Serodynamic Analysis of the Piglets Born from Sows Vaccinated with Modified Live Vaccine or E2 Subunit Vaccine for Classical Swine Fever
Abstract
:1. Introduction
2. Results
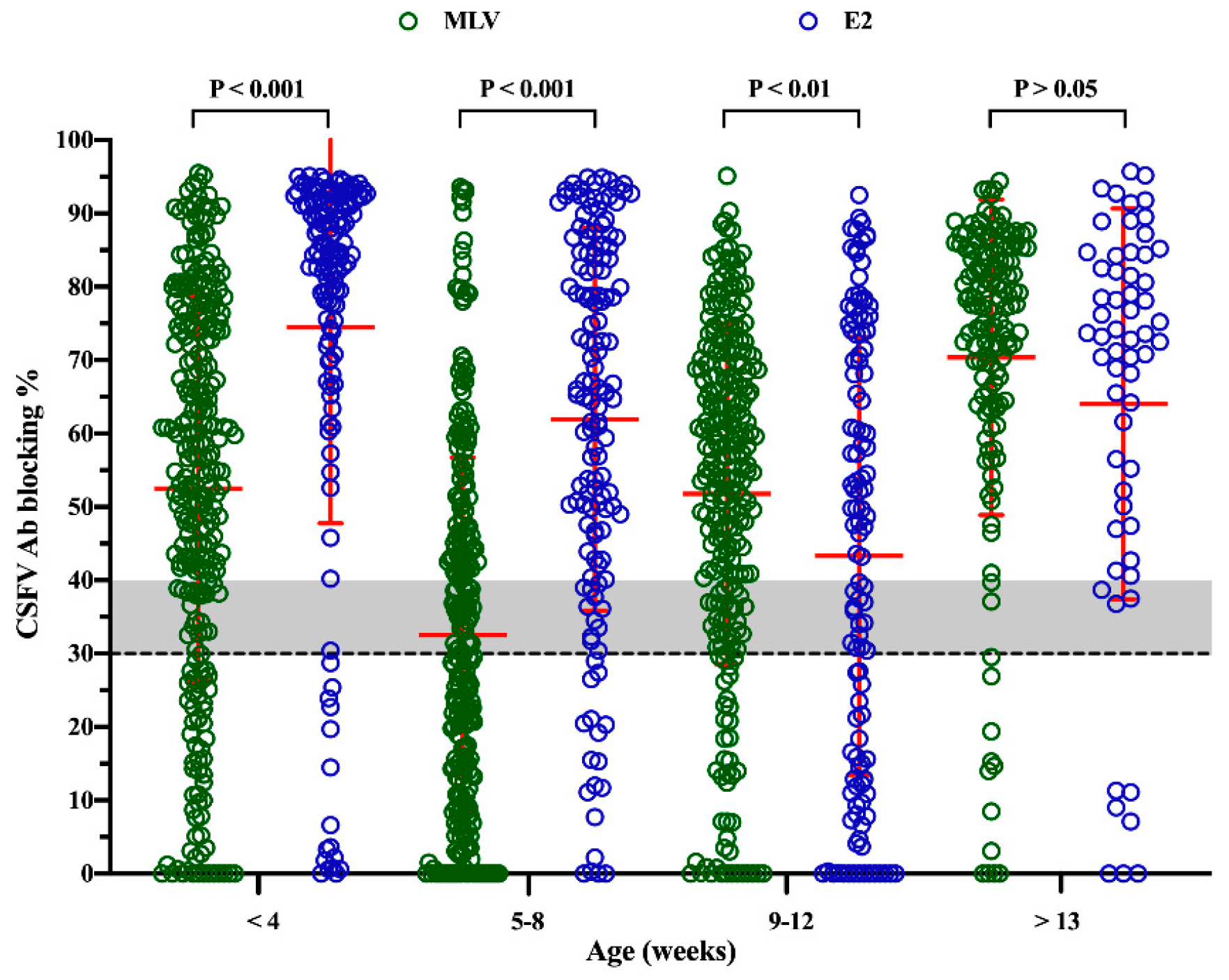
2.1. Levels of CSFV Antibody in Pigs of Different Ages from Sows Immunized with Different Types of CSFV Vaccines
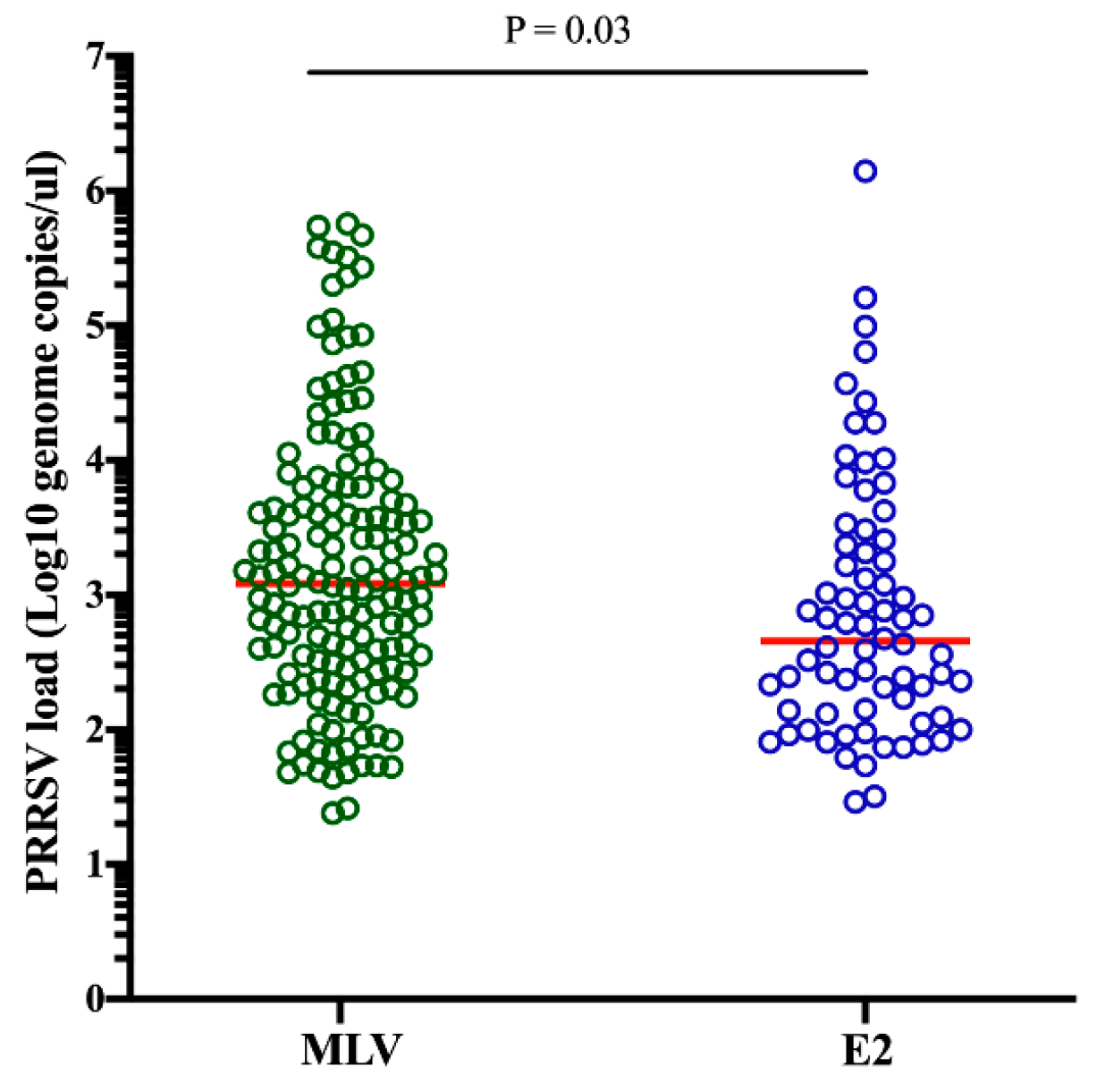
2.2. Correlation of the PRRSV Load and Level of CSFV MDA in the Piglets without CSFV Vaccination from Different Groups
2.3. Viral Load and Detection Rate of PRRSV in the Piglets without CSFV Vaccination from Different Groups
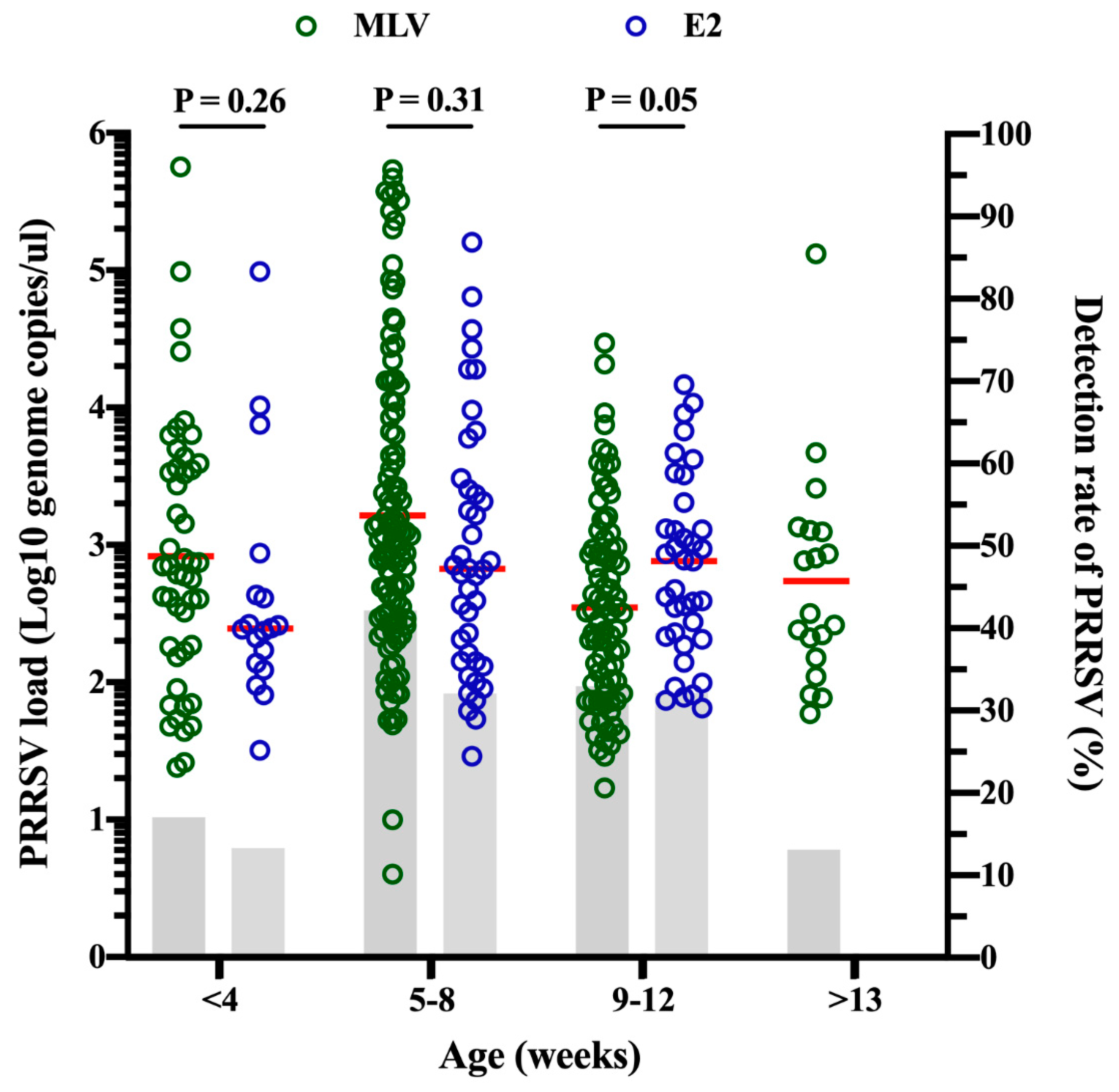
2.4. Viral Load and Detection Rate of PRRSV in Different Age Groups
3. Discussion
4. Materials and Methods
4.1. Sample Source and Processing
4.2. Sample Preparation and PRRSV Real-Time PCR
4.3. Serologic Assessment
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval and Consent to Participate
References
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Muerhoff, A.S.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P.; et al. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef]
- Ma, S.M.; Mao, Q.; Yi, L.; Zhao, M.Q.; Chen, J.D. Apoptosis, Autophagy, and Pyroptosis: Immune Escape Strategies for Persistent Infection and Pathogenesis of Classical Swine Fever Virus. Pathogens 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohorquez, J.A.; Munoz-Gonzalez, S.; Perez-Simo, M.; Munoz, I.; Rosell, R.; Coronado, L.; Domingo, M.; Ganges, L. Foetal Immune Response Activation and High Replication Rate during Generation of Classical Swine Fever Congenital Infection. Pathogens 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever-An Updated Review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Moennig, V.; Floegel-Niesmann, G.; Greiser-Wilke, I. Clinical signs and epidemiology of classical swine fever: A review of new knowledge. Vet. J. 2003, 165, 11–20. [Google Scholar] [CrossRef]
