Evaluation of Volatile Organic Compounds Obtained from Breath and Feces to Detect Mycobacterium tuberculosis Complex in Wild Boar (Sus scrofa) in Doñana National Park, Spain
Abstract
1. Introduction
2. Results
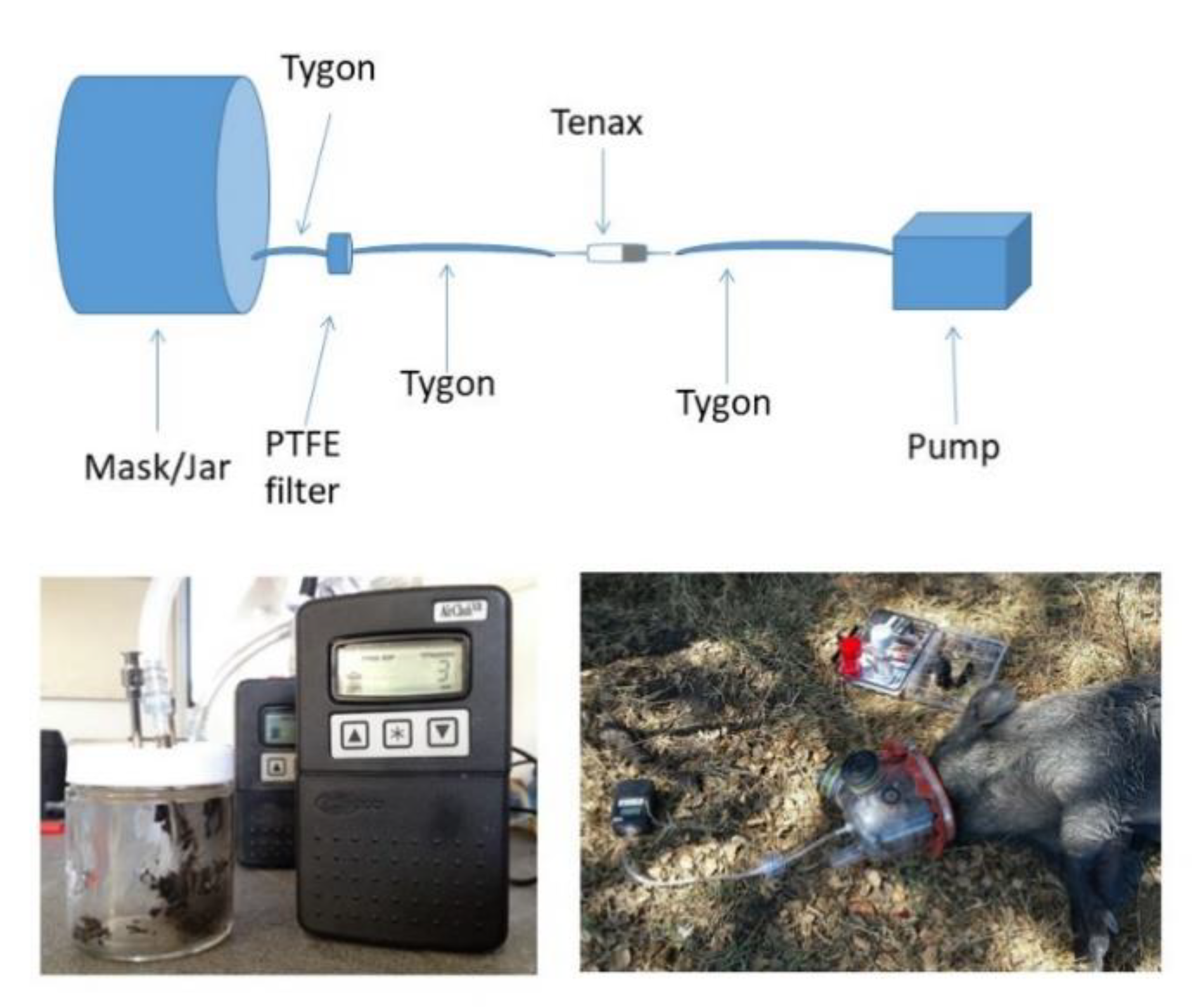
2.1. Assessment of Volatile Organic Compound Collection
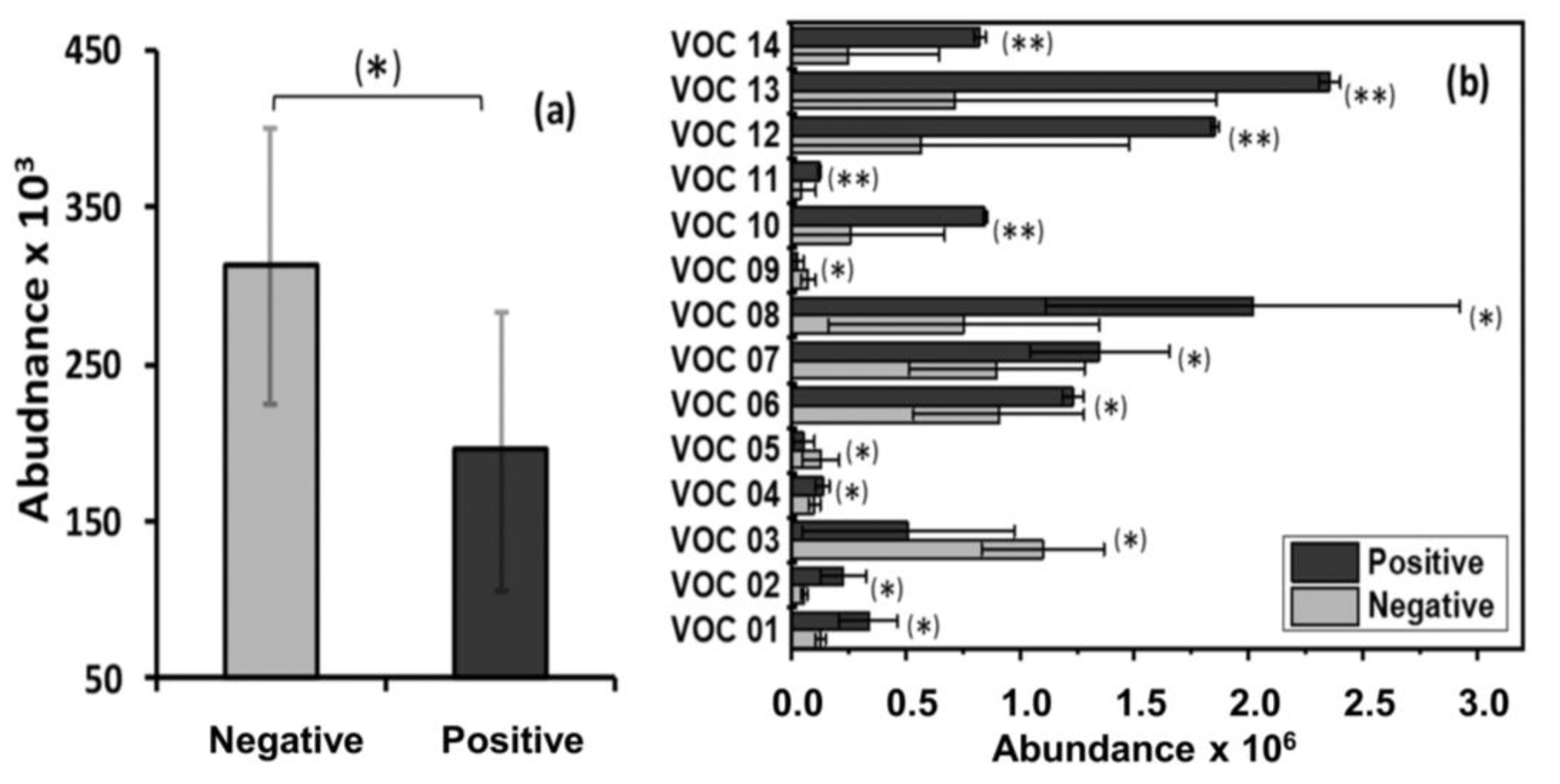
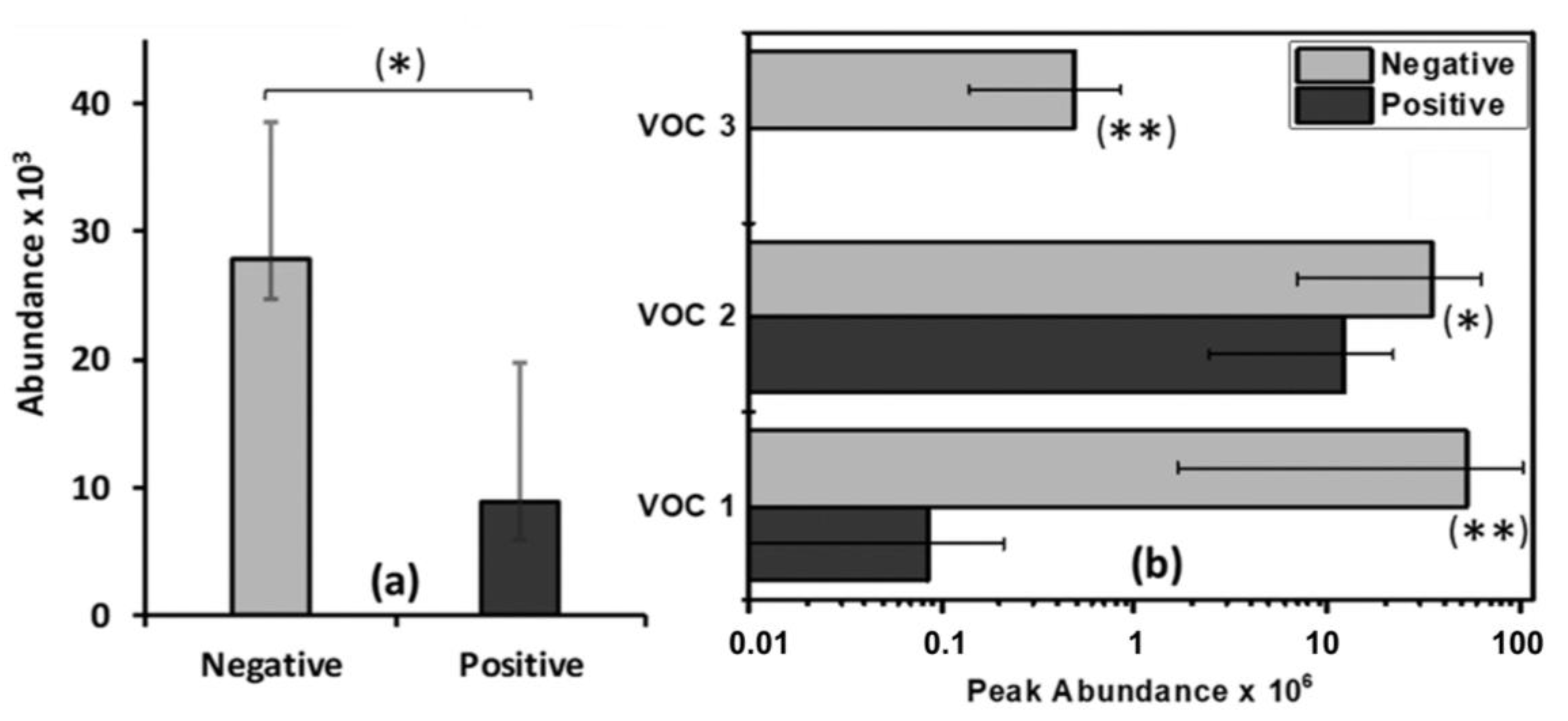
2.2. Animal Disease and VOC Sample Status
2.3. Significant Volatile Organic Compounds
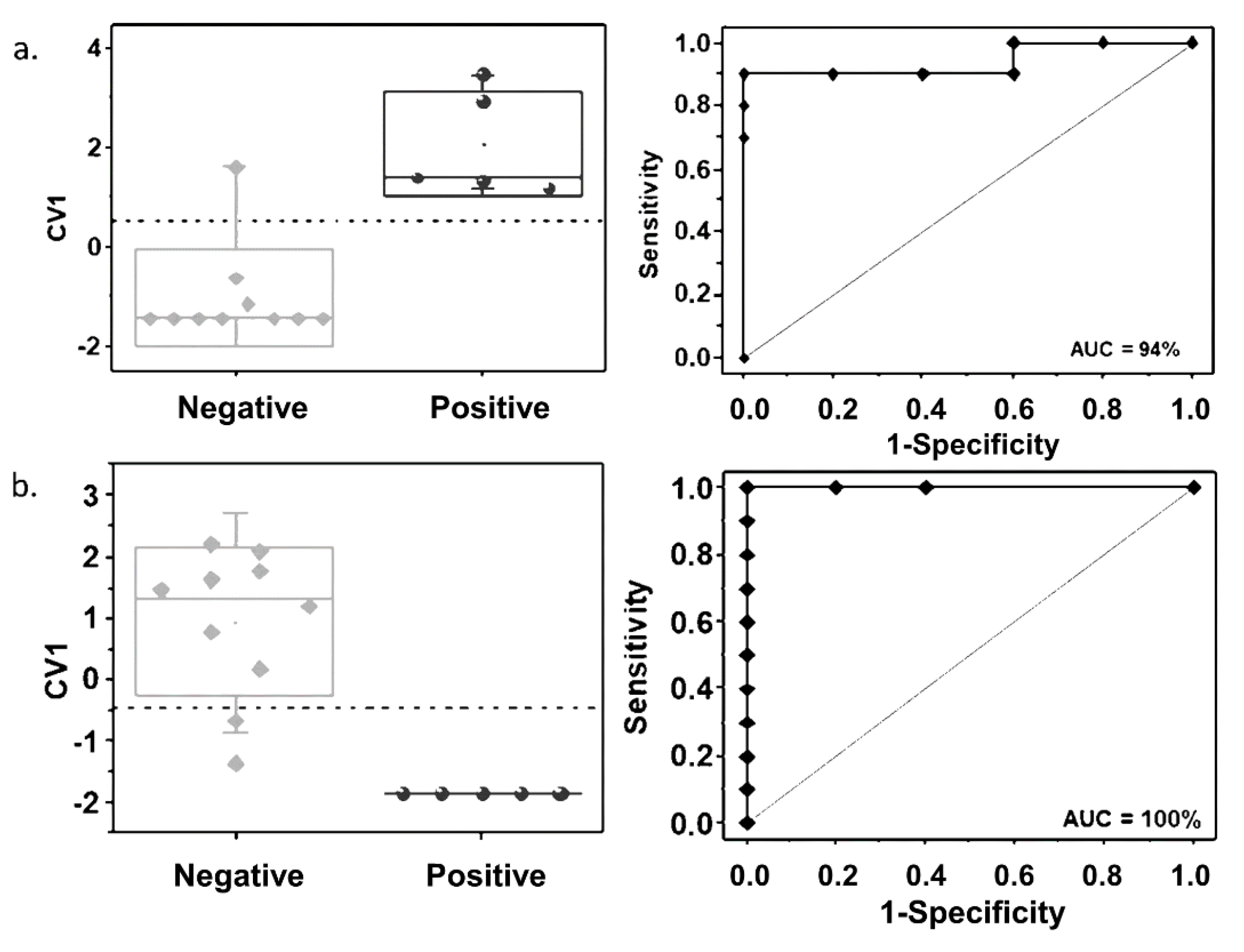
2.4. Classification Models for MTBC Prediction
3. Discussion
4. Methods
4.1. Assessment of Volatile Organic Compound Collection
4.2. Volatile Organic Compound Analysis of Assessment Samples
4.3. Wild Boar Field Study
4.4. Volatile Organic Compound Analysis of Field Samples
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hardstaff, J.L.; Marion, G.; Hutchings, M.; White, P.C. Evaluating the tuberculosis hazard posed to cattle from wildlife across Europe. Res. Veter- Sci. 2014, 97, S86–S93. [Google Scholar] [CrossRef] [PubMed]
- Gortázar, C.; Delahay, R.J.; McDonald, R.A.; Boadella, M.; Wilson, G.J.; Acevedo, P.; Gavier-Widen, D. The status of tuberculosis in European wild mammals. Mammal Rev. 2011, 42, 193–206. [Google Scholar] [CrossRef]
- Barasona, J.A.; Díez-Delgado, I.; Aznar, J.; Vicente, J.; Gortázar, C.; Torres, M.J. Environmental presence of Mycobacterium tuberculosis complex in aggregation points at the wildlife/livestock interface. Transbound. Emerg. Dis. 2016, 64, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.K.; Rice, S.; Maurer, D.; Stahl, R.; Waters, W.R.; Palmer, M.V.; Nol, P.; Rhyan, J.C.; Vercauteren, K.C.; Koziel, J.A. Use of fecal volatile organic compound analysis to discriminate between non-vaccinated and BCG—vaccinated cattle prior to and after Mycobacterium bovis challenge. PLoS ONE 2017, 12, e0179914. [Google Scholar] [CrossRef] [PubMed]
- Stahl, R.S.; Ellis, C.K.; Nol, P.; Waters, W.R.; Palmer, M.; Vercauteren, K.C. Fecal volatile organic compound profiles from white-tailed deer (Odocoileus virginianus) as indicators of Mycobacterium bovis exposure or Mycobacterium bovis Bacille Calmette-Guerin (BCG) vaccination. PLoS ONE 2015, 10, e0129740. [Google Scholar] [CrossRef]
- Phillips, M.; Basa-Dalay, V.; Bothamley, G.; Cataneo, R.N.; Lam, P.K.; Natividad, M.P.R.; Schmitt, P.; Wai, J. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis 2010, 90, 145–151. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Condos, R.; Erickson, G.A.R.; Greenberg, J.; La Bombardi, V.; Munawar, M.I.; Tietje, O. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis 2007, 87, 44–52. [Google Scholar] [CrossRef]
- Fend, R.; Kolk, A.H.J.; Bessant, C.; Buijtels, P.; Klatser, P.; Woodman, A.C. Prospects for clinical application of electronic-nose technology to early detection of Mycobacterium tuberculosis in culture and sputum. J. Clin. Microbiol. 2006, 44, 2039–2045. [Google Scholar] [CrossRef]
- Teixeira, R.C.; Rodríguez, M.; De Romero, N.J.; Bruins, M.; Gómez, R.; Yntema, J.B.; Abente, G.C.; Gerritsen, J.W.; Wiegerinck, W.; Bejerano, D.P.; et al. The potential of a portable, point-of-care electronic nose to diagnose tuberculosis. J. Infect. 2017, 75, 441–447. [Google Scholar] [CrossRef]
