Microsatellite Investigations of Multiple Echinococcus granulosus Sensu Stricto Cysts in Single Hosts Reveal Different Patterns of Infection Events between Livestock and Humans
Abstract
1. Introduction
2. Results
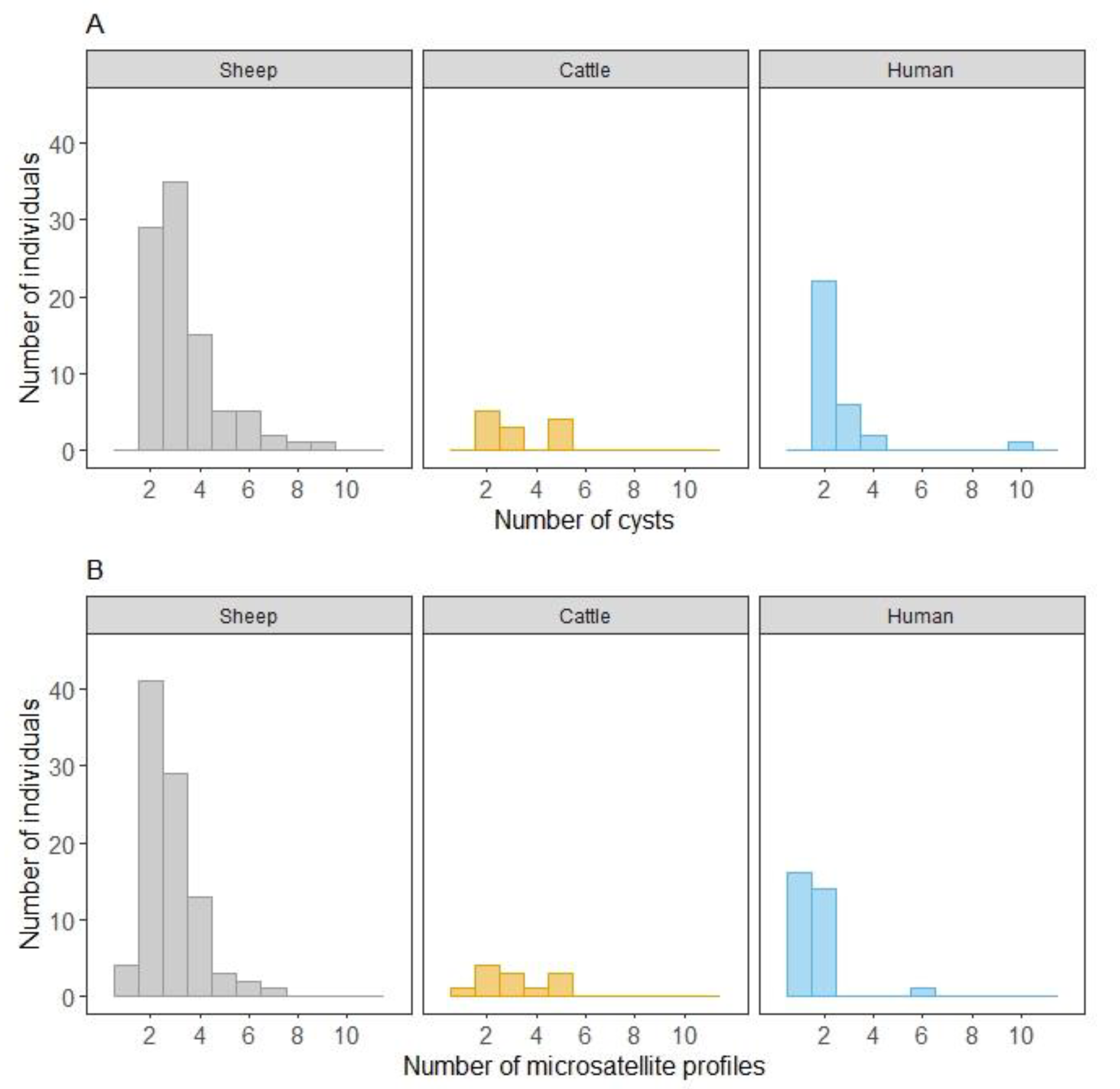
2.1. Number of Cysts and Organ Localization per Host Species
2.2. Microsatellite Genetic Diversity in a Single Host According to Host Species and Country
3. Discussion
4. Materials and Methods
4.1. Ethics Statements
4.2. Isolate Sampling
4.3. PCR Amplification, Microsatellite Fragment Size Analyses and Profile Interpretation
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casulli, A. Recognising the substantial burden of neglected pandemics cystic and alveolar echinococcosis. Lancet Glob. Health 2020, 8, e470–e471. [Google Scholar] [CrossRef]
- Chahed, M.K.; Bellali, H.; Touini, H.; Cherif, R.; Ben Safta, Z.; Essoussi, M.; Kilani, T. L’incidence chirurgicale du kyste hydatique en Tunisie: Résultats de l’enquête 2001–2005 et tendance évolutive entre 1977–2005. Arch. Inst. Pasteur Tunis 2010, 87, 43–52. [Google Scholar] [PubMed]
- WHO Regional Office for Europe; Copenhagen; Denmark. Centralized Information System for Infectious Diseases, Communicable Diseases, Surveillance and Response. Available online: http://data.euro.who.int/cisid/Default.aspx?TabID=405358 (accessed on 15 May 2020).
