Fighting Fusarium Pathogens in the Era of Climate Change: A Conceptual Approach
Abstract
1. Introduction
2. FHB Counteracting and Preventive Measures
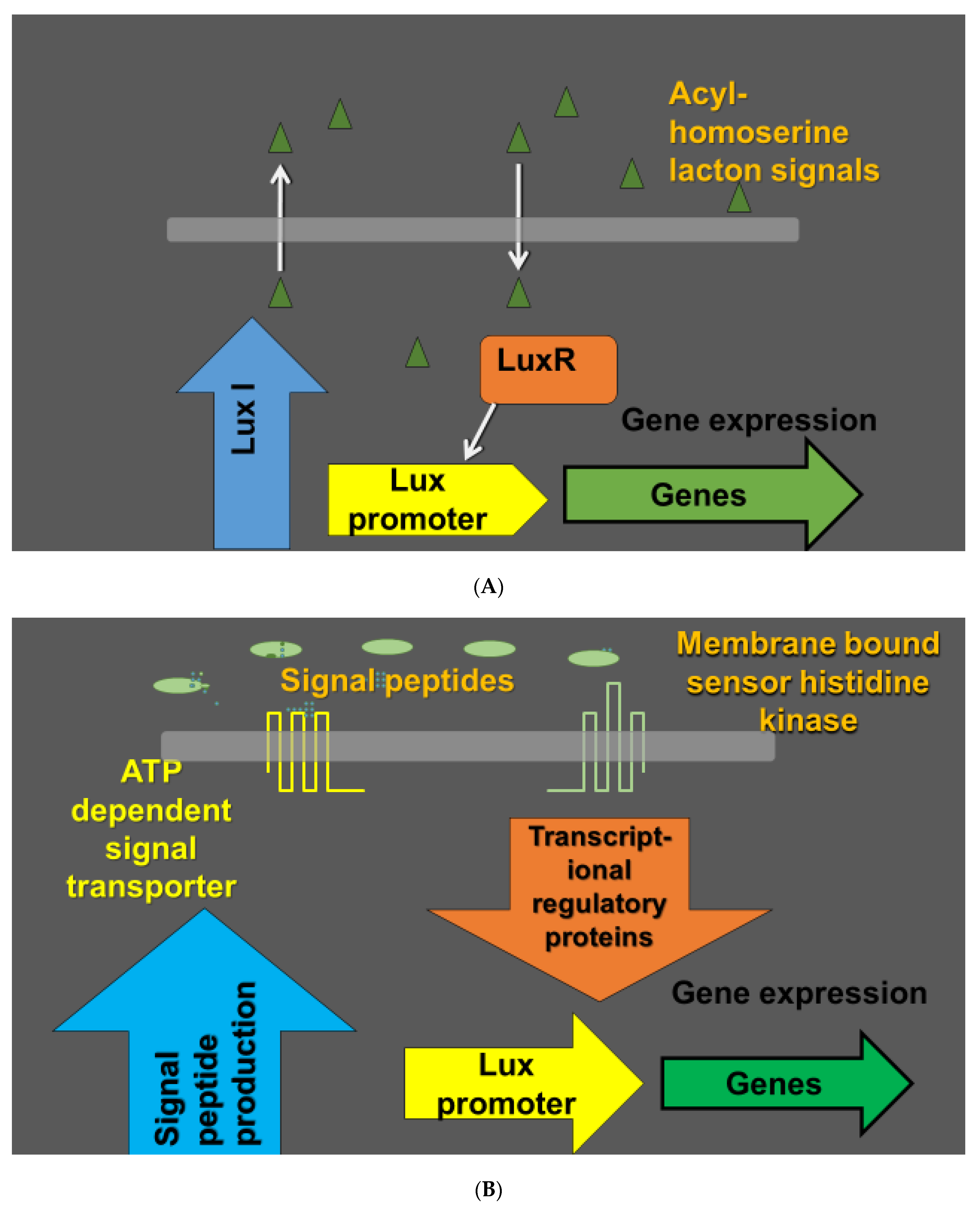
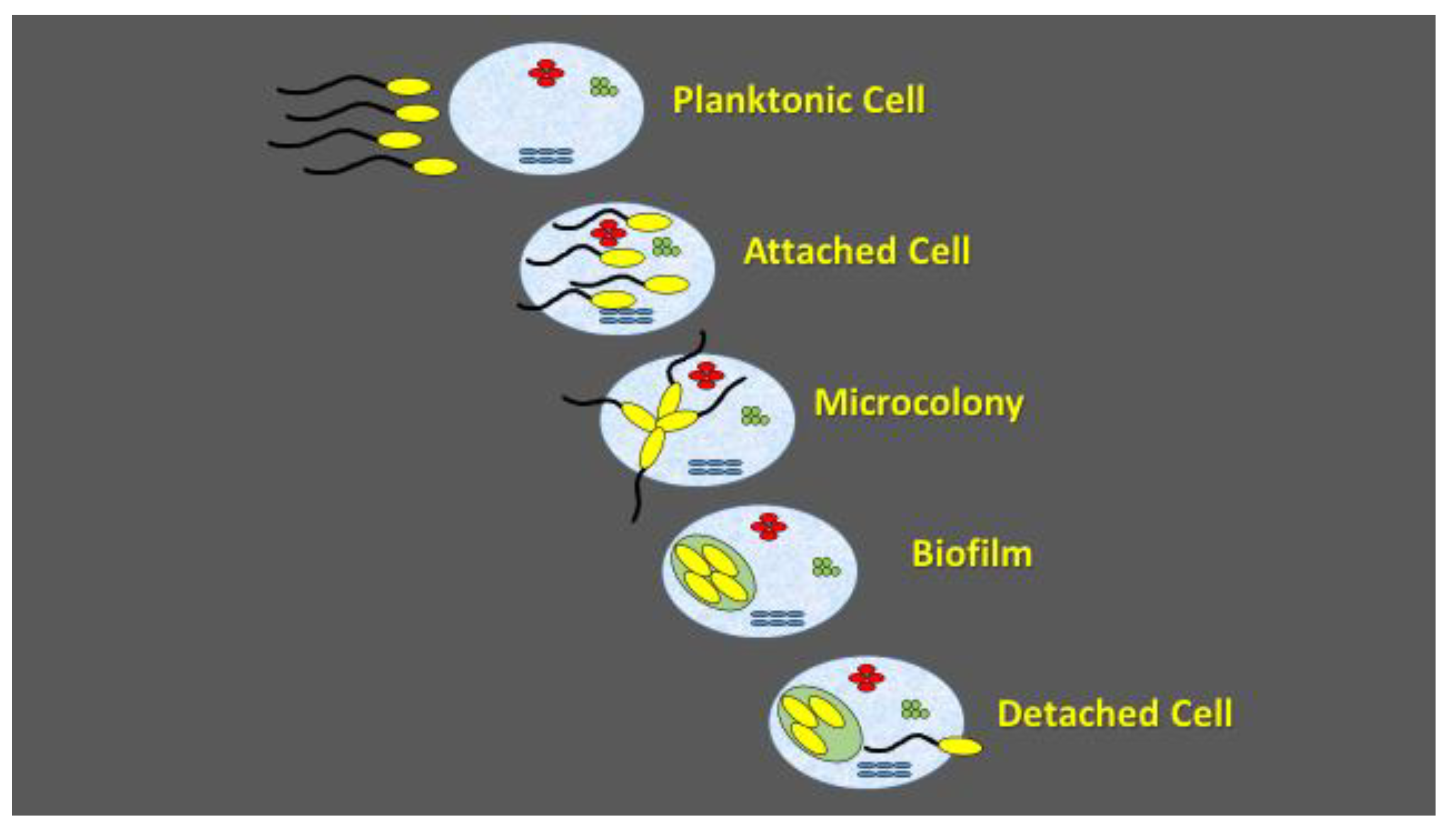
3. Biocontrol
4. The Phyllosphere
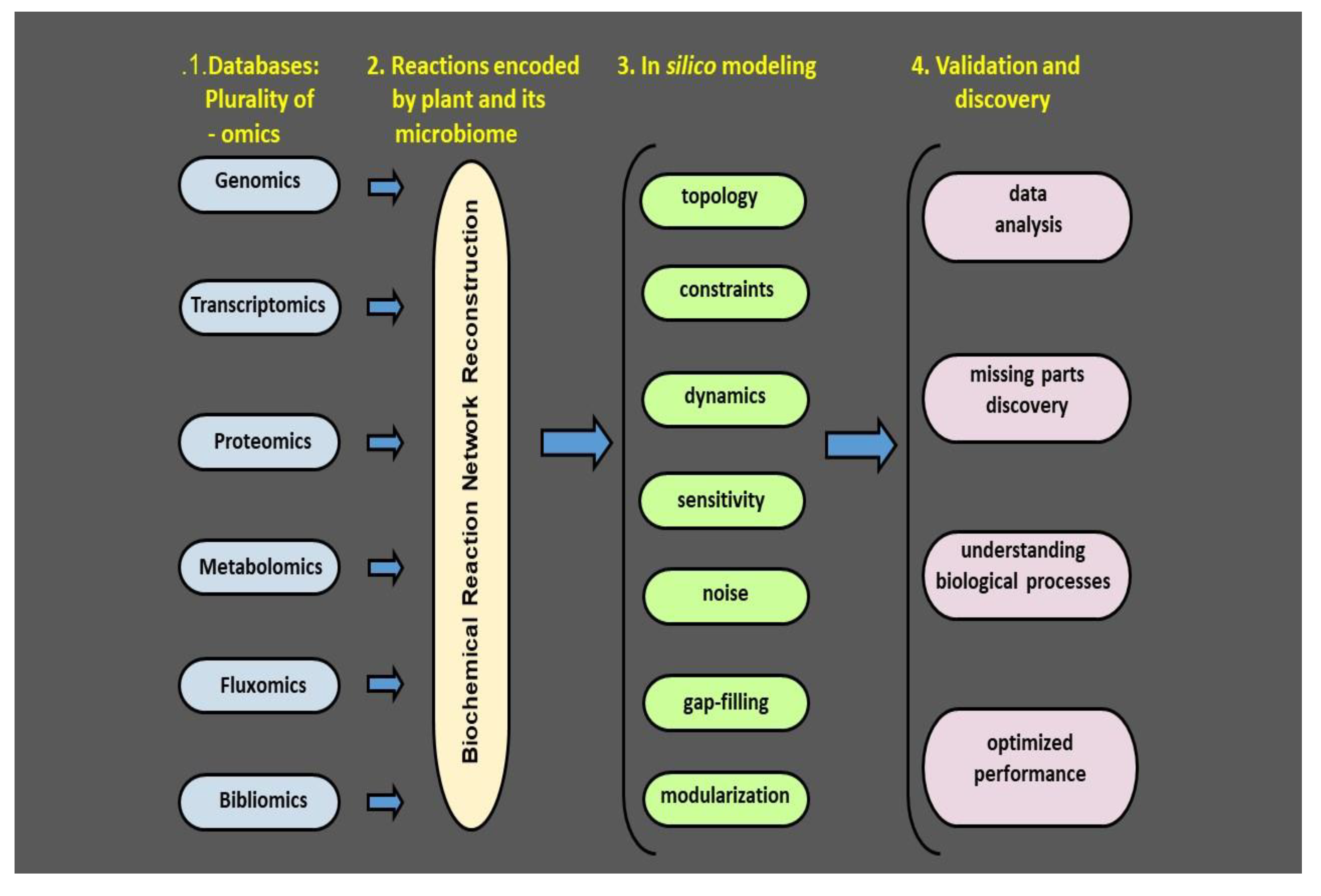
5. Concept of Plant Health
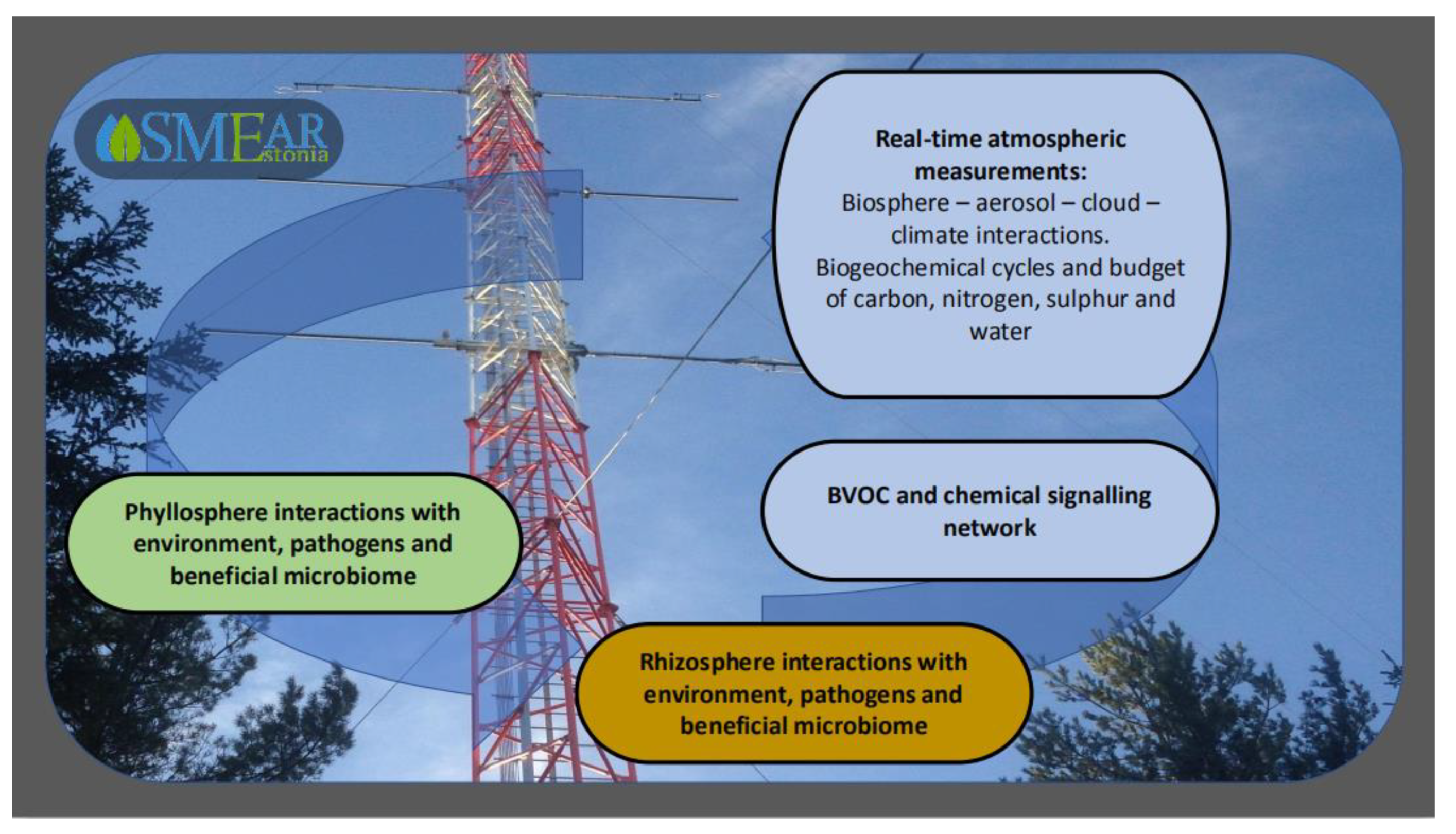
6. Atmospheric Context
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Bebber, D.P.; Holmes, T.; Smith, D.; Gurr, S.J. Economic and physical determinants of the global distributions of crop pests and pathogens. New Phytol. 2014, 202, 901–910. [Google Scholar] [CrossRef]
- Wallace, J.M.; Held, I.M.; Thompson, D.W.J.; Trenberth, K.E.; Walsh, J.E. Global warming and winter weather. Science 2014, 343, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; Van Der Schrier, G.; Jones, P.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2013, 4, 17–22. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kahru, A.; Mander, Ü.; Nõges, P.; Nõges, T.; Uvikene, A. Interacting environmental and chemical regional environmental change tresses under global change in temperate aquatic ecosystems: Stress responses, adaptation, and scaling. Reg. Environ. Chang. 2017, 17, 2061–2077. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Kennedy, T.; Naeem, S.; Howe, K.M.; Knops, J.M.H.; Tilman, D.; Reich, P.B. Biodiversity as a barrier to ecological invasion. Nature 2002, 417, 636–638. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United States). FAOSTAT Online Data; United Nations: Rome, Italy, 2014.
- Organisation for Economic Co-Operation and Development. OECD-FAO Agricultural Outlook 2013; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2013.
- Timmusk, S.; El-Daim, I.A.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.A.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef]
- Geiser, D.M.; Aoki, T.; Bacon, C.W.; Baker, S.E.; Bhattacharyya, M.K.; Brandt, M.E.; Brown, D.W.; Burgess, L.W.; Chulze, S.; Coleman, J.J.; et al. One fungus, one name: Defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 2013, 103, 400–408. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; E Hammond-Kosack, K.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, R.; Ul-Hassan, Z.; Al-Thani, R.; Balmas, V.; Jaoua, S. Application of low-fermenting yeast lachancea thermotolerans for the control of toxigenic fungi aspergillus parasiticus, penicillium verrucosum and Fusarium graminearum and their mycotoxins. Toxins 2018, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium molds and mycotoxins: Potential species-specific effects. Toxins 2018, 10, 244. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Decundo, J.M.; Martínez, M.; Dieguez, S.N.; Moreyra, F.; Moreno, M.V.; Stenglein, S.A. Natural contamination with mycotoxins produced by Fusarium graminearum and Fusarium poae in malting barley in argentina. Toxins 2018, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Keese, C.; Meyer, U.; Starke, A.; Wrenzycki, C.; Dänicke, S.; Rehage, J. Chronic effects of Fusarium mycotoxins in rations with or without increased concentrate proportion on the insulin sensitivity in lactating dairy cows. Toxins 2018, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.J.; Knox, J.P.; Boraston, A.B. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr. Opin. Struct. Boil. 2013, 23, 669–677. [Google Scholar] [CrossRef]
- Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A review. Agron. Sustain. Dev. 2012, 33, 97–111. [Google Scholar] [CrossRef]
- Champeil, A.; Fourbet, J.-F.; Doré, T. Effects of grain sampling procedures on Fusarium mycotoxin assays in wheat grains. J. Agric. Food Chem. 2004, 52, 6049–6054. [Google Scholar] [CrossRef]
- Karron, E.; Runno-Paurson, E.; Lõiveke, H.; Islamov, B.; Kütt, M.-L.; Talve, T.; Lauringson, E.; Horak, H.; Edesi, L.; Niinemets, Ü. Application of widely used fungicides does not necessarily affect grain yield, and incidence of Fusarium spp. and mycotoxins DON, HT-2 and T-2 in spring barley in northern climates. Kvas. Prumysl 2020, 66, 215–223. [Google Scholar] [CrossRef]
- Vaughan, M.; Backhouse, D.; Del Ponte, E.M. Climate change impacts on the ecology of Fusarium graminearum species complex and susceptibility of wheat to Fusarium head blight: A review. World Mycotoxin J. 2016, 9, 685–700. [Google Scholar] [CrossRef]
- Gauthier, L.; Atanasova-Penichon, V.; Chereau, S.; Richard-Forget, F. Metabolomics to decipher the chemical defense of cereals against fusarium graminearum and deoxynivalenol accumulation. Int. J. Mol. Sci. 2015, 16, 24839–24872. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Haber, S. Overview of some recent research developments in Fusarium head blight of wheat. Can. J. Plant Pathol. 2013, 35, 149–174. [Google Scholar] [CrossRef]
