Effect of Litter Treatment on Campylobacter jejuni in Broilers and on Cecal Microbiota
Abstract
1. Introduction
2. Results
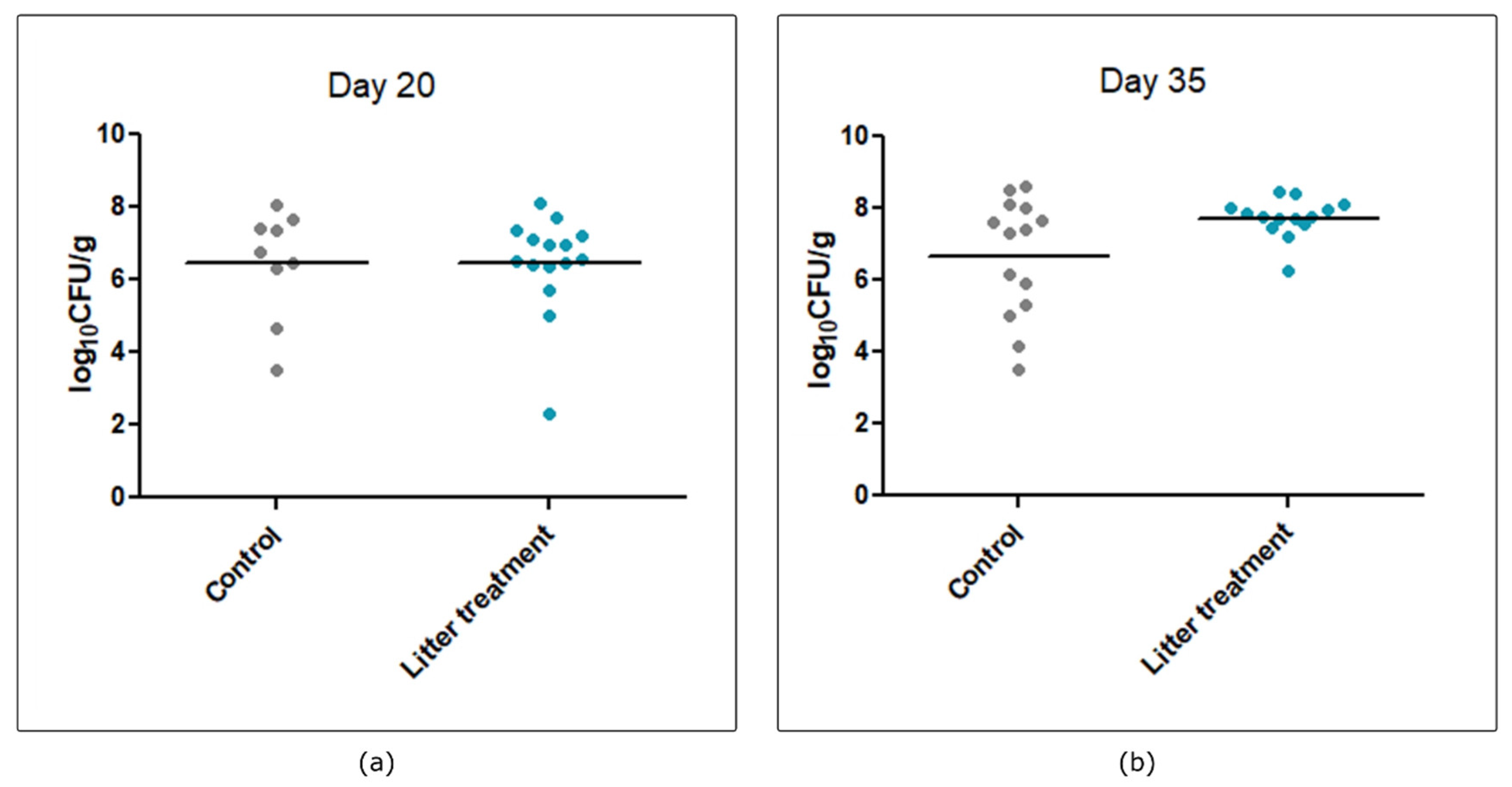
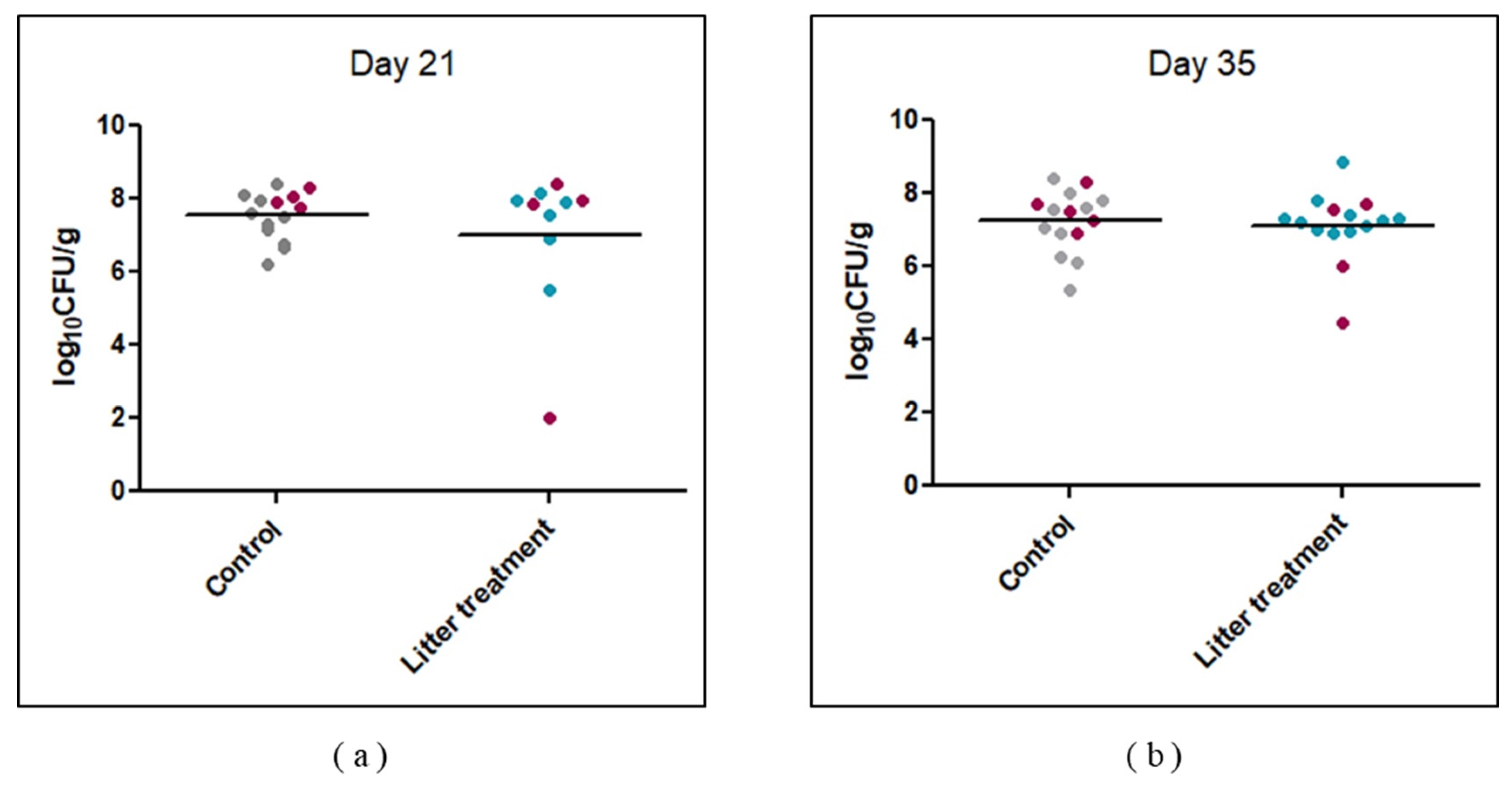
2.1. Impact of Litter Treatment on Broilers’ Technical Performances and Their Colonization by Campylobacter
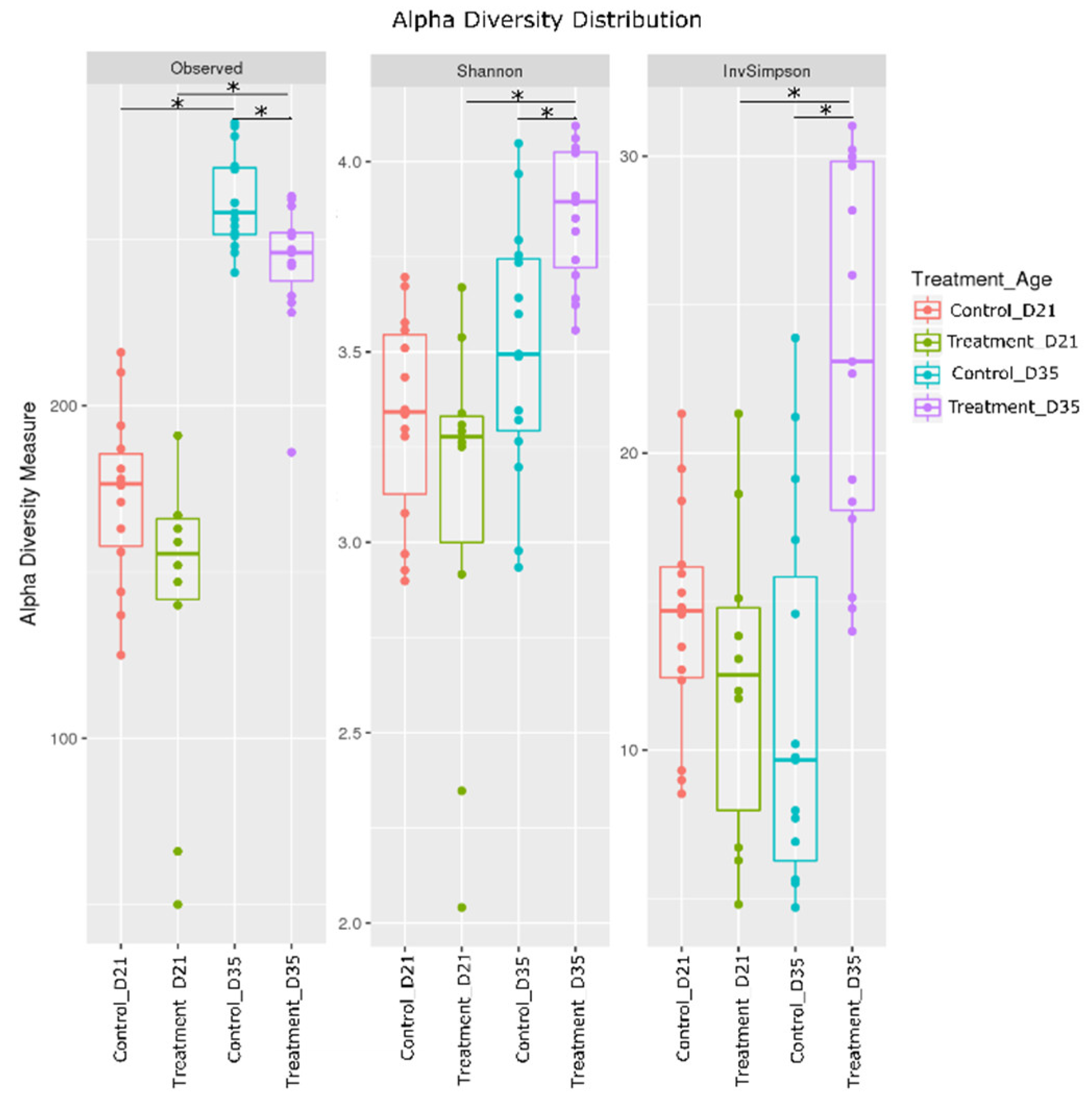
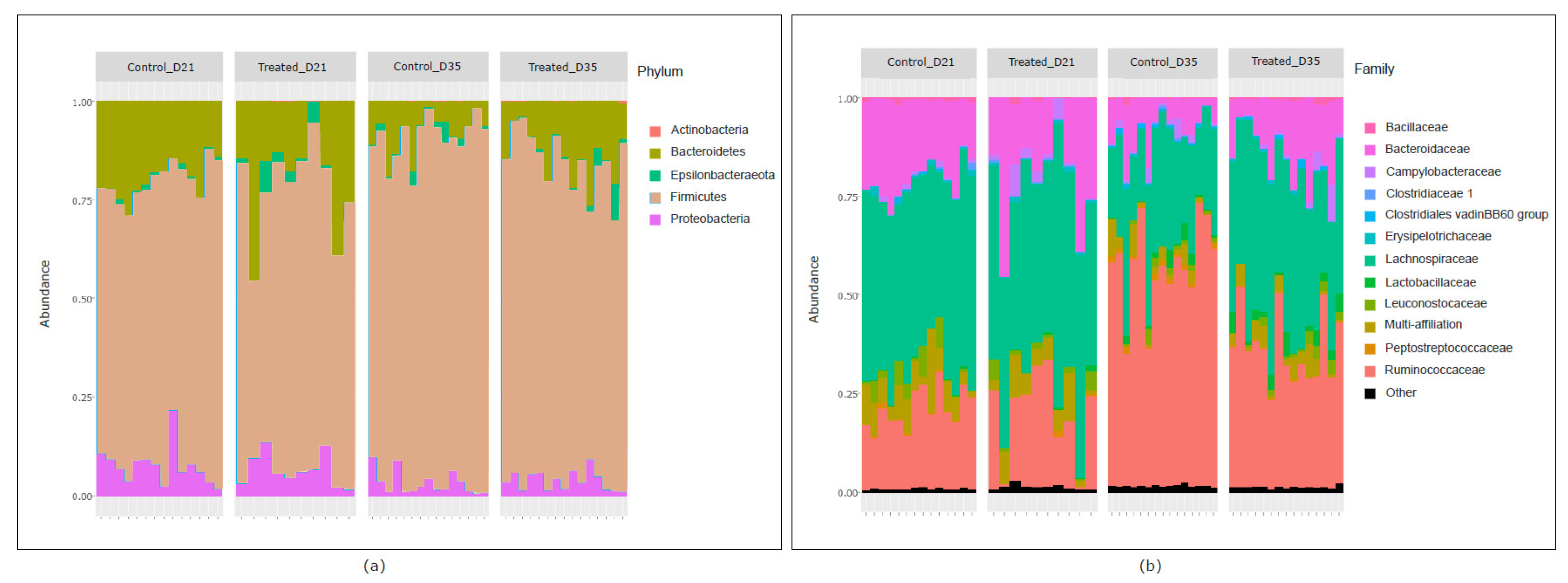
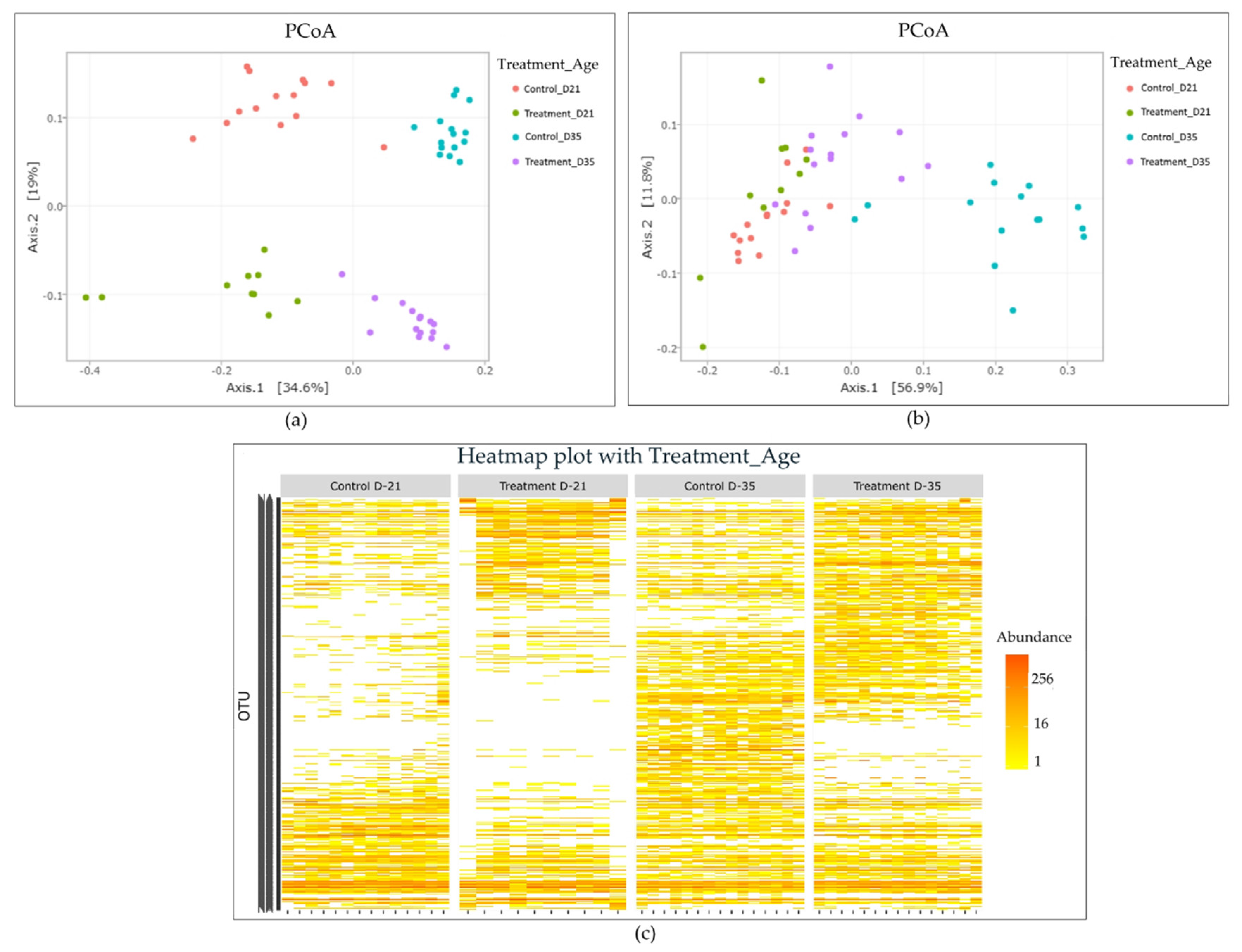
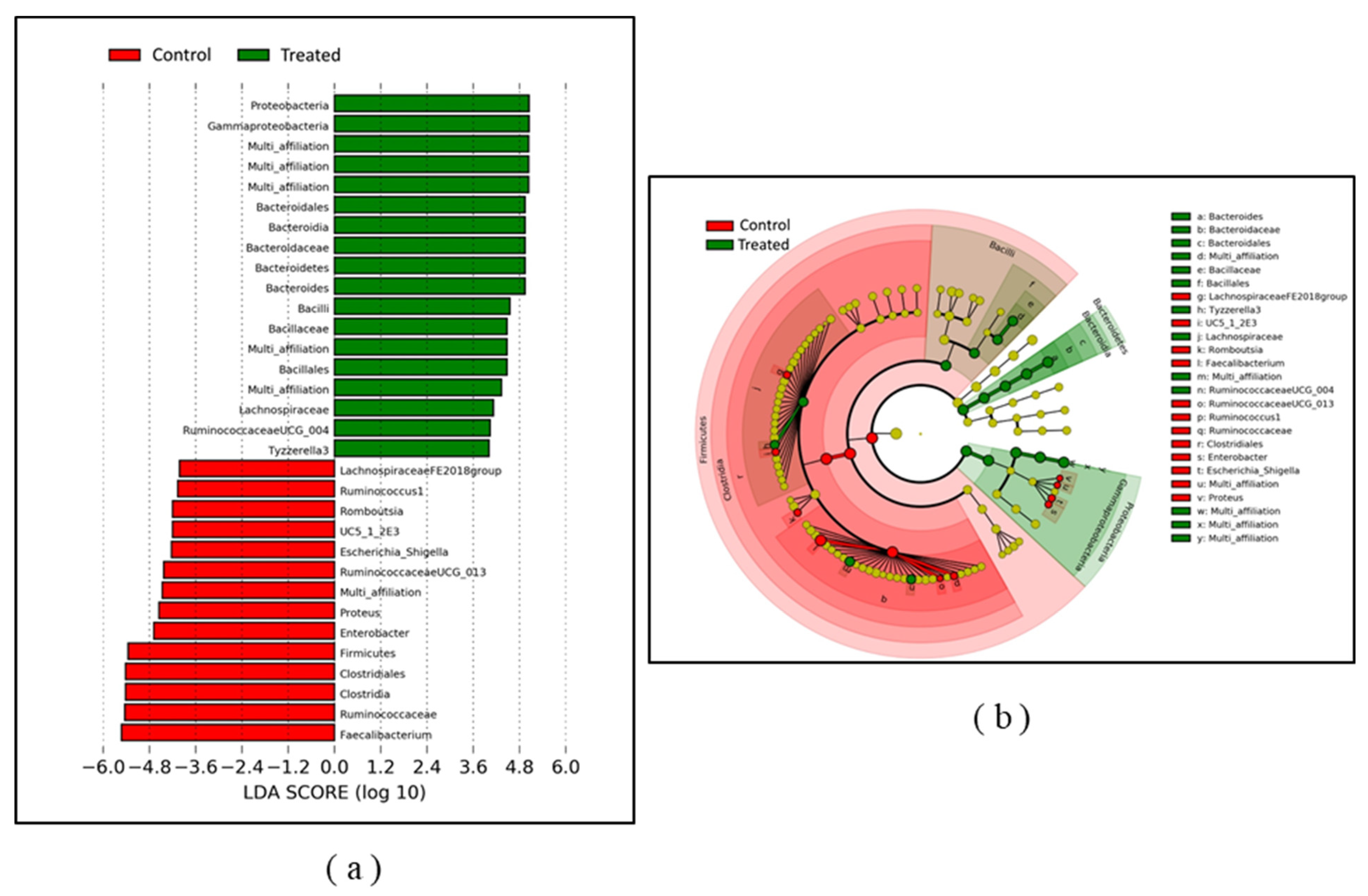
2.2. Effect of Litter Treatment on the Composition of Broilers’ Cecal Microbiota