- Brundu, D.; Piseddu, T.; Stegel, G.; Masu, G.; Ledda, S.; Masala, G. Retrospective study of human cystic echinococcosis in Italy based on the analysis of hospital discharge records between 2001 and 2012. Acta Trop. 2014, 140, 91–96. [Google Scholar] [CrossRef] [PubMed]
- van Cauteren, D.; Millon, L.; de Valk, H.; Grenouillet, F. Retrospective study of human cystic echinococcosis over the past decade in France, using a nationwide hospital medical information database. Parasitol. Res. 2016, 115, 4261–4265. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef] [PubMed]
- Huttner, M.; Nakao, M.; Wassermann, T.; Siefert, L.; Boomker, J.D.; Dinkel, A.; Sako, Y.; Mackenstedt, U.; Romig, T.; Ito, A. Genetic characterization and phylogenetic position of Echinococcus felidis (Cestoda: Taeniidae) from the African lion. Int. J. Parasitol. 2008, 38, 861–868. [Google Scholar] [CrossRef]
- Nakao, M.; McManus, D.P.; Schantz, P.M.; Craig, P.S.; Ito, A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology 2007, 134, 713–722. [Google Scholar] [CrossRef]
- Nakao, M.; Li, T.; Han, X.; Ma, X.; Xiao, N.; Qiu, J.; Wang, H.; Yanagida, T.; Mamuti, W.; Wen, H.; et al. Genetic polymorphisms of Echinococcus tapeworms in China as determined by mitochondrial and nuclear DNA sequences. Int. J. Parasitol. 2010, 40, 379–385. [Google Scholar] [CrossRef]
- Romig, T.; Ebi, D.; Wassermann, M. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet. Parasitol. 2015, 213, 76–84. [Google Scholar] [CrossRef]
- Alvarez Rojas, C.A.; Romig, T.; Lightowlers, M.W. Echinococcus granulosus sensu lato genotypes infecting humans—Review of current knowledge. Int. J. Parasitol. 2014, 44, 9–18. [Google Scholar] [CrossRef]
- Cardona, G.A.; Carmena, D. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Vet. Parasitol. 2013, 192, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Chapter Six—Global Distribution of Alveolar and Cystic Echinococcosis. In Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 95, pp. 315–493. [Google Scholar]
- Casulli, A.; Interisano, M.; Sreter, T.; Chitimia, L.; Kirkova, Z.; La Rosa, G.; Pozio, E. Genetic variability of Echinococcus granulosus sensu stricto in Europe inferred by mitochondrial DNA sequences. Infect. Genet. Evol. 2012, 12, 377–383. [Google Scholar] [CrossRef]
- Vural, G.; Baca, A.U.; Gauci, C.G.; Bagci, O.; Gicik, Y.; Lightowlers, M.W. Variability in the Echinococcus granulosus cytochrome C oxidase 1 mitochondrial gene sequence from livestock in Turkey and a re-appraisal of the G1-3 genotype cluster. Vet. Parasitol. 2008, 154, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Boufana, B.; Lahmar, S.; Rebai, W.; Ben Safta, Z.; Jebabli, L.; Ammar, A.; Kachti, M.; Aouadi, S.; Craig, P.S. Genetic variability and haplotypes of Echinococcus isolates from Tunisia. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 706–714. [Google Scholar] [CrossRef]
