term within the TB host community. The effects of the TB determinants are not straightforward but operate in complex interactions, often related to the ecology of the hosts.
- The effect of wild boar density on TB epidemiology mediated by sows may determine a high incidence in maternal groups from a very early age.
- Stochastic factors may determine the presence of TBL by interacting with the host’s natural life history, as evidenced in male red deer.
- The association of the presence of TB in fallow deer with that of cattle may indicate interspecies transmission (in either direction) and similar exposure to risk factors mediated by ecological overlap (they both use the same pasture areas).
- In DNP, as well as in other protected areas, hunting is not allowed and there are no natural predators. Therefore, the health surveillance and population control of wild ungulates and comprehensive management of cattle stocks, including the application of the national TB programme, must be a priority to control the disease at the wildlife–livestock interface. Since increases in the diversity and density of the host community of TB increases the ability of the system to maintain and transmit the pathogen in Mediterranean assemblages, expanding the distribution of cattle over other areas of the park, as demanded by breeders, could have a negative effect on the distribution and rates of TB.
- This long-term study provides a deeper understanding of the main drivers of a shared pathogen, MTC, which may help government agencies to develop improved risk-management strategies. We encourage administrations to conduct long-term studies and integrated monitoring and also to consider the impact of this issue on public health.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barasona, J.A.; Gortázar, C.; de la Fuente, J.; Vicente, J. Host richness increases tuberculosis disease risk in game-managed areas. Microorganisms 2019, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.; Glitezenstein, J.S.; Jones, C.G.; Kolasa, J.; Likens, G.E.; McDonnell, M.J.; Parker, G.G.; Pickett, S.T.A. Long-term ecological studies: An illustrated account of their design, operation, and importance to ecology. Occas. Publ. Inst. Ecosyst. Stud. 1986, 2, 1–38. [Google Scholar]
- McDonald, J.L.; Robertson, A.; Silk, M.J. Wildlife disease ecology from the individual to the population: Insights from a long-term study of a naturally infected European badger population. J. Anim. Ecol. 2017, 87, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Barasona, J.A.; Acevedo, P.; Ruiz-Fons, J.F.; Boadella, M.; Diez-Delgado, I.; Beltran-Beck, B.; González-Barrio, D.; Queirós, J.; Montoro, V.; et al. Temporal trend of tuberculosis in wild ungulates from mediterranean Spain. Transbound. Emerg. Dis. 2013, 60, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Molina-López, R.A.; Casal, J.; Darwich, L. Causes of morbidity in wild raptor populations admitted at a wildlife rehabilitation centre in Spain from 1995–2007: A long term retrospective study. PLoS ONE 2011, 6, e24603. [Google Scholar] [CrossRef]
- Delahay, R.J.; Walker, N.; Smith, G.S.; Wilkinson, D.; Clifton-Hadley, R.S.; Cheeseman, C.L.; Tomlinson, A.J.; Chambers, M.A. Long-term temporal trends and estimated transmission rates for Mycobacterium bovis infection in an undisturbed high-density badger (Meles meles) population. Epidemiol. Infect. 2013, 141, 1445–1456. [Google Scholar] [CrossRef]
- Vicente, J.; Delahay, R.J.; Walker, N.J.; Cheeseman, C.L. Social organization and movement influence the incidence of bovine tuberculosis in an undisturbed high-density badger Meles meles population. J. Anim. Ecol. 2007, 76, 348–360. [Google Scholar] [CrossRef]
- Gortázar, C.; Delahay, R.J.; Mcdonald, R.A.; Boadella, M.; Wilson, G.J.; Gavier-Widen, D.; Acevedo, P. The status of tuberculosis in European wild mammals. Mamm. Rev. 2012, 42, 193–206. [Google Scholar] [CrossRef]
- Humblet, M.-F.; Boschiroli, M.L.; Saegerman, C. Classification of worldwide bovine tuberculosis risk factors in cattle: A stratified approach. Vet. Res. 2009, 40, 50. [Google Scholar] [CrossRef]
- Kukielka, E.; Barasona, J.A.; Cowie, C.E.; Drewe, J.A.; Gortazar, C.; Cotarelo, I.; Vicente, J. Spatial and temporal interactions between livestock and wildlife in South Central Spain assessed by camera traps. Prev. Vet. Med. 2013, 112, 213–221. [Google Scholar] [CrossRef]