- Postel, A.; Austermann-Busch, S.; Petrov, A.; Moennig, V.; Becher, P. Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 248–261. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.H.; Kaewprom, K.; Wang, S.Y.; Lin, C.F.; Yang, C.Y.; Chiou, M.T.; Lin, C.N. Outbreak of Porcine Reproductive and Respiratory Syndrome Virus 1 in Taiwan. Viruses 2020, 12, 316. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.H.; Shih, H.C.; Wang, S.Y.; Lin, C.F.; Yang, C.Y.; Chiou, M.T.; Lin, C.N. Emergence of a virulent porcine reproductive and respiratory syndrome virus in Taiwan in 2018. Transbound. Emerg. Dis. 2019, 66, 1138–1141. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Alekseev, K.; Jung, K.; Fang, Y.; Saif, L.J. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral. Immunol. 2010, 23, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Albina, E.; Carrat, C.; Charley, B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interferon Cytokine Res. 1998, 18, 485–490. [Google Scholar] [CrossRef]
- Dwivedi, V.; Manickam, C.; Patterson, R.; Dodson, K.; Murtaugh, M.; Torrelles, J.B.; Schlesinger, L.S.; Renukaradhya, G.J. Cross-protective immunity to porcine reproductive and respiratory syndrome virus by intranasal delivery of a live virus vaccine with a potent adjuvant. Vaccine 2011, 29, 4058–4066. [Google Scholar] [CrossRef]
- Suradhat, S.; Thanawongnuwech, R.; Poovorawan, Y. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2003, 84, 453–459. [Google Scholar] [CrossRef]
- Huang, Y.L.; Deng, M.C.; Wang, F.I.; Huang, C.C.; Chang, C.Y. The challenges of classical swine fever control: Modified live and E2 subunit vaccines. Virus Res. 2014, 179, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.L.; Tsai, K.J.; Deng, M.C.; Liu, H.M.; Huang, C.C.; Wang, F.I.; Chang, C.Y. In Vivo Demonstration of the Superior Replication and Infectivity of Genotype 2.1 with Respect to Genotype 3.4 of Classical Swine Fever Virus by Dual Infections. Pathogens 2020, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Suradhat, S.; Damrongwatanapokin, S. The influence of maternal immunity on the efficacy of a classical swine fever vaccine against classical swine fever virus, genogroup 2.2, infection. Vet. Microbiol. 2003, 92, 187–194. [Google Scholar] [CrossRef]