- Debeljak, Z.; Boufana, B.; Interisano, M.; Vidanovic, D.; Kulisic, Z.; Casulli, A. First insights into the genetic diversity of Echinococcus granulosus sensu stricto (s.s.) in Serbia. Vet. Parasitol. 2016, 223, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kinkar, L.; Laurimae, T.; Acosta-Jamett, G.; Andresiuk, V.; Balkaya, I.; Casulli, A.; Gasser, R.B.; van der Giessen, J.; Gonzalez, L.M.; Haag, K.L.; et al. Global phylogeography and genetic diversity of the zoonotic tapeworm Echinococcus granulosus sensu stricto genotype G1. Int. J. Parasitol. 2018, 48, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Laatamna, A.E.; Ebi, D.; Brahimi, K.; Bediaf, K.; Wassermann, M.; Souttou, K.; Romig, T. Frequency and genetic diversity of Echinococcus granulosus sensu stricto in sheep and cattle from the steppe region of Djelfa, Algeria. Parasitol. Res. 2019, 118, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Laurimae, T.; Kinkar, L.; Andresiuk, V.; Haag, K.L.; Ponce-Gordo, F.; Acosta-Jamett, G.; Garate, T.; Gonzalez, L.M.; Saarma, U. Genetic diversity and phylogeography of highly zoonotic Echinococcus granulosus genotype G1 in the Americas (Argentina, Brazil, Chile and Mexico) based on 8279 bp of mtDNA. Infect. Genet. Evol. 2016, 45, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, T.; Mohammadzadeh, T.; Kamhawi, S.; Nakao, M.; Sadjjadi, S.M.; Hijjawi, N.; Abdel-Hafez, S.K.; Sako, Y.; Okamoto, M.; Ito, A. Genetic polymorphisms of Echinococcus granulosus sensu stricto in the Middle East. Parasitol. Int. 2012, 61, 599–603. [Google Scholar] [CrossRef]
- Díaz, Á.; Fernández, C.; Pittini, Á.; Seoane, P.I.; Allen, J.E.; Casaravilla, C. The laminated layer: Recent advances and insights into Echinococcus biology and evolution. Exp. Parasitol. 2015, 158, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Lymbery, A.J. and Thompson, R.C.A. Genetic differences between cysts of Echinococcus granulosus from the same host. Int. J. Parasitol. 1989, 19, 961–964. [Google Scholar] [CrossRef]
- Almeida, F.B.; Rodrigues-Silva, R.; Neves, R.H.; Romani, E.L.S.; Machado-Silva, J.R. Intraspecific variation of Echinococcus granulosus in livestock from Peru. Vet. Parasitol. 2007, 143, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Oudni-M’rad, M.; Cabaret, J.; M’rad, S.; Chaâbane-Banaoues, R.; Mekki, M.; Zmantar, S.; Nouri, A.; Mezhoud, H.; Babba, H. Genetic relationship between the Echinococcus granulosus sensu stricto cysts located in lung and liver of hosts. Infect. Genet. Evol. 2016, 44, 356–360. [Google Scholar] [CrossRef]
- M’rad, S.; Oudni-M’rad, M.; Boubaker, G.; Bouazzi, L.; Gorcii, M.; Nouri, A.; Mezhoud, H.; Babba, H. Retrospective study of the distribution and the fertility of hydatid cysts in the child in Tunisia. Pathol. Biol. 2012, 60, 166–169. [Google Scholar] [CrossRef]
- Oudni-M’rad, M.; M’rad, S.; Babba, H. Molecular and Epidemiology Data on Cystic Echinococcosis in Tunisia. In Current Topics in Echinococcosis; InTech: London, UK, 2015; pp. 56–74. [Google Scholar]
- Umhang, G.; Richomme, C.; Bastid, V.; Boucher, J.M.; de Garam, C.P.; Itie-Hafez, S.; Danan, C.; Boue, F. National survey and molecular diagnosis of Echinococcus granulosus sensu lato in livestock in France, 2012. Parasitology 2020, 147, 667–672. [Google Scholar] [CrossRef]
- Umhang, G.; Chihai, O.; Boue, F. Molecular characterization of Echinococcus granulosus in a hyperendemic European focus, the Republic of Moldova. Parasitol. Res. 2014, 113, 4371–4376. [Google Scholar] [CrossRef]
- Umhang, G.; Richomme, C.; Boucher, J.M.; Hormaz, V.; Boue, F. Prevalence survey and first molecular characterization of Echinococcus granulosus in France. Parasitol. Res. 2013, 112, 1809–1812. [Google Scholar] [CrossRef]
- Hidalgo, C.; Stoore, C. Multiple haplotypes of Echinococcus granulosus sensu stricto in single naturally infected intermediate hosts. Parasitol. Res. 2019, 119, 763–770. [Google Scholar] [CrossRef]