3. Discussion
4. Materials and Methods
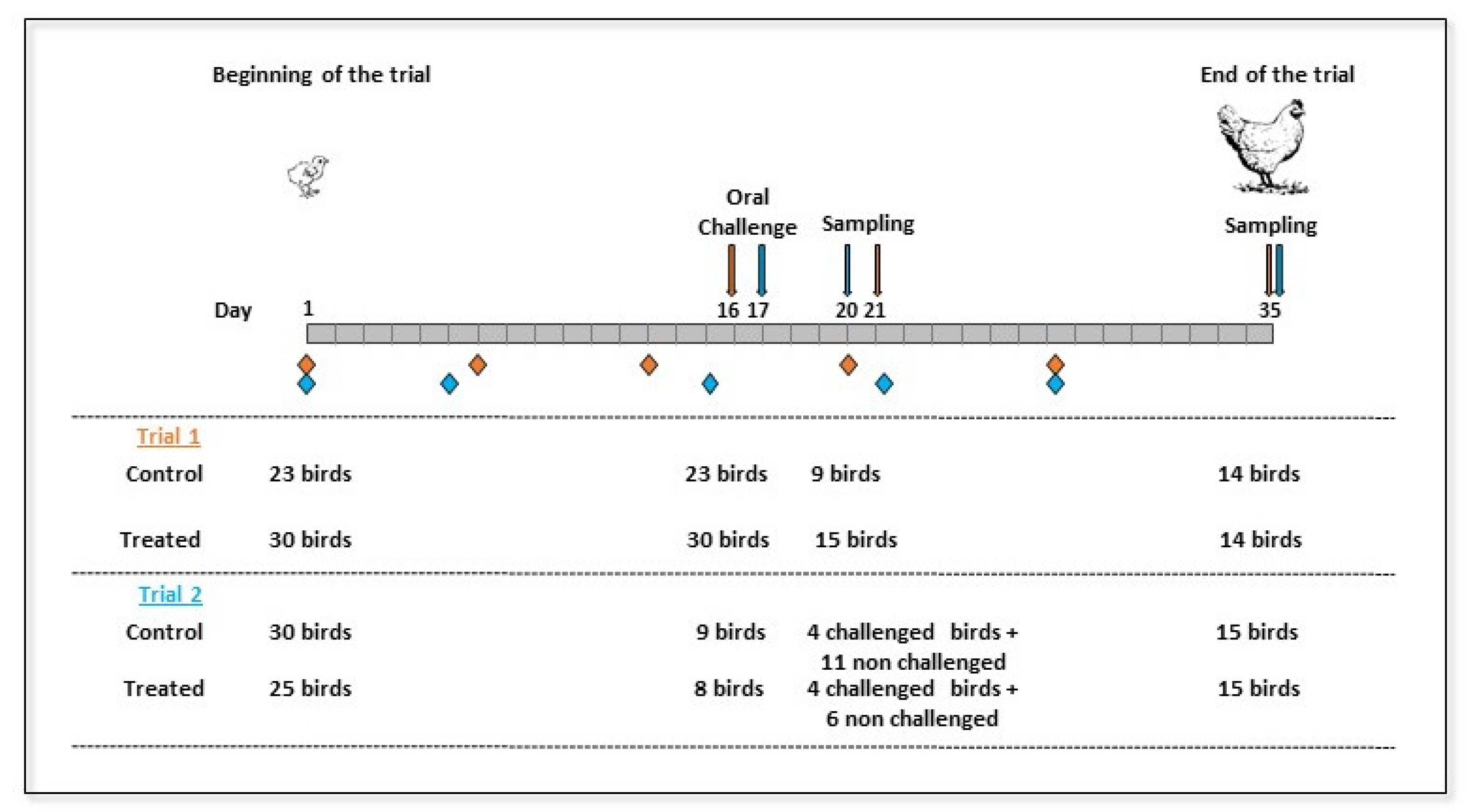
4.1. In-Vivo Experimental Design
4.1.1. C. jejuni Culture Condition
4.1.2. Poultry and Housing Conditions
4.1.3. Chick Colonization Assay
4.1.4. Cecal Enumeration of Campylobacter spp.
4.1.5. Statistical Analyses
4.2. Cecal Microbiota Analyses
4.2.1. DNA Extraction
4.2.2. PCR Amplification of Bacterial 16S Ribosomal Genes and MiSeq Illumina Sequencing
4.2.3. Sequence Analyses
4.2.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EFSA. Scientific report on the