- Cowie, C.E.; Hutchings, M.R.; Barasona, J.A.; Gortázar, C.; Vicente, J.; White, P.C.L. Interactions between four species in a complex wildlife: Livestock disease community: Implications for Mycobacterium bovis maintenance and transmission. Eur. J. Wildl. Res. 2016, 62, 51–64. [Google Scholar] [CrossRef]
- Caron, A.; Cross, P.C.; du Toit, J.T. Ecological implications of Bovine Tuberculosis in African Buffalo herds. Ecol. Appl. 2003, 13, 1338–1345. [Google Scholar] [CrossRef]
- Barasona, J.A.; Vicente, J.; Díez-Delgado, I.; Aznar, J.; Gortázar, C.; Torres, M.J. Environmental Presence of Mycobacterium tuberculosis Complex in Aggregation Points at the Wildlife/Livestock Interface. Transbound. Emerg. Dis. 2017, 64, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, A.; de Juan, L.; Montero, N.; Sanchez, C.; Galka, M.; Delso, C.; Alvarez, J.; Romero, B.; Bezos, J.; Vela, A.I.; et al. Bovine Tuberculosis (Mycobacterium bovis) in Wildlife in Spain. J. Clin. Microbiol. 2004, 42, 2602–2608. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Höfle, U.; Garrido, J.M.; Fernández-De-Mera, I.G.; Juste, R.; Barral, M.; Gortazar, C. Wild boar and red deer display high prevalences of tuberculosis-like lesions in Spain. Vet. Res. 2006, 37, 107–119. [Google Scholar] [CrossRef]
- Martín-Hernando, M.P.; Höfle, U.; Vicente, J.; Ruiz-Fons, F.; Vidal, D.; Barral, M.; Garrido, J.M.; de la Fuente, J.; Gortazar, C. Lesions associated with Mycobacterium tuberculosis complex infection in the European wild boar. Tuberculosis 2007, 87, 360–367. [Google Scholar] [CrossRef]
- Naranjo, V.; Gortazar, C.; Vicente, J.; de la Fuente, J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 2008, 127, 1–9. [Google Scholar] [CrossRef]
- Barasona, J.A.; Mulero-Pázmány, M.; Acevedo, P.; Negro, J.J.; Torres, M.J.; Gortázar, C.; Vicente, J. Unmanned aircraft systems for studying spatial abundance of ungulates: Relevance to spatial epidemiology. PLoS ONE 2014, 9, 1–17. [Google Scholar] [CrossRef]
- Gortazar, C.; Torres, M.J.; Acevedo, P.; Aznar, J.; Negro, J.J.; de la Fuente, J.; Vicente, J. Fine-tuning the space, time, and host distribution of mycobacteria in wildlife. BMC Microbiol. 2011, 11, 27. [Google Scholar] [CrossRef]
- León-Vizcaíno, L. Outbreak of tuberculosis caused by Mycobacterium bovis in wild boars. In Proceedings of the 32nd international symposium on diseases of zoo and wild animals, Eskilstuna, Sweden, 23–27 May 1990; pp. 185–190. [Google Scholar]
- Briones, V.; De Juan, L.; Sanchez, C.; Vela, A.I.; Galka, M.; Montero, N.; Goyache, J.; Aranaz, A.; Mateos, A.; Domínguez, L. Bovine tuberculosis and the endangered Iberian Lynx. Emerg. Infect. Dis. 2000, 6, 189–191. [Google Scholar] [CrossRef]
- Perez, J.; Mozos, E.; Calzada, J.; Leon-Vizcaino, L.; Cubero, M.J.; Velarde, J. Tuberculosis in an Iberian lynx (Lynx pardina). Vet. Rec. 2001, 148, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Romero, B.; Aranaz, A.; Sandoval, Á.; Álvarez, J.; de Juan, L.; Bezos, J.; Sánchez, C.; Galka, M.; Fernández, P.; Mateos, A.; et al. Persistence and molecular evolution of Mycobacterium bovis population from cattle and wildlife in Doñana National Park revealed by genotype variation. Vet. Microbiol. 2008, 132, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Gortázar, C.; Torres, M.J.; Vicente, J.; Acevedo, P.; Reglero, M.; de la Fuente, J.; Negro, J.J.; Aznar-Martín, J. Bovine tuberculosis in Doñana Biosphere Reserve: The role of wild ungulates as disease reservoirs in the last Iberian lynx strongholds. PLoS ONE 2008, 3, e2776. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Latham, M.C.; Acevedo, P.; Armenteros, J.A.; Latham, A.D.M.; Gortazar, C.; Carro, F.; Soriguer, R.C.; Vicente, J. Spatiotemporal interactions between wild boar and cattle: Implications for cross-species disease transmission. Vet. Res. 2014, 45, 122. [Google Scholar] [CrossRef]
- Martín-Atance, P.; León-Vizcaíno, L.; Palomares, F.; Revilla, E.; González-Candela, M.; Calzada, J.; Cubero-Pablo, M.J.; Delibes, M. Antibodies to Mycobacterium bovis in wild carnivores from Doñana National Park (Spain). J. Wildl. Dis. 2006, 42, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Gobierno de España. Ministerio de Agricultura, Pesca y Alimentación. Available online: https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/sanidad-animal/enfermedades/tuberculosis/Tuberculosis_bovina.aspx (accessed on 29 April 2017).