- Valot, B.; Knapp, J.; Umhang, G.; Grenouillet, F.; Millon, L. Genomic characterization of EmsB microsatellite loci in Echinococcus multilocularis. Infect. Genet. Evol. 2015, 32, 338–341. [Google Scholar] [CrossRef]
- Knapp, J.; Damy, S.; Brillaud, J.; Tissot, J.D.; Navion, J.; Melior, R.; Afonso, E.; Hormaz, V.; Gottstein, B.; Umhang, G.; et al. EWET: Data collection and interface for the genetic analysis of Echinococcus multilocularis based on EmsB microsatellite. PLoS ONE 2017, 12, e0183849. [Google Scholar] [CrossRef] [PubMed]
- Umhang, G.; Grenouillet, F.; Bastid, V.; M’rad, S.; Valot, B.; Oudni-M’rad, M.; Babba, H.; Boué, F. Investigating the genetic diversity of Echinococcus granulosus sensu stricto with new microsatellites. Parasitol. Res. 2018, 117, 2743–2755. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.; Menezes da Silva, A.; Akhan, O.; Müllhaupt, B.; Vizcaychipi, K.A.; Budke, C.; Vuitton, D.A. Chapter Four—The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. In Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 96, pp. 259–369. [Google Scholar]
- Chaâbane-Banaoues, R.; Oudni-M’rad, M.; Cabaret, J.; M’rad, S.; Mezhoud, H.; Babba, H. Infection of dogs with Echinococcus granulosus: Causes and consequences in an hyperendemic area. Parasites Vectors 2015, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Oudni-M’rad, M.; Chaabane-Banaoues, R.; M’rad, S.; Trifa, F.; Mezhoud, H.; Babba, H. Gastrointestinal parasites of canids, a latent risk to human health in Tunisia. Parasites Vectors 2017, 10, 280. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Deplazes, P.; Casulli, A. Reinventing the Wheel of Echinococcus granulosus sensu lato Transmission to Humans. Trends. Parasitol. 2020, 36, 427–434. [Google Scholar] [CrossRef]
- Velasco-Tirado, V.; Romero-Alegría, Á.; Belhassen-García, M.; Alonso-Sardón, M.; Esteban-Velasco, C.; López-Bernús, A.; Carpio-Perez, A.; Jimenez López, M.F.; Muñoz Bellido, J.L.; Muro, A.; et al. Recurrence of cystic echinococcosis in an endemic area: A retrospective study. BMC Infect. Dis. 2017, 17, 455. [Google Scholar] [CrossRef]
- Abdelraouf, A.; El-Aal, A.A.A.; Shoeib, E.Y.; Attia, S.S.; Hanafy, N.A.; Hassani, M.; Shoman, S. Clinical and serological outcomes with different surgical approaches for human hepatic hydatidosis. Rev. Soc. Bras. Med. Trop. 2015, 48, 587–593. [Google Scholar] [CrossRef]
- El Malki, H.O.; El Mejdoubi, Y.; Souadka, A.; Zakri, B.; Mohsine, R.; Ifrine, L.; Abouqal, R.; Belkouchi, A. Does primary surgical management of liver hydatid cyst influence recurrence? J. Gastrointest. Surg. 2010, 14, 1121–1127. [Google Scholar] [CrossRef]
- Dinkel, A.; Njoroge, E.M.; Zimmermann, A.; Walz, M.; Zeyhle, E.; Elmahdi, I.E.; Mackenstedt, U.; Romig, T. A PCR system for detection of species and genotypes of the Echinococcus granulosus-complex, with reference to the epidemiological situation in eastern Africa. Int. J. Parasitol. 2004, 34, 645–653. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial sequencing. Mol. Biochem. Parasit. 1992, 54, 165–173. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning. A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; Volume 3. [Google Scholar]
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 15 May 2020).