European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Ivarsson, S.; Lofdahl, M.; Nauta, M.J. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiol. Infect. 2013, 141, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Van Cauteren, D.; De Valk, H.; Sommen, C.; King, L.A.; Jourdan-Da Silva, N.; Weill, F.X.; Le Hello, S.; Megraud, F.; Vaillant, V.; Desenclos, J.C. Community Incidence of Campylobacteriosis and Nontyphoidal Salmonellosis, France, 2008–2013. Foodborne Pathog. Dis. 2015, 12, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Mughini Gras, L.; Smid, J.H.; Wagenaar, J.A.; de Boer, A.G.; Havelaar, A.H.; Friesema, I.H.; French, N.P.; Busani, L.; van Pelt, W. Risk factors for campylobacteriosis of chicken, ruminant, and environmental origin: A combined case-control and source attribution analysis. PLoS ONE 2012, 7, e42599. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B.M.; Schielke, A.; Didelot, X.; Kops, F.; Breidenbach, J.; Willrich, N.; Golz, G.; Alter, T.; Stingl, K.; Josenhans, C.; et al. A combined case-control and molecular source attribution study of human Campylobacter infections in Germany, 2011–2014. Sci. Rep. 2017, 7, 5139. [Google Scholar] [CrossRef] [PubMed]
- Thepault, A.; Rose, V.; Quesne, S.; Poezevara, T.; Beven, V.; Hirchaud, E.; Touzain, F.; Lucas, P.; Meric, G.; Mageiros, L.; et al. Ruminant and chicken: Important sources of campylobacteriosis in France despite a variation of source attribution in 2009 and 2015. Sci. Rep. 2018, 8, 9305. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- EFSA_Panel_on_Biological_Hazards_(BIOHAZ). Scientific Opinion on Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010, 8, 1437. [Google Scholar] [CrossRef]