- Laguna, E.; Barasona, J.A.; Triguero-Ocaña, R.; Mulero-Pázmány, M.; Negro, J.J.; Vicente, J.; Acevedo, P. The relevance of host overcrowding in wildlife epidemiology: A new spatially explicit aggregation index. Ecol. Indic. 2018, 84, 695–700. [Google Scholar] [CrossRef]
- Triguero-Ocaña, R.; Barasona, J.A.; Carro, F.; Soriguer, R.C.; Vicente, J.; Acevedo, P. Spatio-temporal trends in the frequency of interspecific interactions between domestic and wild ungulates from Mediterranean Spain. PLoS ONE 2019, 14, e0211216. [Google Scholar] [CrossRef]
- Triguero-Ocaña, R.; Martínez-López, B.; Vicente, J.; Barasona, J.A.; Martínez-Guijosa, J.; Acevedo, P. Dynamic network of interactions in the wildlife-livestock interface in mediterranean spain: An epidemiological point of view. Pathogens 2020, 9, 1–16. [Google Scholar] [CrossRef]
- Schmitt, S.M.; O’Brien, D.J.; Bruning-Fann, C.S.; Fitzgerald, S.D. Bovine Tuberculosis in Michigan Wildlife and Livestock. Ann. N. Y. Acad. Sci. 2002, 969, 262–268. [Google Scholar] [CrossRef]
- Katale, B.Z.; Mbugi, E.V.; Siame, K.K.; Keyyu, J.D.; Kendall, S.; Kazwala, R.R.; Dockrell, H.M.; Fyumagwa, R.D.; Michel, A.L.; Rweyemamu, M.; et al. Isolation and Potential for Transmission of Mycobacterium bovis at Human–livestock–wildlife Interface of the Serengeti Ecosystem, Northern Tanzania. Transbound. Emerg. Dis. 2017, 64, 815–825. [Google Scholar] [CrossRef]
- Glaser, L.; Carstensen, M.; Shaw, S.; Robbe-Austerman, S.; Wunschmann, A.; Grear, D.; Stuber, T.; Thomsen, B. Descriptive Epidemiology and Whole Genome Sequencing Analysis for an Outbreak of Bovine Tuberculosis in Beef Cattle and White-Tailed Deer in Northwestern Minnesota. PLoS ONE 2016, 11, e0145735. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Ryoo, S.; Lee, H.; Kim, N.; Lee, H.; Park, S.; Song, W.; Kim, J.-T.; Lee, H.S.; Myung Kim, J. Isolation of Mycobacterium bovis from Free-Ranging Wildlife in South Korea. J. Wildl. Dis. 2017, 53, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Boadella, M.; Vicente, J.; Ruiz-Fons, F.; de la Fuente, J.; Gortázar, C. Effects of culling Eurasian wild boar on the prevalence of Mycobacterium bovis and Aujeszky’s disease virus. Prev. Vet. Med. 2012, 107, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.; White, A.; Lurz, P.W.W.; Gortázar, C.; Díez-Delgado, I.; Boots, M. The Critical Role of Infectious Disease in Compensatory Population Growth in Response to Culling. Am. Nat. 2019, 194, E1–E12. [Google Scholar] [CrossRef]
- Barasona, J.A.; Torres, M.J.; Aznar, J.; Gortázar, C.; Vicente, J. DNA Detection Reveals Mycobacterium tuberculosis Complex Shedding Routes in Its Wildlife Reservoir the Eurasian Wild Boar. Transbound. Emerg. Dis. 2017, 64, 906–915. [Google Scholar] [CrossRef]
- Santos, N.; Almeida, V.; Gortázar, C.; Correia-Neves, M. Patterns of Mycobacterium tuberculosis-complex excretion and characterization of super-shedders in naturally-infected wild boar and red deer. Vet. Res. 2015, 46, 129. [Google Scholar] [CrossRef]
- Gortazar, C.; Vicente, J.; Samper, S.; Garrido, J.M.; Fernández-De-Mera, I.G.; Gavín, P.; Juste, R.A.; Martín, C.; Acevedo, P.; De La Puente, M.; et al. Molecular characterization of Mycobacterium tuberculosis complex isolates from wild ungulates in south-central Spain. Vet. Res. 2005, 36, 43–52. [Google Scholar] [CrossRef]
- Vicente, J.; Höfle, U.; Garrido, J.M.; Fernández-de-mera, I.G.; Acevedo, P.; Juste, R.; Barral, M.; Gortazar, C. Risk factors associated with the prevalence of tuberculosis-like lesions in fenced wild boar and red deer in south central Spain. Vet. Res. 2007, 38, 451–464. [Google Scholar] [CrossRef]