- Karlsson, I.; Friberg, H.; Steinberg, C.; Persson, P. Fungicide effects on fungal community composition in the wheat phyllosphere. PLoS ONE 2014, 9, e111786. [Google Scholar] [CrossRef] [PubMed]
- Bürstmayr, M.; Steiner, B.; Wagner, C.; Schwarz, P.; Brugger, K.; Barabaschi, D.; Volante, A.; Valè, G.; Cattivelli, L.; Bürstmayr, H. High-resolution mapping of the pericentromeric region on wheat chromosome arm 5AS harbouring the Fusarium head blight resistance QTL Qfhs.ifa-5A. Plant Biotechnol. J. 2017, 16, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Samad-Zamini, M.; Schweiger, W.; Nussbaumer, T.; Mayer, K.F.; Bürstmayr, H. Time-course expression QTL-atlas of the global transcriptional response of wheat to Fusarium graminearum. Plant Biotechnol. J. 2017, 15, 1453–1464. [Google Scholar] [CrossRef]
- Steiner, B.; Bürstmayr, H.; Michel, S.; Schweiger, W.; Lemmens, M. Breeding strategies and advances in line selection for Fusarium head blight resistance in wheat. Trop. Plant Pathol. 2017, 42, 165–174. [Google Scholar] [CrossRef]
- Jia, H.; Zhou, J.; Xue, S.; Li, G.; Yan, H.; Ran, C.; Zhang, Y.; Shi, J.; Jia, L.; Wang, X.; et al. A journey to understand wheat Fusarium head blight resistance in the Chinese wheat landrace Wangshuibai. Crop. J. 2018, 6, 48–59. [Google Scholar] [CrossRef]
- Zhu, X.; Zhong, S.; Chao, S.; Gu, Y.Q.; Kianian, S.F.; Elias, E.; Cai, X. Toward a better understanding of the genomic region harboring Fusarium head blight resistance QTL Qfhs.ndsu-3AS in durum wheat. Theor. Appl. Genet. 2015, 129, 31–43. [Google Scholar] [CrossRef]
- Bürstmayr, H.; Steiner, B.; Hartl, L.; Griesser, M.; Angerer, N.; Lengauer, D.; Miedaner, T.; Schneider, B.; Lemmens, M. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. II. Resistance to fungal penetration and spread. Theor. Appl. Genet. 2003, 107, 503–508. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Juroszek, P.; Von Tiedemann, A. Climate change and potential future risks through wheat diseases: A review. Eur. J. Plant Pathol. 2012, 136, 21–33. [Google Scholar] [CrossRef]
- Valverde-Bogantes, E.; Bianchini, A.; Herr, J.R.; Rose, D.J.; Wegulo, S.N.; Hallen-Adams, H.E. Recent population changes of Fusarium head blight pathogens: Drivers and implications. Can. J. Plant Pathol. 2019, 1–15. [Google Scholar] [CrossRef]
- Stoytcheva, M. Pesticides in the Modern World—Pesticides Use and Management; BoD–Books on Demand: Norderstedt, Germany, 2011. [Google Scholar]