- Hue, O.; Le Bouquin, S.; Laisney, M.J.; Allain, V.; Lalande, F.; Petetin, I.; Rouxel, S.; Quesne, S.; Gloaguen, P.Y.; Picherot, M.; et al. Prevalence of and risk factors for Campylobacter spp. contamination of broiler chicken carcasses at the slaughterhouse. Food Microbiol. 2010, 27, 992–999. [Google Scholar] [CrossRef]
- Hue, O.; Allain, V.; Laisney, M.J.; Le Bouquin, S.; Lalande, F.; Petetin, I.; Rouxel, S.; Quesne, S.; Gloaguen, P.Y.; Picherot, M.; et al. Campylobacter contamination of broiler caeca and carcasses at the slaughterhouse and correlation with Salmonella contamination. Food Microbiol. 2011, 28, 862–868. [Google Scholar] [CrossRef]
- Hansson, I.; Pudas, N.; Harbom, B.; Engvall, E.O. Within-flock variations of Campylobacter loads in caeca and on carcasses from broilers. Int. J. Food Microbiol. 2010, 141, 51–55. [Google Scholar] [CrossRef] [PubMed]
- The European Commission. COMMISSION REGULATION (EU) 2017/1495 of 23 August 2017 amending Regulation (EC) No 2073/2005 as regards Campylobacter in broiler carcases. Off. J. Eur. Union 2017, L218, 1–6.