- Suradhat, S.; Damrongwatanapokin, S.; Thanawongnuwech, R. Factors critical for successful vaccination against classical swine fever in endemic areas. Vet. Microbiol. 2007, 119, 1–9. [Google Scholar] [CrossRef]
- van Oirschot, J.T. Vaccinology of classical swine fever: From lab to field. Vet. Microbiol. 2003, 96, 367–384. [Google Scholar] [CrossRef]
- Vandeputte, J.; Too, H.L.; Ng, F.K.; Chen, C.; Chai, K.K.; Liao, G.A. Adsorption of colostral antibodies against classical swine fever, persistence of maternal antibodies, and effect on response to vaccination in baby pigs. Am. J. Vet. Res. 2001, 62, 1805–1811. [Google Scholar] [CrossRef]
- Blome, S.; Moss, C.; Reimann, I.; Konig, P.; Beer, M. Classical swine fever vaccines-State-of-the-art. Vet. Microbiol. 2017, 206, 10–20. [Google Scholar] [CrossRef]
- Dewulf, J.; Laevens, H.; Koenen, F.; Vanderhallen, H.; Mintiens, K.; Deluyker, H.; de Kruif, A. An experimental infection with classical swine fever in E2 sub-unit marker-vaccine vaccinated and in non-vaccinated pigs. Vaccine 2000, 19, 475–482. [Google Scholar] [CrossRef]
- Floegel-Niesmann, G. Classical swine fever (CSF) marker vaccine. Trial III. Evaluation of discriminatory ELISAs. Vet. Microbiol. 2001, 83, 121–136. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Truong, D.A.; Ly, V.D.; Vu, H.T.; Hoang, T.V.; Nguyen, C.T.; Chu, N.T.; Nguyen, V.T.; Nguyen, D.T.; Miyazawa, K.; et al. The potential efficacy of the E2-subunit vaccine to protect pigs against different genotypes of classical swine fever virus circulating in Vietnam. Clin. Exp. Vaccine Res. 2020, 9, 26–39. [Google Scholar] [CrossRef]
- Klinkenberg, D.; Moormann, R.J.; de Smit, A.J.; Bouma, A.; de Jong, M.C. Influence of maternal antibodies on efficacy of a subunit vaccine: Transmission of classical swine fever virus between pigs vaccinated at 2 weeks of age. Vaccine 2002, 20, 3005–3013. [Google Scholar] [CrossRef]
- Kavanova, L.; Matiaskova, K.; Leva, L.; Nedbalcova, K.; Matiasovic, J.; Faldyna, M.; Salat, J. Concurrent infection of monocyte-derived macrophages with porcine reproductive and respiratory syndrome virus and Haemophilus parasuis: A role of IFNalpha in pathogenesis of co-infections. Vet. Microbiol. 2018, 225, 64–71. [Google Scholar] [CrossRef]