- Barasona, J.A.; Acevedo, P.; Diez-Delgado, I.; Queiros, J.; Carrasco-García, R.; Gortazar, C.; Vicente, J. Tuberculosis-associated death among adult wild boars, Spain, 2009–2014. Emerg. Infect. Dis. 2016, 22, 2178–2180. [Google Scholar] [CrossRef]
- Morris, R.S.; Pfeiffer, D.U.; Jackson, R. The epidemiology of Mycobacterium bovis infections. Vet. Microbiol. 1994, 40, 153–177. [Google Scholar] [CrossRef]
- Braza, F.; Alvarez, F. Habitat use by Red Deer and Fallow Deer in Doñana National Park. Misc. Zool. Barc. 1987, 11, 363–367. [Google Scholar]
- Lazo, A. Ranging behaviour of feral cattle (Bos taurus) in Doñana National Park, S.W. Spain. J. Zool. 1995, 236, 359–369. [Google Scholar] [CrossRef]
- Cowie, C.E.; Beck, B.B.; Gortazar, C.; Vicente, J.; Hutchings, M.R.; Moran, D.; White, P.C.L. Risk factors for the detected presence of Mycobacterium bovis in cattle in south central Spain. Eur. J. Wildl. Res. 2014, 60, 113–123. [Google Scholar] [CrossRef]
- Cooper, S.M.; Scott, H.M.; de la Garza, G.R.; Deck, A.L.; Cathey, J.C. Distribution and interspecies contact of feral swine and cattle on rangeland in south Texas: Implications for disease transmission. J. Wildl. Dis. 2010, 46, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Renwick, A.R.; White, P.C.L.; Bengis, R.G. Bovine tuberculosis in southern African wildlife: A multi-species host–pathogen system. Epidemiol. Infect. 2007, 135, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Munyeme, M.; Muma, J.B.; Skjerve, E.; Nambota, A.M.; Phiri, I.G.K.; Samui, K.L.; Dorny, P.; Tryland, M. Risk factors associated with bovine tuberculosis in traditional cattle of the livestock/wildlife interface areas in the Kafue basin of Zambia. Prev. Vet. Med. 2008, 85, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Ballari, S.A.; Barrios-García, M.N. A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges. Mamm. Rev. 2014, 44, 124–134. [Google Scholar] [CrossRef]
- Barasona, J.A.; Risalde, M.A.; Ortíz, J.A.; Gonzalez-Barrio, D.; Che-Amat, A.; Pérez-Sancho, M.; Vargas-Castillo, L.; Xeidakis, A.; Jurado-Tarifa, E.; Gortázar, C. Disease-mediated piglet mortality prevents wild boar population growth in fenced overabundant settings. Eur. J. Wildl. Res. 2020, 66, 26. [Google Scholar] [CrossRef]
- Carranza, J. Ciervo—Cervus elaphus. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Barja, I., Eds.; Museo Nacional de Ciencias Naturales—CSIC: Madrid, Spain, 2017; pp. 1–34. [Google Scholar] [CrossRef]
- Carranza, J.; Alvarez, F.; Redondo, T. Territoriality as a mating strategy in red deer. Anim. Behav. 1990, 40, 79–88. [Google Scholar] [CrossRef]
- Westneat, D.F.; Birkhead, T.R. Alternative hypotheses linking the immune system and mate choice for good genes. Proc. R. Soc. Biol. Sci. 1998, 265, 1065–1073. [Google Scholar] [CrossRef]
- Lochmiller, R.L.; Deerenberg, C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos 2000, 88, 87–98. [Google Scholar] [CrossRef]
- Malo, A.; Roldan, E.R.; Garde, J.; Soler, A.; Vicente, J.; Gortazar, C.; Gomendio, M. What does testosterone do for red deer males? Proc. R. Soc. B Biol. Sci. 2009, 276, 971–980. [Google Scholar] [CrossRef]
- Vicente, J.; Pérez-Rodríguez, L.; Gortazar, C. Sex, age, spleen size, and kidney fat of red deer relative to infection intensities of the lungworm Elaphostrongylus cervi. Naturwissenschaften 2007, 94, 581–587. [Google Scholar] [CrossRef] [PubMed]
- De la Peña, E.; Martín, J.; Barja, I.; Pérez-Caballero, R.; Acosta, I.; Carranza, J. The immune challenge of mating effort: Steroid hormone profile, dark ventral patch and parasite burden in relation to intrasexual competition in male Iberian red deer. Integr. Zool. 2020, 1749–4877.12427. [Google Scholar] [CrossRef]