- Palazzini, J.M.; Torres, A.M.; Chulze, S.N. Biological Control of Fusarium Head Blight of Wheat: From Selection to Formulation; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2013; pp. 191–204. [Google Scholar]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A. Antifungal activity of bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Shearer, C.R.; Limay-Rios, V.; Zhou, T.; Raizada, M.N. Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front. Plant Sci. 2015, 6, 781. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Boland, G.; Zhou, T. Concurrent selection for microbial suppression of Fusarium graminearum, Fusarium head blight and deoxynivalenol in wheat. J. Appl. Microbiol. 2009, 106, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Copolovici, D.; Copolovici, L.; Teder, T.; Nevo, E.; Behers, L. Paenibacillus polymyxa biofilm polysaccharides antagonise Fusarium graminearum. Sci. Rep. 2019, 9, 662. [Google Scholar] [CrossRef]
- Timmusk, S.; Kim, S.-B.; Nevo, E.; El Daim, A.; Ek, B.; Bergquist, J.; Behers, L. Sfp-type PPTase inactivation promotes bacterial biofilm formation and ability to enhance wheat drought tolerance. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L. Risk Levels of invasive Fusarium oxysporum f. sp. in areas suitable for date palm (phoenix dactylifera) cultivation under various climate change projections. PLoS ONE 2013, 8, e83404. [Google Scholar] [CrossRef]
- El Daim, I.A.A.; Häggblom, P.; Karlsson, M.; Stenström, E.; Timmusk, S. Paenibacillus polymyxa A26 Sfp-type PPTase inactivation limits bacterial antagonism against Fusarium graminearum but not of F. culmorum in kernel assay. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Ortega, R.A.; Mahnert, A.; Müller, H.; Berg, G. The plant is crucial: Specific composition and function of the phyllosphere microbiome of indoor ornamentals. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- Lasík, J. Microbiology of the phyllosphere. Folia Microbiol. 1987, 32, 453. [Google Scholar] [CrossRef]
- Rudrappa, T.; Biedrzycki, M.L.; Bais, H. Causes and consequences of plant-associated biofilms. FEMS Microbiol. Ecol. 2008, 64, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Dave, R.; Venugopalan, V.P. Pumping iron to keep fit: Modulation of siderophore secretion helps efficient aromatic utilization in Pseudomonas putida KT2440. Microbiology 2014, 160, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Zeriouh, H.; De Vicente, A.; Pérez-García, A.; Romero, D.; Romero, D. Surfactin triggers biofilm formation ofBacillus subtilisin melon phylloplane and contributes to the biocontrol activity. Environ. Microbiol. 2013, 16, 2196–2211. [Google Scholar] [CrossRef]