- Peled, N.; Ionescu, R.; Nol, P.; Barash, O.; McCollum, M.; Vercauteren, K.; Koslow, M.; Stahl, R.; Rhyan, J.; Haick, H. Detection of volatile organic compounds in cattle naturally infected with Mycobacterium bovis. Sens. Actuators B Chem. 2012, 171, 588–594. [Google Scholar] [CrossRef]
- Fend, R.; Geddes, R.; Lesellier, S.; Vordermeier, H.-M.; Corner, L.A.L.; Gormley, E.; Costello, E.; Hewinson, R.G.; Marlin, D.J.; Woodman, A.C.; et al. Use of an electronic nose to diagnose Mycobacterium bovis infection in badgers and cattle. J. Clin. Microbiol. 2005, 43, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Spooner, A.D.; Bessant, C.; Turner, C.; Knobloch, H.; Chambers, M. Evaluation of a combination of SIFT-MS and multivariate data analysis for the diagnosis of Mycobacterium bovis in wild badgers. Analyst 2009, 134, 1922. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Franchina, F.A.; Mellors, T.R.; Aliyeva, M.; Wagner, J.; Daphtary, N.; A Lundblad, L.K.; Fortune, S.M.; Rubin, E.J.; Hill, J.E. Towards the use of breath for detecting mycobacterial infection: A case study in a murine model. J. Breath Res. 2018, 12, 026008. [Google Scholar] [CrossRef] [PubMed]
- Costello, B.D.L.; Amann, A.; Alkateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 14001. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.; Hung, I.; Yen, J.; Hwang, B.; Soong, W. Determination of benzene and alkylbenzenes in milk by purge and trap gas chromatography. Toxicol. Environ. Chem. 1998, 67, 1–7. [Google Scholar] [CrossRef]