| Country of Origin | Host Species | Number of Cysts per Host | Number of Hosts | Number of Hosts Ranked by Number of Microsatellite Profiles Observed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||
| France | sheep | 2 | 15 | 15 | ||||||
| 3 | 15 | 7 | 8 | |||||||
| 4 | 7 | 1 | 4 | 2 | ||||||
| 5 | 1 | 1 | ||||||||
| 6 | 3 | 1 | 2 | |||||||
| 7 | 1 | 1 | ||||||||
| Tunisia | sheep | 2 | 14 | 2 | 12 | |||||
| 3 | 20 | 1 | 6 | 13 | ||||||
| 4 | 8 | 2 | 6 | |||||||
| 5 | 4 | 1 | 1 | 2 | ||||||
| 6 | 2 | 1 | 1 | |||||||
| 7 | 1 | 1 | ||||||||
| 8 | 1 | 1 | ||||||||
| 9 | 1 | 1 | ||||||||
| Tunisia | cattle | 2 | 5 | 1 | 4 | |||||
| 3 | 3 | 3 | ||||||||
| 5 | 4 | 1 | 3 | |||||||
| Tunisia | humans | 2 | 22 | 10 | 12 | |||||
| 3 | 6 | 4 | 2 | |||||||
| 4 | 2 | 2 | ||||||||
| 10 | 1 | 1 | ||||||||
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Species (Ref = France) | |||
| Tunisia | 0.5 | 0.2–1.4 | 0.2 |
| No. Cysts | 2.6 | 1.7–4.4 | <0.001 |
| Variables | Probabilities for a Host to Have at Least Two Cysts with | |||||
|---|---|---|---|---|---|---|
| Similar Microsatellite Profiles | Different Microsatellite Profiles | |||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Species (Ref = Sheep) | ||||||
| Human | 8.8 | 3.2–26.1 | <0.001 | 0 | 0–0.1 | <0.001 |
| No. Cysts | 2.7 | 1.8–4.6 | <0.001 | 1.1 | 0.7–1.7 | 0.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’rad, S.; Oudni-M’rad, M.; Bastid, V.; Bournez, L.; Mosbahi, S.; Nouri, A.; Babba, H.; Grenouillet, F.; Boué, F.; Umhang, G. Microsatellite Investigations of Multiple Echinococcus granulosus Sensu Stricto Cysts in Single Hosts Reveal Different Patterns of Infection Events between Livestock and Humans. Pathogens 2020, 9, 444. https://doi.org/10.3390/pathogens9060444
M’rad S, Oudni-M’rad M, Bastid V, Bournez L, Mosbahi S, Nouri A, Babba H, Grenouillet F, Boué F, Umhang G. Microsatellite Investigations of Multiple Echinococcus granulosus Sensu Stricto Cysts in Single Hosts Reveal Different Patterns of Infection Events between Livestock and Humans. Pathogens. 2020; 9(6):444. https://doi.org/10.3390/pathogens9060444
Chicago/Turabian StyleM’rad, Selim, Myriam Oudni-M’rad, Vanessa Bastid, Laure Bournez, Sana Mosbahi, Abdelallatif Nouri, Hamouda Babba, Frédéric Grenouillet, Franck Boué, and Gérald Umhang. 2020. "Microsatellite Investigations of Multiple Echinococcus granulosus Sensu Stricto Cysts in Single Hosts Reveal Different Patterns of Infection Events between Livestock and Humans" Pathogens 9, no. 6: 444. https://doi.org/10.3390/pathogens9060444
APA StyleM’rad, S., Oudni-M’rad, M., Bastid, V., Bournez, L., Mosbahi, S., Nouri, A., Babba, H., Grenouillet, F., Boué, F., & Umhang, G. (2020). Microsatellite Investigations of Multiple Echinococcus granulosus Sensu Stricto Cysts in Single Hosts Reveal Different Patterns of Infection Events between Livestock and Humans. Pathogens, 9(6), 444. https://doi.org/10.3390/pathogens9060444