- Tsai, K.Y. Classical Swine Fever E2 Subunit Marker Vaccine: Field Trial and Assessment of Protective Efficacy. Master’s Thesis, National Chung Hsing University, Taichung, Taiwan, 2002. [Google Scholar]
- Lipowski, A.; Drexler, C.; Pejsak, Z. Safety and efficacy of a classical swine fever subunit vaccine in pregnant sows and their offspring. Vet. Microbiol. 2000, 77, 99–108. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lai, Y.C.; Wang, H.C.; Chien, M.S.; Lee, W.C. Monitoring maternal antibody from sows boosted with classical swine fever E2 subunit vaccine. In Proceedings of the 6th Asian Pig Veterinary Society Congress, Ho Chi Minh City, Vietnam, 23–25 September 2013. [Google Scholar]
- Li, H.; Yang, H. Infection of porcine reproductive and respiratory syndrome virus suppresses the antibody response to classical swine fever virus vaccination. Vet. Microbiol. 2003, 95, 295–301. [Google Scholar] [CrossRef]
- Wang, X.; Mu, G.; Dang, R.; Yang, Z. Up-regulation of IL-10 upon PRRSV vaccination impacts on the immune response against CSFV. Vet. Microbiol. 2016, 197, 68–71. [Google Scholar] [CrossRef]
- Suradhat, S.; Kesdangsakonwut, S.; Sada, W.; Buranapraditkun, S.; Wongsawang, S.; Thanawongnuwech, R. Negative impact of porcine reproductive and respiratory syndrome virus infection on the efficacy of classical swine fever vaccine. Vaccine 2006, 24, 2634–2642. [Google Scholar] [CrossRef]
- Choe, S.; Kim, J.H.; Kim, K.S.; Song, S.; Cha, R.M.; Kang, W.C.; Kim, H.J.; Park, G.N.; Shin, J.; Jo, H.N.; et al. Adverse Effects of Classical Swine Fever Virus LOM Vaccine and Jeju LOM Strains in Pregnant Sows and Specific Pathogen-Free Pigs. Pathogens 2019, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Choe, S.; Le, V.P.; Shin, J.; Kim, J.H.; Kim, K.S.; Song, S.; Cha, R.M.; Park, G.N.; Nguyen, T.L.; Hyun, B.H.; et al. Pathogenicity and Genetic Characterization of Vietnamese Classical Swine Fever Virus: 2014-2018. Pathogens 2020, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Wu, C.M.; Liao, C.M.; Chen, K.C.; You, C.C.; Wang, Y.W.; Huang, C.; Chien, M.S. The impact of porcine circovirus associated diseases on live attenuated classical swine fever vaccine in field farm applications. Vaccine 2019, 37, 6535–6542. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Pang, V.F.; Deng, M.C.; Chang, C.Y.; Jeng, C.R. Porcine circovirus type 2 decreases the infection and replication of attenuated classical swine fever virus in porcine alveolar macrophages. Res. Vet. Sci. 2014, 96, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Pang, V.F.; Lin, C.M.; Tsai, Y.C.; Chia, M.Y.; Deng, M.C.; Chang, C.Y.; Jeng, C.R. Porcine circovirus type 2 (PCV2) infection decreases the efficacy of an attenuated classical swine fever virus (CSFV) vaccine. Vet. Res. 2011, 42, 115. [Google Scholar] [CrossRef] [Green Version]
- Chinsakchai, S.; Molitor, T.W. Immunobiology of pseudorabies virus infection in swine. Vet. Immunol. Immunopathol. 1994, 43, 107–116. [Google Scholar] [CrossRef]
- Lin, W.H.; Shih, H.C.; Lin, C.F.; Yang, C.Y.; Lin, C.N.; Chiou, M.T. Genotypic analyses and virulence characterization of Glaesserella parasuis isolates from Taiwan. PeerJ 2019, 7, e6960. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.I.; Jeoung, H.Y.; Kim, B.; Song, J.Y.; Kim, J.; Kim, H.Y.; Cho, I.S.; Woo, G.H.; Lee, J.B.; An, D.J. Impact of porcine reproductive and respiratory syndrome virus and porcine circovirus-2 infection on the potency of the classical swine fever vaccine (LOM strain). Vet. Microbiol. 2016, 193, 36–41. [Google Scholar] [CrossRef]
- Chiou, M.T.; Su, P.C.; Chuang, M.S.; Lin, C.N. Porcine circovirus type 2 infection status in sick or moribund pigs in Taiwan. Taiwan Vet. J. 2004, 30, 163–168. [Google Scholar]
- Yang, C.Y.; Chang, T.C.; Lin, C.N.; Tsai, C.P.; Chiou, M.T. Study of infectious agents involved in porcine respiratory disease complex in Taiwan. Taiwan Vet. J. 2007, 33, 40–46. [Google Scholar]