- Pleil, J.; Lindstrom, A.B. Exhaled human breath measurement method for assessing exposure to halogenated volatile organic compounds. Clin. Chem. 1997, 43, 723–730. [Google Scholar] [CrossRef]
- Phillips, M.; Greenberg, J.; Awad, J. Metabolic and environmental origins of volatile organic compounds in breath. J. Clin. Pathol. 1994, 47, 1052–1053. [Google Scholar] [CrossRef]
- Pleil, J. Role of exhaled breath biomarkers in environmental health science. J. Toxicol. Environ. Heal. Part B 2008, 11, 613–629. [Google Scholar] [CrossRef]
- Ciganek, M.; Neča, J. Chemical characterization of volatile organic compounds on animal farms. Veterinární Medicína 2008, 53, 641–651. [Google Scholar] [CrossRef]
- Garner, C.E.; Smith, S.; Costello, B.D.L.; White, P.; Spencer, R.; Probert, C.S.J.; Ratcliffe, N.M. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007, 21, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Bayn, A.; Nol, P.; Tisch, U.; Rhyan, J.; Ellis, C.K.; Haick, H. Detection of volatile organic compounds in Brucella abortus-seropositive bison. Anal. Chem. 2013, 85, 11146–11152. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Latham, M.C.; Acevedo, P.; Armenteros, J.A.; Latham, A.D.M.; Gortázar, C.; Carro, F.; Soriguer, R.C.; Vicente, J. Spatiotemporal interactions between wild boar and cattle: Implications for cross-species disease transmission. Veter-Res. 2014, 45, 122. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Olvera, J.R.L.; Beltran-Beck, B.; Gortázar, C.; Vicente, J. Trap-effectiveness and response to tiletamine-zolazepam and medetomidine anaesthesia in Eurasian wild boar captured with cage and corral traps. BMC Veter- Res. 2013, 9, 107. [Google Scholar] [CrossRef]
- Gortázar, C.; Torres, M.J.; Vicente, J.; Acevedo, P.; Reglero, M.; De La Fuente, J.; Negro, J.J.; Martín, J.A. Bovine tuberculosis in Doñana Biosphere Reserve: The role of wild ungulates as disease reservoirs in the last Iberian lynx strongholds. PLoS ONE 2008, 3, e2776. [Google Scholar] [CrossRef]
- Scott, S.M.; James, D.; Ali, Z. Data analysis for electronic nose systems. Microchim. Acta 2006, 156, 183–207. [Google Scholar] [CrossRef]
- Tisch, U.; Aluf, Y.; Ionescu, R.; Nakhleh, M.; Bassal, R.; Axelrod, N.; Robertman, R.; Tessler, Y.; Finberg, J.P.; Haick, H. Detection of asymptomatic nigrostriatal dopaminergic lesion in rats by exhaled air analysis using carbon nanotube sensors. ACS Chem. Neurosci. 2011, 3, 161–166. [Google Scholar] [CrossRef]
| Day | ||||
|---|---|---|---|---|
| Sample Source | Flow Rate | 1 | 2 | 3 |
| Breath | 100 mL/min for 1 min | |||
| 100 mL/min for 2 min | ||||
| 200 mL/min for 1 min | √ | |||
| 200 mL/min for 2 min | √ | |||
| 1L/min for 1 min | √ | √ | ||
| 1L/min for 2 min | √ | √ | ||
| Feces | 200 mL/min for 2 min | |||
| 200 mL/min for 5 min | ||||
| 200 mL/min for 10 min | √ | √ | √ | |
| Age | Nº of Animals | |||
|---|---|---|---|---|
| MTBC-Negative | MTBC-Positive | |||
| Male | Female | Male | Female | |
| Adult | 2 | 4 | 4 | 2 |
| Sub-adult | - | 2 | 3 | 1 |