- García-Gutiérrez, L.; Zeriouh, H.; Romero, D.; Cubero, J.; De Vicente, A.; Pérez-García, A. The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate-and salicylic acid-dependent defence responses. Microb. Biotechnol. 2013, 6, 264–274. [Google Scholar] [CrossRef]
- Zeriouh, H.; Romero, D.; García-Gutiérrez, L.; Cazorla, F.M.; De Vicente, A.; Pérez-García, A. The iturin-like lipopeptides are essential components in the biological control arsenal of bacillus subtilis against bacterial diseases of cucurbits. Mol. Plant Microbe Interact. 2011, 24, 1540–1552. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Genet. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Lambais, M.R.; Crowley, D.; Cury, J.C.; Bull, R.C.; Rodrigues, R.R. Bacterial diversity in tree canopies of the atlantic forest. Science 2006, 312, 1917. [Google Scholar] [CrossRef]
- Timmusk, S.; Seisenbaeva, G.; Behers, L. Titania (TiO2) nanoparticles enhance the performance of growth-promoting rhizobacteria. Sci. Rep. 2018, 8, 617. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2017, 8, 373. [Google Scholar] [CrossRef]
- Grube, M.; Schmid, F.; Berg, G. Black fungi and associated bacterial communities in the phyllosphere of grapevine. Fungal Boil. 2011, 115, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Genet. 2013, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, P.B.; Chai, Y.; Vlamakis, H.; Losick, R.; Kolter, R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc. Natl. Acad. Sci. USA 2013, 110, E1621–E1630. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Boil. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Nevo, E. Plant root associated biofilms: Perspectives for natural product mining. In Bacteria in Agrobiology: Plant Nutrient Management; Springer Science and Business Media LLC: Berlin, Germany, 2011; pp. 285–300. [Google Scholar]
- Stone, B.W.G.; Weingarten, E.A.; Jackson, C.R. The Role of the Phyllosphere Microbiome in Plant Health and Function. Annual Plant. Reviews Online 2018, 1, 533–556. [Google Scholar] [CrossRef]
