Organic Amendments Modulate Soil Microbiota and Reduce Virus Disease Incidence in the TSWV-Tomato Pathosystem
Abstract
1. Introduction
2. Results
2.1. Effect of OAs on TSWV Inoculated Plants
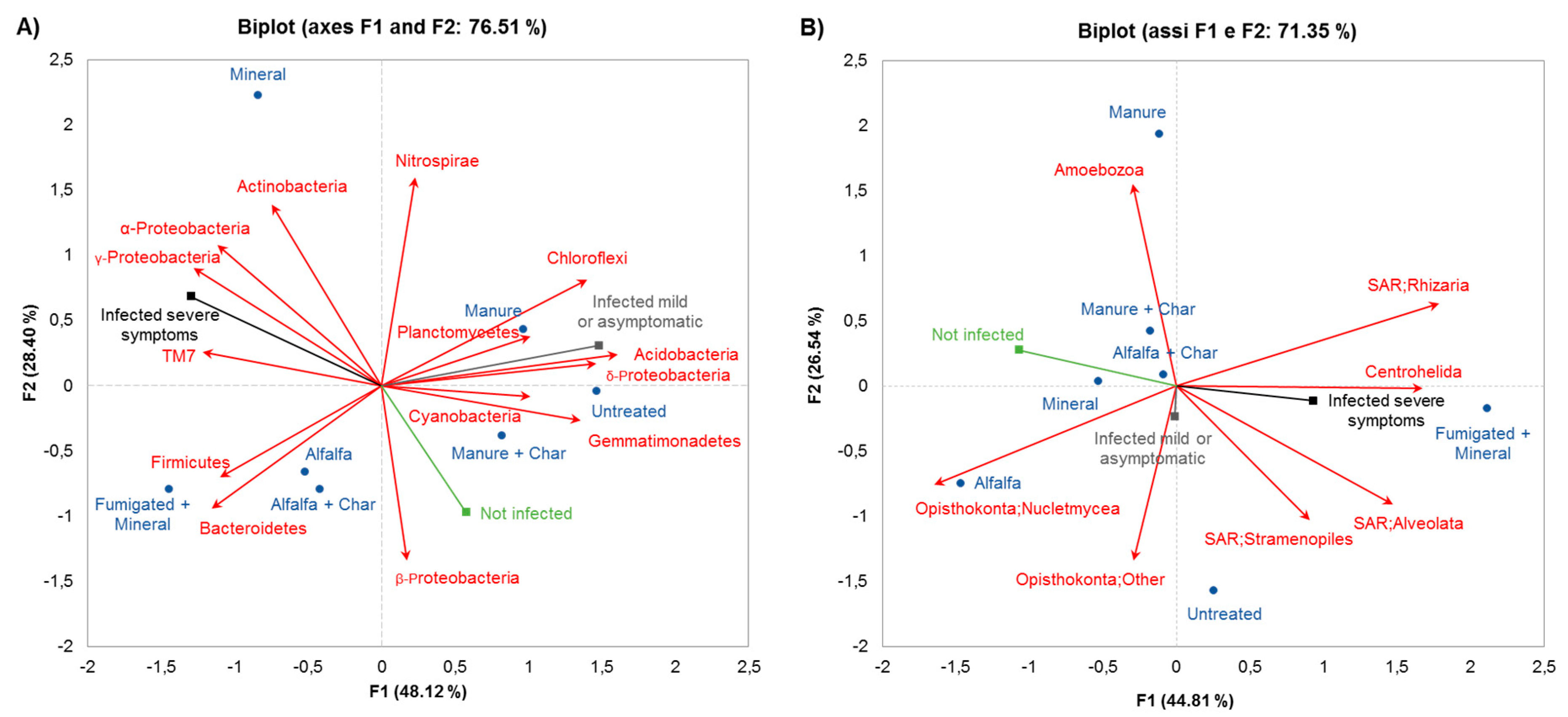
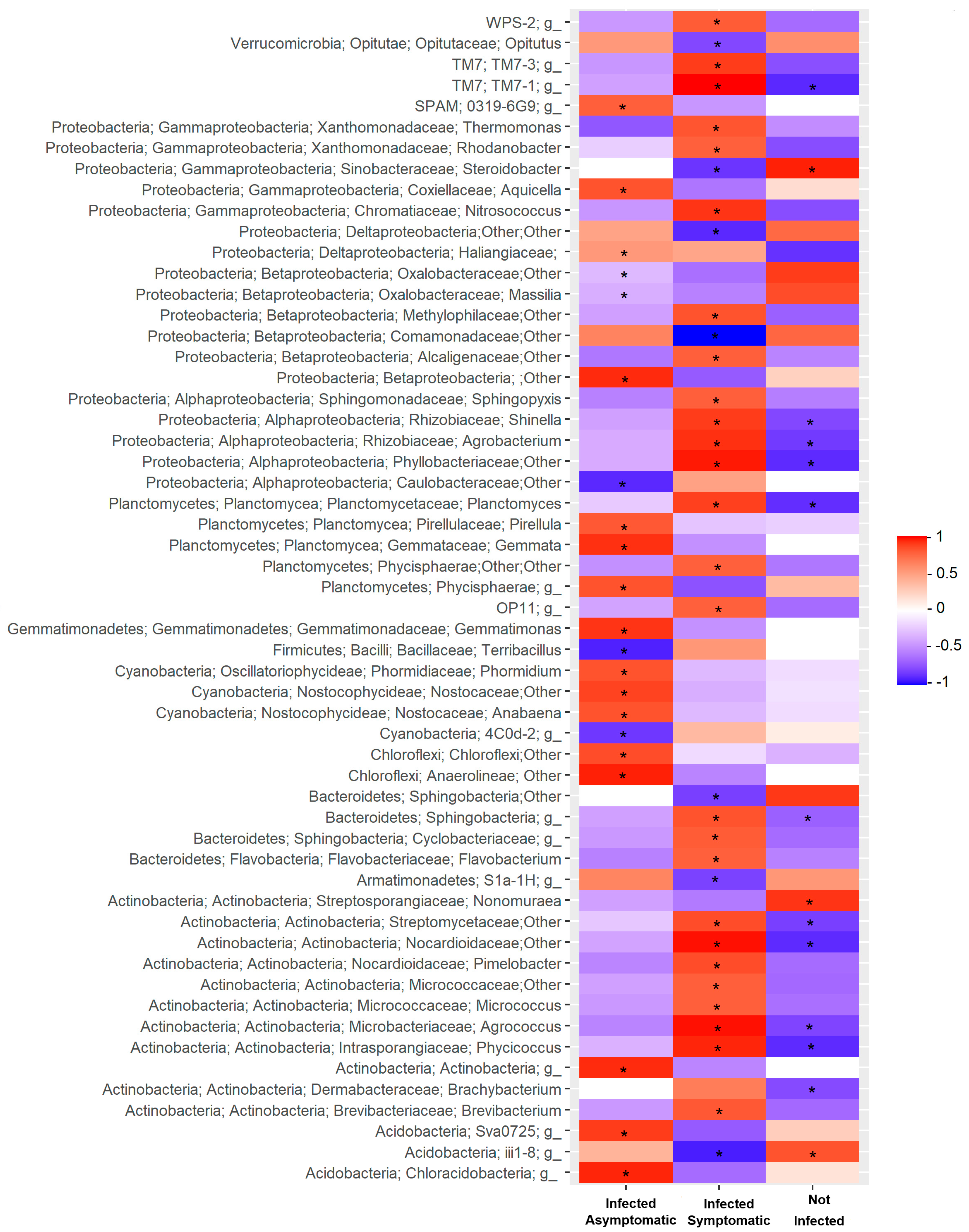
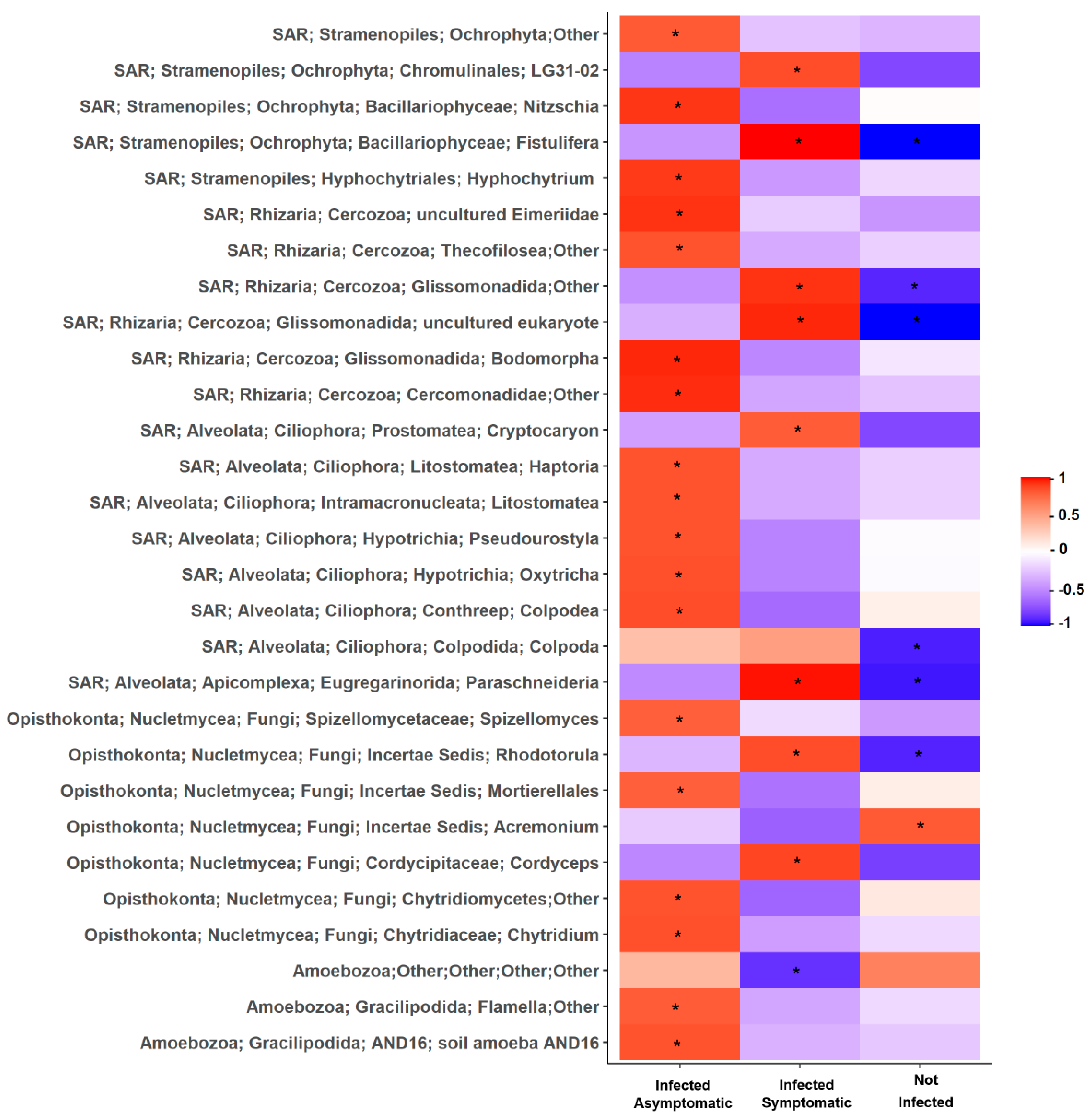
2.2. Relationship Between Soil Microbiota Composition, TSWV Incidence, and Symptom Expression
3. Discussion
4. Materials and Methods
4.1. Soil Treatments and Microbial Analysis
4.2. TSWV-Tomato Experiment
4.3. Assessment of TSWV Infection
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota: A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Stockdale, E.; Shepherd, M.; Fortune, S.; Cuttle, S. Soil fertility in organic farming systems-fundamentally different? Soil Use Manag. 2002, 18, 301–308. [Google Scholar] [CrossRef]
- Torres, I.F.; Bastida, F.; Hernández, T.; García, C. The effects of fresh and stabilized pruning wastes on the biomass, structure and activity of the soil microbial community in a semiarid climate. Appl. Soil Ecol. 2015, 89, 1–9. [Google Scholar] [CrossRef]
- Bronick, C.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Albiach, R.; Canet, R.; Pomares, F.; Ingelmo, F. Organic matter components and aggregate stability after the application of different amendments to a horticultural soil. Bioresour. Technol. 2001, 76, 125–129. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, L.; Zhang, Y.; Yang, L.; Yu, C.; Yin, G.; Doane, T.A.; Wu, Z.; Zhu, P.; Ma, X. Biochar improves soil aggregate stability and water availability in a mollisol after three years of field application. PLoS ONE 2016, 11, e0154091. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- García-Gil, J.C.; Plaza, C.; Soler-Rovira, P.; Polo, A. Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol. Biochem. 2000, 32, 1907–1913. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mader, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Hiddink, G.A.; Van Bruggen, A.H.C.; Termorshuizen, A.J.; Raaijmakers, J.M.; Semenov, A.V. Effect of organic management of soils on suppressiveness to Gaeumannomyces graminis var. Tritici and its antagonist, Pseudomonas fluorescens. Eur. J. Plant Pathol. 