- Galván, I.; Solano, F.; Zougagh, M.; de Andrés, F.; Murtada, K.; Ríos, Á.; de la Peña, E.; Carranza, J. Unprecedented high catecholamine production causing hair pigmentation after urinary excretion in red deer. Cell. Mol. Life Sci. 2019, 76, 397–404. [Google Scholar] [CrossRef]
- Rehbein, S.; Visser, M.; Jekel, I.; Silaghi, C. Endoparasites of the fallow deer (Dama dama) of the Antheringer Au in Salzburg, Austria. Wien. Klin. Wochenschr. 2014, 126, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Santos, C.; Valente, T.; Gortázar, C.; Almeida, V.; Correia-Neves, M. Widespread Environmental Contamination with Mycobacterium tuberculosis Complex Revealed by a Molecular Detection Protocol. PLoS ONE 2015, 10, e0142079. [Google Scholar] [CrossRef]
- Mulero-Pázmány, M.; Barasona, J.Á.; Acevedo, P.; Vicente, J.; Negro, J.J. Unmanned Aircraft Systems complement biologging in spatial ecology studies. Ecol. Evol. 2015, 5, 4808–4818. [Google Scholar] [CrossRef]
- Focardi, S.; Pecchioli, E. Social cohesion and foraging decrease with group size in fallow deer (Dama dama). Behav. Ecol. Sociobiol. 2005, 59, 84–91. [Google Scholar] [CrossRef]
- Tucker, C.B. Behaviour of cattle. In The Ethology of Domestic Animals, 2nd ed.; Jensen, P., Biology, I., Eds.; Linköping University: Linköping, Sweden, 2009. [Google Scholar]
- Díaz-Delgado, R.; Aragonés, D.; Afán, I.; Bustamante, J. Long-Term Monitoring of the Flooding Regime and Hydroperiod of Doñana Marshes with Landsat Time Series (1974–2014). Remote Sens. 2016, 8, 775. [Google Scholar] [CrossRef]
- Fernández-Delgado, C. Conservation management of a European natural area: Doñana National Park, Spain. Princ. Conserv. Biol. 1997, 458–467. [Google Scholar] [CrossRef]
- Mitchell, B. Growth Layers in Dental Cement for Determining the Age of Red Deer (Cervus elaphus L.). J. Anim. Ecol. 2017, 36, 279–293. [Google Scholar] [CrossRef]
- Sáez-Royuela, C.; Gomáriz, R.P.; Tellería, J.L. Age-Determination of European Wild Boar. Wildl. Soc. Bull. 1989, 17, 326–329. [Google Scholar]
- Sáenz de Buruaga, M.; Lucio, A.J.; Purroy, F.J. Reconocimiento de Sexo y Edad en Especies Cinegéticas; Diputación Foral de Álava: Vitoria, Spain, 1991. [Google Scholar]
- Estación Biológica de Doñana-CSIC. Equipo de Seguimiento del Espacio Nacional de Doñana. Available online: http://icts.ebd.csic.es/datos-meteorologicos (accessed on 12 April 2017).
- Rodriguez-Hidalgo, P.; Gortazar, C.; Tortosa, F.S.; Rodriguez-Vigal, C.; Fierro, Y.; Vicente, J. Effects of density, climate, and supplementary forage on body mass and pregnancy rates of female red deer in Spain. Oecologia 2010, 164, 389–398. [Google Scholar] [CrossRef] [PubMed]
- QGIS Development Team QGIS. Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: https://qgis.org/es/site/ (accessed on 16 May 2017).
- Consejería de Medio Ambiente y Ordenación del Territorio, Andalucía, España: Red de Información Ambiental de Andalucía, REDIAM. Available online: http://www.juntadeandalucia.es/medioambiente/site/rediam/ (accessed on 15 May 2017).
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.B.; Marques, T.A.; Burnham, K.P. Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling: Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. GLM and GAM for Count Data. In Statistics for Biology and Health; Gail, M., Krickeberg, K., Samet, J.M., Eds.; Springer: New York, NY, USA, 2009; Volume 21, pp. 209–243. ISBN 9780387874579. [Google Scholar]
- George, D.; Mallery, P. IBM SPSS for Windows Statistics 19 Step by Step: A Simple Guidance and Reference, 12th ed.; Routledge: Abingdon, UK, 2012. [Google Scholar]
- R Core Team (R Foundation for Statistical Computing). R: A Language and Environment for Statistical Computing. 2019. Available online: https://www.r-project.org/ (accessed on 23 June 2017).