- Shank, E.A.; Kolter, R. New developments in microbial interspecies signaling. Curr. Opin. Microbiol. 2009, 12, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Shank, E.A.; Klepac-Ceraj, V.; Collado-Torres, L.; Powers, G.E.; Losick, R.; Kolter, R. Interspecies interactions that result in Bacillus subtilis forming biofilms are mediated mainly by members of its own genus. Proc. Natl. Acad. Sci. USA 2011, 108, E1236–E1243. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Liu, C.; Yang, A.; Qiao, J. Quorum sensing for population-level control of bacteria and potential therapeutic applications. Cell. Mol. Life Sci. 2019, 77, 1319–1343. [Google Scholar] [CrossRef]
- Ryu, C.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- De Vrieze, M.; Pandey, P.; Bucheli, T.D.; Varadarajan, A.R.; Ahrens, C.H.; Weisskopf, L.; Bailly, A. Volatile organic compounds from native potato-associated pseudomonas as potential anti-oomycete agents. Front. Microbiol. 2015, 6, 875. [Google Scholar] [CrossRef]
- Niinemets, Ü. What Are Plant—Released Biogenic Volatiles and How They Participate in Landscape-to Global-Level Processes? Springer Science and Business Media LLC: Berlin, Germany, 2018; pp. 29–56. [Google Scholar]
- Jiang, Y.; Ye, J.; Veromann, L.-L.; Niinemets, Ü.; Veromann-Jürgenson, L.-L. Scaling of photosynthesis and constitutive and induced volatile emissions with severity of leaf infection by rust fungus (Melampsora larici-populina) inPopulus balsamiferavar.suaveolens. Tree Physiol. 2016, 36, 856–872. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Vlami, M.; De Souza, J.T. Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek 2002, 81, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, M.; Andrews, J.S.; Biggs, C.; Eboigbodin, K.E.; Elliott, D.R.; Rolfe, S.A.; Scholes, J.; Ojeda, J.; Romero-Gonzalez, M.E.; Edyvean, R.G.J.; et al. The polymer physics and chemistry of microbial cell attachment and adhesion. Faraday Discuss. 2008, 139, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Chu, F.; Kolter, R.; Losick, R. Bistability and biofilm formation in Bacillus subtilis. Mol. Microbiol. 2007, 67, 254–263. [Google Scholar] [CrossRef]
- Liang, T.-W.; Wang, S.-L. Recent advances in exopolysaccharides from paenibacillus spp.: Production, isolation, structure, and bioactivities. Mar. Drugs 2015, 13, 1847–1863. [Google Scholar] [CrossRef]
- Timmusk, S.; Zucca, C. The plant microbiome as a resource to increase crop productivity and soil resilience: A systems approach. J. Cameroon Acad. Sci. 2019, 14, 181. [Google Scholar] [CrossRef][Green Version]
- Nevo, E.; Fu, Y.-B.; Pavlicek, T.; Khalifa, S.; Tavasi, M.; Beiles, A. Evolution of wild cereals during 28 years of global warming in Israel. Proc. Natl. Acad. Sci. USA 2012, 109, 3412–3415. [Google Scholar] [CrossRef]