2005, 113, 417–435. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Paudel, I.; Graber, E.R.; Cytryn, E.; Frenkel, O. Linking the belowground microbial composition, diversity and activity to soilborne disease suppression and growth promotion of tomato amended with biochar. Sci. Rep. 2017, 7, 44382. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiong, W.; Zhang, R.; Hang, X.; Wang, D.; Li, R.; Shen, Q. Continuous application of different organic additives can suppress tomato disease by inducing the healthy rhizospheric microbiota through alterations to the bulk soil microflora. Plant Soil 2018, 423, 229–240. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.; Thürig, B.; Bruns, C.; Fuchs, J.G.; Köpke, U.; Laustela, M.; Leifert, C.; Mahlberg, N.; Nietlispach, B.; Schmidt, C.; et al. Soil type, management history, and soil amendments influence the development of soil-borne (Rhizoctonia solani, Pythium ultimum) and air-borne (Phytophthora infestans, Hyaloperonospora parasitica) diseases. Eur. J. Plant Pathol. 2010, 127, 465–481. [Google Scholar] [CrossRef]
- Yan, Z.; Reddy, M.S.; Ryu, C.M.; McInroy, J.; Wilson, M.; Kloepper, J.W. Induced systemic protection against tomato late blight elicited by plant growth-promoting rhizobacteria. Phytopathology 2002, 92, 1329–1333. [Google Scholar] [CrossRef]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef]
- Larkin, R.P.; Griffin, T.S. Control of soilborne potato diseases using brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Tenuta, M.; Lazarovits, G. Soil properties associated with the variable effectiveness of meat and bone meal to kill microsclerotia of Verticillium dahliae. Appl. Soil Ecol. 2004, 25, 219–236. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Hoitink, H.; Boehm, M. Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446. [Google Scholar] [CrossRef]