- Vicente, J.; Apollonio, M.; Blanco-Aguiar, J.A.; Borowik, T.; Brivio, F.; Casaer, J.; Croft, S.; Ericsson, G.; Ferroglio, E.; Gavier-Widen, D.; et al. Science-based wildlife disease response. Science 2019, 364, 943–944. [Google Scholar] [CrossRef]
- Vicente, J.; Palencia, P.; Plhal, R.; Blanco-Aguiar, J.A.; Laguna, E.; Soriguer, R.; López, J.F.; Podgórski, T.; Petrović, K.; Apollonio, M.; et al. Harmonization of the use of hunting statistics for wild boar density estimation in different study areas. EFSA Support. Publ. 2019, 16, 1706E. [Google Scholar] [CrossRef]



| Wild Boar | Fallow Deer | Red Deer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F df(x,y) | Estimate ± SE | p | F df(x,y) | Estimate ± SE | p | F df(x,y) | Estimate ± SE | p | |
| Sex 1 | 1.64 (1,1119) | Female: 1.17 ± 0.69 | 0.20 | 0.12 (1,604) | Female: −0.30 ± 1.88 | 0.73 | 1.64 (1,612) | Female: 2.25 ± 1.62 | 0.20 |
| Age 2 | 3.88 (2,1199) | 2 years: 2.79 ± 1.32 ≥3 years: 2.2 ± 1.01 | 0.02 | 0.04 (2,604) | 2 years: 1.27 ± 4.43 ≥3 years: 1.47 ± 4.25 | 0.96 | 0.15 (2,612) | 2 years: 2.24 ± 2.85 ≥3 years: 1.44 ± 2.66 | 0.86 |
| DW | 3.66 (1,1199) | 0 ± 0 | 0.05 | 0.51 (1,604) | 0 ± 0 | 0.48 | 0.08 (1,612) | −0 ± 0 | 0.78 |
| DE | 6.10 (1,1199) | −0 ± 0 | 0.01 | 0.80 (1,604) | −0 ± 0 | 0.37 | 5.24 (1,612) | −0 ± 0 | 0.02 |
| Open habitats | 12.21 (1,1199) | 0.26 ± 0.08 | <0.01 | 0.61 (1,604) | 0.09 ± 0.12 | 0.44 | 2.40 (1,612) | 0.11 ± 0.07 | 0.12 |
| Watercourse vegetation | 0.24 (1,1199) | −0.04 ± 0.08 | 0.63 | 1.68 (1,604) | −0.15 ± 0.11 | 0.20 | 0.09 (1,612) | −0.03 ± 0.1 | 0.76 |
| Previous Rainfall | 0.23 (1,1199) | −0 ± 0 | 0.63 | 0.09 (1,604) | 0 ± 0.01 | 0.77 | 0.09 (1,612) | 0 ± 0 | 0.76 |
| Red deer density | 0.04 (1,1199) | −0.01 ± 0.03 | 0.84 | 0.14 (1,604) | −0.02 ± 0.04 | 0.70 | 1.79 (1,612) | −0.36 ± 0.26 | 0.18 |
| Fallow deer density | 0.34 (1,1199) | 0.04 ± 0.06 | 0.56 | 0.26 (1,604) | −0.24 ± 0.57 | 0.61 | 0.02 (1,612) | 0.01 ± 0.04 | 0.88 |
| Wild boar density | 3.23 (1,1199) | −1.24 ± 0.68 | 0.07 | 0.26 (1,604) | 0.11 ± 0.22 | 0.61 | 0 (1,612) | 0.002 ± 0.17 | 0.99 |
| Prevalence of red deer TBL | 1.61 (1,1199) | −0.01 ± 0.01 | 0.20 | 0.05 (1,604) | 0 ± 0 | 0.82 | 20.85 (1,612) | 0.05 ± 0.03 | <0.01 |
| Prevalence of fallow deer TBL | 0.30 (1,1199) | 0.004 ± 0.01 | 0.59 | 7.03 (1,604) | 0.01 ± 0.05 | <0.01 | 0 (1,612) | −0 ± 0.01 | 0.97 |
| Prevalence of wild boar TBL | 85.18 (1,1199) | 0.04 ± 0.01 | <0.01 | 0 (1,604) | −0 ± 0.01 | 0.98 | 0.04 (1,612) | −0 ± 0.01 | 0.84 |
| Sex*age | 1.65 (2,1199) | Female* 2 years: −0.91 ± 0.51 Female* ≥3 years: −0.16 ± 0.36 | 0.19 | 0.20 (2,604) | Female* 2 years: −0.11 ± 1.43 Female* ≥3 years: −0.51 ± 1.31 | 0.82 | 0.75 (2,612) | Female* 2 years: −1.72 ± 1.52 Female* ≥3 years: −0.99 ± 1.35 | 0.47 |
| Rainfall*sex | 2.47 (1,1199) | Rainfall*Female: −0 ± 0 | 0.12 | 0.29 (1,604) | Rainfall*Female: 0.001 ± 0.003 | 0.59 | 3.90 (1,612) | Rainfall*Female: −0 ± 0 | 0.05 |