| Juvenile | 4 | 6 | 3 | 2 |
| Total | 6 | 12 | 10 | 5 |
| Age | VOC Number | VOC | Chemical Family | p-Value |
|---|---|---|---|---|
| Adult | ABVOC 1 01 | O-cymene | Aromatic | 0.045 |
| Juvenile | JBVOC 2 01 | Acetic acid, methyl ester | Ester | 0.022 |
| JBVOC 02 | 3-methylpentane | Alkane | 0.018 | |
| JBVOC 03 | Trichloromethane | Alkane Derivative | 0.047 | |
| JBVOC 04 | α-methylstyrene | Aromatic | 0.046 | |
| JBVOC 05 | Decane | Alkane | 0.045 | |
| JBVOC 06 | 4,6,8-trimethyl-1-nonene | Alkene | 0.024 | |
| JBVOC 07 | 1,3-bis(1,1-dimethylethyl)-benzene | Aromatic | 0.033 | |
| JBVOC 08 | 2,5-dimethylhexane-2,5-dihydroperoxide | Alkane Derivative | 0.036 | |
| JBVOC 09 | 2,5-bis(1,1-dimethylethyl)-phenol | Aromatic Derivative | 0.012 | |
| JBVOC 10 | Heptacosane | Alkane | 0.001 | |
| JBVOC 11 | 5-butyl-5-ethylheptadecane | Alkane | 0.003 | |
| JBVOC 12 | 11-decyl-tetracosane | Alkane | 0.002 | |
| JBVOC 13 | 11-(1-ethylpropyl)-heneicosane | Alkane | 0.001 | |
| JBVOC 14 | 3-ethyl-5-(2-ethylbutyl)-octadecane | Alkane | 0.001 |
| Age | VOC Number | VOC | Chemical Family | p-Value |
|---|---|---|---|---|
| Sub-Adult | SAFVOC 1 01 | 10,18-bisnorabieta-8,11,13-triene | Aromatic | 0.048 |
| Juvenile | JFVOC 2 01 | Acetone | Ketone | 0.009 |
| JFVOC 02 | Toluene | Aromatic | 0.041 | |
| JFVOC 03 | 2,6-bis(1,1-dimethylethyl)-4-(1-methylpropyl)-phenol | Aromatic | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nol, P.; Ionescu, R.; Geremariam Welearegay, T.; Barasona, J.A.; Vicente, J.; de Jesus Beleño-Sáenz, K.; Barrenetxea, I.; Jose