- Artursson, V.; Finlay, R.D.; Jansson, J.K. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ. Microbiol. 2006, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, N.; Gutiérrez-Barranquero, J.A.; De Vicente, A.; Cazorla, F.M. Enhancing soil quality and plant health through suppressive organic amendments. Diversity 2012, 4, 475–491. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef]
- Vallad, G.E.; Cooperband, L.; Goodman, R.M. Plant foliar disease suppression mediated by composted forms of paper mill residuals exhibits molecular features of induced resistance. Physiol. Mol. Plant Pathol. 2003, 63, 65–77. [Google Scholar] [CrossRef]
- Parrella, G.; Gognalons, P.; Gebre-Selassie, K.; Vovlas, C.; Marchoux, G. An update of the host range of tomato spotted wilt virus. J. Plant Pathol. 2003, 12, 227–264. [Google Scholar]
- EFSA. Scientific opinion on the risk to plant health posed by Tomato spotted wilt virus to the EU territory with identification and evaluation of risk reduction options. EFSA J. 2012, 10, 3029. [Google Scholar] [CrossRef]
- Pappu, H.R.; Jones, R.A.C.; Jain, R.K. Global status of tospovirus epidemics in diverse cropping systems: Successes achieved and challenges ahead. Virus Res. 2009, 141, 219–236. [Google Scholar] [CrossRef]
- Scholthof, K.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Palloix, A.; Ayme, V.; Moury, B. Durability of plant major resistance genes to pathogens depends on the genetic background, experimental evidence and consequences for breeding strategies. New Phytol. 2009, 183, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Messelink, G.J.; van Maanen, R.; van Steenpaal, S.E.; Janssen, A. Biological control of thrips and whiteflies by a shared predator: Two pests are better than one. Biol. Control 2008, 44, 372–379. [Google Scholar] [CrossRef]
- Saito, T.; Brownbridge, M. Compatibility of soil-dwelling predators and microbial agents and their efficacy in controlling soil-dwelling stages of western flower thrips Frankliniella occidentalis. Biol. Control 2016, 92, 92–100. [Google Scholar] [CrossRef]
- Cesarano, G.; De Filippis, F.; La Storia, A.; Scala, F.; Bonanomi, G. Organic amendment type and application frequency affect crop yields, soil fertility and microbiome composition. Appl. Soil Ecol. 2017, 120, 254–264. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Noble, R.; Coventry, E. Suppression of soil-borne plant diseases with composts: A review. Biocontrol Sci. Technol. 2005, 15, 3–20. [Google Scholar] [CrossRef]