- Tsai, G.T.; Lin, Y.C.; Lin, W.H.; Lin, J.H.; Chiou, M.T.; Liu, H.F.; Lin, C.N. Phylogeographic and genetic characterization of porcine circovirus type 2 in Taiwan from 2001–2017. Sci. Rep. 2019, 9, 10782. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C. Mornitoring Wild Classical Swine Fever Virus Persistent Infection and Antibody Responses of Different Vaccination Programs in Taiwan. Master’s Thesis, National Chung Hsing University, Taichung, Taiwan, 2014. [Google Scholar]
- Lin, C.N.; Lin, W.H.; Hung, L.N.; Wang, S.Y.; Chiou, M.T. Comparison of viremia of type II porcine reproductive and respiratory syndrome virus in naturally infected pigs by zip nucleic acid probe-based real-time PCR. BMC Vet. Res. 2013, 9, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Age (Weeks) | CSFV Vaccine Type | Total | P Value † | |
|---|---|---|---|---|
| MLV | E2 | |||
| <4 | 47/276 (17.03) | 18/135 (13.33) | 65/411 (15.82) | 0.412 |
| 5–8 | 110/261 (42.15) | 42/141 (29.79) | 152/402 (37.81) | 0.019 |
| 9–12 | 86/261 (32.95) | 37/119 (31.09) | 123/380 (32.37) | 0.081 |
| >13 | 19/145 (13.10) | 0/60 (0) | 19/205 (9.27) | N/A * |
| Total | 262/943 (27.78) | 97/455 (21.32) | 359/1398 (25.68) | 0.011 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-C.; Chiou, M.-T.; Lin, C.-N. Serodynamic Analysis of the Piglets Born from Sows Vaccinated with Modified Live Vaccine or E2 Subunit Vaccine for Classical Swine Fever. Pathogens 2020, 9, 427. https://doi.org/10.3390/pathogens9060427
Li Y-C, Chiou M-T, Lin C-N. Serodynamic Analysis of the Piglets Born from Sows Vaccinated with Modified Live Vaccine or E2 Subunit Vaccine for Classical Swine Fever. Pathogens. 2020; 9(6):427. https://doi.org/10.3390/pathogens9060427
Chicago/Turabian StyleLi, Yi-Chia, Ming-Tang Chiou, and Chao-Nan Lin. 2020. "Serodynamic Analysis of the Piglets Born from Sows Vaccinated with Modified Live Vaccine or E2 Subunit Vaccine for Classical Swine Fever" Pathogens 9, no. 6: 427. https://doi.org/10.3390/pathogens9060427
APA StyleLi, Y.-C., Chiou, M.-T., & Lin, C.-N. (2020). Serodynamic Analysis of the Piglets Born from Sows Vaccinated with Modified Live Vaccine or E2 Subunit Vaccine for Classical Swine Fever. Pathogens, 9(6), 427. https://doi.org/10.3390/pathogens9060427