- Vieweger, A.; Döring, T. Assessing health in agriculture—Towards a common research framework for soils, plants, animals, humans and ecosystems. J. Sci. Food Agric. 2014, 95, 438–446. [Google Scholar] [CrossRef]
- E Leach, J.; Triplett, L.R.; Argueso, C.T.; Trivedi, P. Communication in the Phytobiome. Cell 2017, 169, 587–596. [Google Scholar] [CrossRef]
- Hartmann, A.; Fischer, D.; Kinzel, L.; Chowdhury, S.P.; Hofmann, A.; Baldani, J.I.; Rothballer, M. Assessment of the structural and functional diversities of plant microbiota: Achievements and challenges—A review. J. Adv. Res. 2019, 19, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.S.; Palsson, B.O. Systems Properties of the Haemophilus influenzaeRd metabolic genotype. J. Boil. Chem. 1999, 274, 17410–17416. [Google Scholar] [CrossRef] [PubMed]
- Newbery, F.; Qi, A.; Fitt, B.D.L. Modelling impacts of climate change on arable crop diseases: Progress, challenges and applications. Curr. Opin. Plant Boil. 2016, 32, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–pathogen warfare under changing climate conditions. Curr. Boil. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed]
- Noe, S.; Krasnov, D.; Krasnova, A.; Cordey, H.; Niinemets, Ü. Seasonal variation and characterisation of reactive trace gas mixing ratios over a hemi-boreal mixed forest site in Estonia. Boreal Environ. Res. 2016, 21, 332–344. [Google Scholar]
- Rahaman, M.; Chen, D.; Gillani, Z.; Klukas, C.; Chen, M. Advanced phenotyping and phenotype data analysis for the study of plant growth and development. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Kulmala, M. Build a global earth observatory. Nature 2018, 553, 21–23. [Google Scholar] [CrossRef]
- May, N.; Olson, N.E.; Panas, M.G.; Axson, J.L.; Tirella, P.S.; Kirpes, R.M.; Craig, R.; Gunsch, M.J.; China, S.; Laskin, J.; et al. Aerosol emissions from great lakes harmful algal blooms. Environ. Sci. Technol. 2017, 52, 397–405. [Google Scholar] [CrossRef]
- Timmusk, S.; Conrad, J.; Niinemets, Y.; Nevo, E.; Behers, L.; Bergqvist, J. Managing Plant Stress in the Era of Climate Change: Realising the Global Sustainable Development Goals. Available online: http://www.global-engage.com/agricultural-biotechnology/managing-plant-stress-in-the-era-of-climate-change-realising-global-sustainable-development-goals/ed2020 (accessed on 17 February 2020).