- Postma, J.; Schilder, M.T.; Bloem, J.; van Leeuwen-Haagsma, W.K. Soil suppressiveness and functional diversity of the soil microflora in organic farming systems. Soil Biol. Biochem. 2008, 40, 2394–2406. [Google Scholar] [CrossRef]
- Yadessa, G.B.; Van Bruggen, A.H.C.; Ocho, F.L. Effects of different soil amendments on bacterial wilt caused by Ralstonia solanacearum and on the yield of tomato. J. Plant Pathol. 2010, 92, 439–450. [Google Scholar]
- Aliyu, T.H.; Balogun, O.S.; Alade, O.O. Assessment of the effect of rate and time of application of rice-husk powder as an organic amendment on cowpea (Vigna unguiculata L., walp) inoculated with cowpea mottle virus. Agric. Biol. J. North Am. 2011, 2, 74–79. [Google Scholar] [CrossRef]
- Fajinmi, A.A.; Adebode, C.A. Effect of poultry manure on pepper veinal mottle virus (PVMV), yield and agronomic parameters of pepper (Capsicum annuum) in Nigeria. East Afr. J. Sci. 2007, 1, 104–111. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Hu, S.; Van Bruggen, A.H.C. Short-term cover crop decomposition in organic and conventional soils: Characterization of soil C, N, microbial and plant pathogen dynamics. Eur. J. Plant Pathol. 2000, 106, 37–50. [Google Scholar] [CrossRef]
- He, M.; Tian, G.; Semenov, A.M.; van Bruggen, A.H.C. Short-term fluctuations of sugar beet damping-off by Pythium ultimum in relation to changes in bacterial communities after organic amendments to two soils. Phytopathology 2012, 102, 413–420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dick, R.P.; Rasmussen, P.E.; Kerle, E.A. Influence of long-term residue management on soil enzyme activities in relation to soil chemical properties of a wheat-fallow system. Biol. Fertil. Soils 1988, 6, 159–164. [Google Scholar] [CrossRef]
- Kandeler, E. Response of soil microbial biomass, urease and xylanase within particle size fractions to long-term soil management. Soil Biol. Biochem. 1999, 31, 261–273. [Google Scholar] [CrossRef]