| Density*sex | 0.06 (1,1199) | Density*Female −0.04 ± 0.16 | 0.81 | 0 (1,604) | Density*Female −0 ± 0.11 | 0.99 | 0.602 (1,612) | Density*Female 0.03 ± 0.04 | 0.44 |
| Rainfall*age | 1.67 (2,1199) | Rainfall* 2 years: 0 ± 0 Rainfall* ≥3 years: 0 ± 0 | 0.19 | 0.04 (2,604) | Rainfall* 2 years: −0 ± 0.01 Rainfall* ≥3 years: −0 ± 0.01 | 0.96 | 0.67 (2,612) | Rainfall* 2 years: −0 ± 0.01 Rainfall* ≥3 years: −0 ± 0 | 0.51 |
| Density*age | 0.03 (2,1199) | Density* 2 years: 0.03 ± 0.26 Density* ≥3 years: 0.05 ± 0.18 | 0.97 | 0 (2,604) | Density* 2 years: 0.02 ± 0.4 Density* ≥3 years: 0.02 ± 0.36 | 0.99 | 2.24 (2,612) | Density* 2 years: 0.12 ± 0.27 Density* ≥3 years: 0.33 ± 0.23 | 0.11 |
| Prevalence*age | 6.09 (2,119) | Prevalence* 2 years: 0.04 ± 0.01 Prevalence* ≥3 years: 0.03 ± 0.01 | <0.01 | 1.91 (2,604) | Prevalence* 2 years: 0.11 ± 0.05 Prevalence* ≥3 years: 0.08 ± 0.05 | 0.15 | 0.66 (2,612) | Prevalence* 2 years: 0.02 ± 0.03 Prevalence* ≥3 years: −0.01 ± 0 | 0.52 |
| Rainfall*density species studied | 2.58 (1,119) | 0 ± 0 | 0.11 | 0.23 (1,60) | 0 ± 0 | 0.63 | 0.01 (1,61) | 0 ± 0 | 0.91 |
| Wild Boar | Fallow Deer | Red Deer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F df(x,y) | Estimate ± SE | p | F df(x,y) | Estimate ± SE | p | F df(x,y) | Estimate ± SE | p | |
| Sex 1 | 1.11 (1,800) | Female: −0.84 ± 1.06 | 0.29 | 1.93 (1,68) | Female: −8.35 ± 5.95 | 0.17 | 0.004 (1,246) | Female: −14.7 ± 210.89 | 0.95 |
| Age 2 | 0.48 (2,800) | 2 years: −0.59 ± 1.46 ≥3 years: −1.14 ± 1.25 | 0.62 | 0.55 (1,68) | 2 years: 7.05 ± 324.75 ≥3 years: 1.90 ± 324.80 | 0.46 | 0.09 (2,246) | 2 years: −175.87 ± 613.73 ≥3 years: −177.09 ± 613.73 | 0.91 |
| DW | 1.76 (1,800) | −0 ± 0 | 0.19 | 0.13 (1,68) | 0 ± 0 | 0.72 | 1.16 (1,246) | −0 ± 0 | 0.28 |
| DE | 1.14 (1,800) | −0 ± 0 | 0.29 | 0.81 (1,68) | 0 ± 0 | 0.37 | 0.007 (1,246) | 0 ± 0 | 0.93 |
| Open habitats | 1.76 (1,800) | −0.14 ± 0.1 | 0.19 | 0.08 (1,68) | 0.09 ± 0.31 | 0.78 | 0.557 (1,246) | 0.1 ± 0.14 | 0.48 |
| Watercourse vegetation | 2.86 (1,800) | −0.17 ± 0.1 | 0.09 | 0.10 (1,68) | −0.11 ± 0.34 | 0.75 | 0.001 (1,246) | 0.02 ± 0.15 | 0.91 |
| Previous rainfall | 0.38 (1,800) | −0 ± 0 | 0.54 | 0.69 (1,68) | −0.01 ± 0.01 | 0.41 | 0.102 (1,246) | −0.32 ± 1.02 | 0.75 |
| Red deer density | 0.60 (1,800) | 0 ± 0.04 | 0.44 | 0.04 (1,68) | 0.03 ± 0.13 | 0.84 | 0.095 (1,246) | −7.47 ± 23.16 | 0.76 |
| Fallow deer density | 0.26 (1,800) | −0.04 ± 0.08 | 0.61 | 0.60 (1,68) | 2.52 ± 2.21 | 0.44 | 0.447 (1,246) | −0.09 ± 0.08 | 0.27 |
| Wild boar density | 0.38 (1,800) | 0.28 ± 0.99 | 0.54 | 0.02 (1,68) | 0.09 ± 0.73 | 0.90 | 0.426 (1,246) | −0.20 ± 0.28 | 0.47 |
| Bovine Density | 0.31 (1,800) | −0.07 ± 0.13 | 0.58 | 0.26 (1,68) | 0.15 ± 0.29 | 0.61 | 0.044 (1,246) | 0.01 ± 0.11 | 0.60 |