- Ezhova, E.; Ylivinkka, I.; Kuusk, J.; Komsaare, K.; Vana, M.; Krasnova, A.; Noe, S.M.; Arshinov, M.; Belan, B.; Bin Park, S.; et al. Direct effect of aerosols on solar radiation and gross primary production in boreal and hemiboreal forests. Atmos. Chem. Phys. Discuss. 2018, 18, 17863–17881. [Google Scholar] [CrossRef]
- Watanabe, Y. Canopy, leaf surface structure and tree phenology: Arboreal factors influencing aerosol deposition in forests. J. Agric. Meteorol. 2015, 71, 167–173. [Google Scholar] [CrossRef]
- Chen, Y. Ecophysiological responses of winter wheat seedling to aerosol wet deposition of Xi’an area, China. J. Environ. Sci. 2010, 22, 1786–1791. [Google Scholar] [CrossRef]
- Calfapietra, C.; Penuelas, J.; Niinemets, Ü. Urban plant physiology: Adaptation-mitigation strategies under permanent stress. Trends Plant Sci. 2015, 20, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Kanagendran, A.; Pazouki, L.; Li, S.; Liu, B.; Kännaste, A.; Niinemets, Ü. Ozone-triggered surface uptake and stress volatile emissions in Nicotiana tabacum ‘Wisconsin’. J. Exp. Bot. 2017, 69, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Dos Santos, B.M.; Kanagendran, A.; Neilson, E.J.; Niinemets, Ü. Ozone and wounding stresses differently alter the temporal variation in formylated phloroglucinols in eucalyptus globulus leaves. Metabolites 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Harley, P.C.; Niinemets, Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant Cell Environ. 2017, 40, 1984–2003. [Google Scholar] [CrossRef]
- Gustafsson, L.; Eriksson, I. Factors of importance for the epiphytic vegetation of aspen populus tremula with special emphasis on bark chemistry and soil chemistry. J. Appl. Ecol. 1995, 32, 412. [Google Scholar] [CrossRef]
- Garty, J.; Weissman, L.; Tamir, O.; Cohen, Y.; Orlovsky, L.; Beer, S.; Karnieli, A. Comparison of five physiological parameters to assess the vitality of the lichen Ramalina lacera exposed to air pollution. Physiol. Plant. 2000, 109, 410–418. [Google Scholar] [CrossRef]
- Ismail, A.; Wahab, N.A.A.; Latif, M.T.; Said, M.N.M.; Ismail, A.; Zulkifli, A.R. Atmospheric air pollution and roughness of bark as possible factor in increasing density of epiphytic terrestrial algae. Asian J. Appl. Sci. 2016, 4, 256–261. [Google Scholar]
- Lear, G.; Lewis, G. Microbial Biofilms Current Research and Applications; Caister Academic Press: Norfolk, UK, 2012. [Google Scholar]
- Noe, S.M.; Niinemets, Ü.; Krasnova, A.; Krasnov, D.; Motallebi, A.; Kängsepp, V.; Jõgiste, K.; Hõrrak, U.; Komsaare, K.; Mirme, S.; et al. SMEAR Estonia: Perspectives of a large-scale forest ecosystem—Atmosphere research infrastructure. For. Stud. 2015, 63, 56–84. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timmusk, S.; Nevo, E.; Ayele, F.; Noe, S.; Niinemets, Ü. Fighting Fusarium Pathogens in the Era of Climate Change: A Conceptual Approach. Pathogens 2020, 9, 419. https://doi.org/10.3390/pathogens9060419
Timmusk S, Nevo E, Ayele F, Noe S, Niinemets Ü. Fighting Fusarium Pathogens in the Era of Climate Change: A Conceptual Approach. Pathogens. 2020; 9(6):419. https://doi.org/10.3390/pathogens9060419
Chicago/Turabian StyleTimmusk, Salme, Eviatar Nevo, Fantaye Ayele, Steffen Noe, and Ülo Niinemets. 2020. "Fighting Fusarium Pathogens in the Era of Climate Change: A Conceptual Approach" Pathogens 9, no. 6: 419. https://doi.org/10.3390/pathogens9060419
APA StyleTimmusk, S., Nevo, E., Ayele, F., Noe, S., & Niinemets, Ü. (2020). Fighting Fusarium Pathogens in the Era of Climate Change: A Conceptual Approach. Pathogens, 9(6), 419. https://doi.org/10.3390/pathogens9060419