- Bonanomi, G.; Gaglione, S.A.; Cesarano, G.; Sarker, T.C.; Pascale, M.; Scala, F.; Zoina, A. Frequent applications of organic matter to agricultural soil increase fungistasis. Pedosphere 2017, 27, 86–95. [Google Scholar] [CrossRef]
- Zhang, W.; Dick, W.A.; Hoitink, H.A.J. Compost-induced systemic acquired resistance in cucumber to Pythium root rand anthracnose. Phytopathology 1996, 86, 1066–1070. [Google Scholar] [CrossRef]
- Harel, Y.M.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E.R. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar] [CrossRef]
- Mehari, Z.H.; Elad, Y.; Rav-David, D.; Graber, E.R.; Harel, Y.M. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil 2015, 395, 31–44. [Google Scholar] [CrossRef]
- Kandan, A.; Ramiah, M.; Vasanthi, V.J.; Radjacommare, R.; Nandakumar, R.; Ramanathan, A.; Samiyappan, R. Use of Pseudomonas fluorescens-based formulations for management of Tomato spotted wilt virus (TSWV) and enhanced yield in tomato. Biocontrol. Sci. Technol. 2005, 15, 553–569. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, P.S.; Agrawal, L.; Raj, R.; Srivastava, A.; Gupta, S.; Mishra, S.K.; Yadav, S.; Singh, P.C.; Raj, S.K. Paenibacillus lentimorbus inoculation enhances tobacco growth and extenuates the virulence of cucumber mosaic virus. PLoS ONE 2016, 11, e0149980. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Lou, Y.; Shi, M.; Jiang, Y.; Zhou, J.; Sun, Y.; Xue, Q.; Lai, H. Bacillus amyloliquefaciens Ba13 induces plant systemic resistance and improves rhizosphere microecology against tomato yellow leaf curl virus disease. Appl. Soil Ecol. 2019, 137, 154–166. [Google Scholar] [CrossRef]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 2018, 8, 10320. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Hulbert, S.H.; Schroeder, K.L.; Mavrodi, O.; Mavrodi, D.; Dhingra, A.; Schillinger, W.F.; Paulitz, T.C. Role of bacterial communities in the natural suppression of Rhizoctonia solani bare patch disease of wheat (Triticum aestivum L.). Appl. Environ. Microbiol. 2013, 79, 7428–7438. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, D.T.; Poling, S.M. Antimicrobial activity of pyrrocidines from Acremonium zeae against endophytes and pathogens of maize. Phytopathology 2009, 99, 109–115. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, K.A.; Knorr, K.; Jørgensen, L.N.; Nicolaisen, M.; Boelt, B. Exploring the potential of symbiotic fungal endophytes in cereal disease suppression. Biol. Control 2012, 63, 69–78. [Google Scholar] [CrossRef]
- Marra, R.; Vinale, F.; Cesarano, G.; Lombardi, N.; d’Errico, G.; Crasto, A.; Mazzei, P.; Piccolo, A.; Incerti, G.; Woo, S.L.; et al. Biochars from olive mill waste have contrasting effects on plants, fungi and phytoparasitic nematodes. PLoS ONE 2018, 13, e0198728. [Google Scholar] [CrossRef]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Nanni, B.; Lombardi, N.; Rita, A.; Saracino, A.; Scala, F. Biochar as plant growth promoter: Better off alone or mixed with organic amendments? Front. Plant Sci. 2017, 8, 1570. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Amonette, J.E.M.; Ippolito, J.A.; Lima, I.M.; Gaskin, J.; Das, K.C.; Steiner, C.; Ahmedna, M. Biochars impact on soil-moisture storage in an ultisol and two aridisols. Soil Sci. 2012, 177, 310–320. [Google Scholar] [CrossRef]
- Liu, J.; Schulz, H.; Brandl, S.; Miehtke, H.; Huwe, B.; Glaser, B. Short-term effect of biochar and compost on soil fertility and water status of a dystric cambisol in NE Germany under field conditions. J. Plant Nutr. Soil Sci. 2012, 175, 698–707. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Postma, J.; Clematis, F.; Nijhuis, E.H.; Someus, E. Efficacy of four phosphate-mobilizing bacteria applied with an animal bone charcoal formulation in controlling Pythium aphanidermatum and Fusarium oxysporum f. sp. Radicis Lycopersici in tomato. Biol. Control 2013, 67, 284–291. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Cytryn, E.; Graber, E.R.; Frenkel, O. Activating biochar by manipulating the bacterial and fungal microbiome through pre-conditioning. New Phytol. 2018, 219, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Kammann, C.; Glaser, B.; Schmidt, H.P. Combining biochar and organic amendments. In Biochar in European Soils and Agriculture: Science and Practice; Shackley, S., Ruysschaert, G., Zwart, K., Glaser, B., Eds.; Routledge: London, UK, 2016; pp. 136–164. [Google Scholar]