| Red deer generalized prevalence of TBL | 0.06 (1,800) | −0.01 ± 0.02 | 0.80 | 0.50 (1,68) | −0.03 ± 0.04 | 0.49 | 6.13 (1,246) | 0.05 ± 0.02 | 0.01 |
| Fallow deer generalized prevalence of TBL | 2.72 (1,800) | −0.02 ± 0.01 | 0.10 | 6.05 (1,68) | 0.07 ± 0.03 | 0.02 | 0.15 (1,246) | −0 ± 0.01 | 0.70 |
| Wild boar generalized prevalence of TBL | 59.74 (1,800) | 0.07 ± 0.01 | <0.01 | 0.05 (1,68) | −0.01 ± 0.03 | 0.82 | 0.33 (1,246) | −0.01 ± 0.01 | 0.57 |
| Sex*age | 0.44 (2,800) | Female* 2 years: −0.51 ± 0.57 Female* ≥3 years: −0.17 ± 0.45 | 0.65 | 0.22 (2,68) | Female* ≥3 years: 0.80 ± 1.69 | 0.64 | 0.25 (2,246) | Female* 2 years: −14.85 ± 210.89 Female* ≥3 years: 15.68 ± 210.88 | 0.78 |
| Rainfall*sex | 0.27 (1,800) | Rainfall* Female: 0 ± 0 | 0.60 | 1.80 (1,68) | Rainfall* Female 0.02 ± 0.01 | 0.19 | 0.04 (1,246) | Rainfall* Female: −0 ± 0 | 0.84 |
| Density*sex | 4.13 (1,800) | Density* Female 0.37 ± 0.18 | 0.04 | 0.50 (1,68) | Density* Female −0.30 ± 0.42 | 0.48 | 1.14 (1,246) | Density* Female −0.07 ± 0.06 | 0.29 |
| Rainfall*age | 0.27 (2,800) | Rainfall* 2 years: 0 ± 0 Rainfall* ≥3 years: 0 ± 0 | 0.60 | 1.37 (2,68) | Rainfall* ≥3 years: 0.01 ± 0.01 | 0.25 | 0.17 (2,246) | Rainfall* 2 years: 0.33 ± 1.02 Rainfall*≥3 years: 0.33 ± 1.02 | 0.84 |
| Density*age | 0.55 (2,800) | Density* 2 years: 0.28 ± 0.27 Density* ≥3 years: 0.15 ± 0.22 | 0.58 | 2.89 (2,68) | Density* ≥3 years: −1.97 ± 1.16 | 0.09 | 0.06 (2,246) | Density* 2 years: 7.75 ± 23.2 Density* ≥3 years: 7.75 ± 23.2 | 0.94 |
| Rainfall*density species studied | 0.31 (1,800) | −0 ± 0 | 0.58 | 0.17 (1,68) | −0 ± 0 | 0.68 | 1.47 (1,246) | −0 ± 0 | 0.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso, P.; Barasona, J.A.; Acevedo, P.; Palencia, P.; Carro, F.; Negro, J.J.; Torres, M.J.; Gortázar, C.; Soriguer, R.C.; Vicente, J. Long-Term Determinants of Tuberculosis in the Ungulate Host Community of Doñana National Park. Pathogens 2020, 9, 445. https://doi.org/10.3390/pathogens9060445
Barroso P, Barasona JA, Acevedo P, Palencia P, Carro F, Negro JJ, Torres MJ, Gortázar C, Soriguer RC, Vicente J. Long-Term Determinants of Tuberculosis in the Ungulate Host Community of Doñana National Park. Pathogens. 2020; 9(6):445. https://doi.org/10.3390/pathogens9060445
Chicago/Turabian StyleBarroso, Patricia, José A. Barasona, Pelayo Acevedo, Pablo Palencia, Francisco Carro, Juan José Negro, María José Torres, Christian Gortázar, Ramón C. Soriguer, and Joaquín Vicente. 2020. "Long-Term Determinants of Tuberculosis in the Ungulate Host Community of Doñana National Park" Pathogens 9, no. 6: 445. https://doi.org/10.3390/pathogens9060445
APA StyleBarroso, P., Barasona, J. A., Acevedo, P., Palencia, P., Carro, F., Negro, J. J., Torres, M. J., Gortázar, C., Soriguer, R. C., & Vicente, J. (2020). Long-Term Determinants of Tuberculosis in the Ungulate Host Community of Doñana National Park. Pathogens, 9(6), 445. https://doi.org/10.3390/pathogens9060445