Torres, M.; Ionescu, F.; Rhyan, J. Evaluation of Volatile Organic Compounds Obtained from Breath and Feces to Detect Mycobacterium tuberculosis Complex in Wild Boar (Sus scrofa) in Doñana National Park, Spain. Pathogens 2020, 9, 346. https://doi.org/10.3390/pathogens9050346
Nol P, Ionescu R, Geremariam Welearegay T, Barasona JA, Vicente J, de Jesus Beleño-Sáenz K, Barrenetxea I, Jose Torres M, Ionescu F, Rhyan J. Evaluation of Volatile Organic Compounds Obtained from Breath and Feces to Detect Mycobacterium tuberculosis Complex in Wild Boar (Sus scrofa) in Doñana National Park, Spain. Pathogens. 2020; 9(5):346. https://doi.org/10.3390/pathogens9050346
Chicago/Turabian StyleNol, Pauline, Radu Ionescu, Tesfalem Geremariam Welearegay, Jose Angel Barasona, Joaquin Vicente, Kelvin de Jesus Beleño-Sáenz, Irati Barrenetxea, Maria Jose Torres, Florina Ionescu, and Jack Rhyan. 2020. "Evaluation of Volatile Organic Compounds Obtained from Breath and Feces to Detect Mycobacterium tuberculosis Complex in Wild Boar (Sus scrofa) in Doñana National Park, Spain" Pathogens 9, no. 5: 346. https://doi.org/10.3390/pathogens9050346
APA StyleNol, P., Ionescu, R., Geremariam Welearegay, T., Barasona, J. A., Vicente, J., de Jesus Beleño-Sáenz, K., Barrenetxea, I., Jose Torres, M., Ionescu, F., & Rhyan, J. (2020). Evaluation of Volatile Organic Compounds Obtained from Breath and Feces to Detect Mycobacterium tuberculosis Complex in Wild Boar (Sus scrofa) in Doñana National Park, Spain. Pathogens, 9(5), 346. https://doi.org/10.3390/pathogens9050346