- Dijkstra, J.; de Jager, C.P. Practical Plant Virology: Protocols and Exercises; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Li, R.; Khafipour, E.; Krause, D.O.; Entz, M.H.; de Kievit, T.R.; Fernando, W.G.D. Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities. PLoS ONE 2012, 7, e51897. [Google Scholar] [CrossRef]
- Chaudhry, V.; Rehman, A.; Mishra, A.; Chauhan, P.S.; Nautiyal, C.S. Changes in bacterial community structure of agricultural land due to long-term organic and chemical amendments. Microb. Ecol. 2012, 64, 450–460. [Google Scholar] [CrossRef]
- Bonanomi, G.; Cesarano, G.; Antignani, V.; Di Maio, C.; De Filippis, F.; Scala, F. Conventional farming impairs Rhizoctonia solani disease suppression by disrupting soil food web. J. Phytopathol. 2018, 1, 1–11. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanomi, G.; Alioto, D.; Minutolo, M.; Marra, R.; Cesarano, G.; Vinale, F. Organic Amendments Modulate Soil Microbiota and Reduce Virus Disease Incidence in the TSWV-Tomato Pathosystem. Pathogens 2020, 9, 379. https://doi.org/10.3390/pathogens9050379
Bonanomi G, Alioto D, Minutolo M, Marra R, Cesarano G, Vinale F. Organic Amendments Modulate Soil Microbiota and Reduce Virus Disease Incidence in the TSWV-Tomato Pathosystem. Pathogens. 2020; 9(5):379. https://doi.org/10.3390/pathogens9050379
Chicago/Turabian StyleBonanomi, Giuliano, Daniela Alioto, Maria Minutolo, Roberta Marra, Gaspare Cesarano, and Francesco Vinale. 2020. "Organic Amendments Modulate Soil Microbiota and Reduce Virus Disease Incidence in the TSWV-Tomato Pathosystem" Pathogens 9, no. 5: 379. https://doi.org/10.3390/pathogens9050379
APA StyleBonanomi, G., Alioto, D., Minutolo, M., Marra, R., Cesarano, G., & Vinale, F. (2020). Organic Amendments Modulate Soil Microbiota and Reduce Virus Disease Incidence in the TSWV-Tomato Pathosystem. Pathogens, 9(5), 379. https://doi.org/10.3390/pathogens9050379