- Sibanda, N.; McKenna, A.; Richmond, A.; Ricke, S.C.; Callaway, T.; Stratakos, A.C.; Gundogdu, O.; Corcionivoschi, N. A Review of the Effect of Management Practices on Campylobacter Prevalence in Poultry Farms. Front. Microbiol. 2018, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Guyard-Nicodeme, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sanchez, J.; Vesseur, P.; Martin, A.; Medel, P.; et al. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 2016, 95, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Gracia, M.I.; Millan, C.; Sanchez, J.; Guyard-Nicodeme, M.; Mayot, J.; Carre, Y.; Csorbai, A.; Chemaly, M.; Medel, P. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period: Part B. Poult. Sci. 2016, 95, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M.; Guyard-Nicodeme, M.; Dory, D.; Chemaly, M. Control strategies against Campylobacter at the poultry production level: Biosecurity measures, feed additives and vaccination. J. Appl. Microbiol. 2016, 120, 1139–1173. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.J.; Guyard-Nicodeme, M.; Messaoudi, S.; Chemaly, M.; Cappelier, J.M.; Dousset, X.; Haddad, N. Recent Advances in Screening of Anti-Campylobacter Activity in Probiotics for Use in Poultry. Front. Microbiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Saint-Cyr, M.J.; Haddad, N.; Taminiau, B.; Poezevara, T.; Quesne, S.; Amelot, M.; Daube, G.; Chemaly, M.; Dousset, X.; Guyard-Nicodeme, M. Use of the potential probiotic strain Lactobacillus salivarius SMXD51 to control Campylobacter jejuni in broilers. Int. J. Food Microbiol. 2017, 247, 9–17. [Google Scholar] [CrossRef]
- Guyard-Nicodeme, M.; Huneau-Salaun, A.; Tatone, F.A.; Skiba, F.; Quentin, M.; Quesne, S.; Poezevara, T.; Chemaly, M. Effect of Feed Additives on Productivity and Campylobacter spp. Loads in Broilers Reared under Free Range Conditions. Front. Microbiol. 2017, 8, 828. [Google Scholar] [CrossRef]
- Kassem, I.I.; Sanad, Y.; Gangaiah, D.; Lilburn, M.; Lejeune, J.; Rajashekara, G. Use of bioluminescence imaging to monitor Campylobacter survival in chicken litter. J. Appl. Microbiol. 2010, 109, 1988–1997. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, A.; Letellier, A.; Yergeau, E.; Larriviere-Gauthier, G.; Fravalo, P. Lack of Evidence That Selenium-Yeast Improves Chicken Health and Modulates the Caecal Microbiota in the Context of Colonization by Campylobacter jejuni. Front. Microbiol. 2017, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Montrose, M.S.; Shane, S.M.; Harrington, K.S. Role of litter in the transmission of Campylobacter jejuni. Avian Dis. 1985, 29, 392–399. [Google Scholar] [CrossRef]
- Newell, D.G.; Fearnley, C. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003, 69, 4343–4351. [Google Scholar] [CrossRef]
- Shreeve, J.E.; Toszeghy, M.; Pattison, M.; Newell, D.G. Sequential spread of Campylobacter infection in a multipen broiler house. Avian Dis. 2000, 44, 983–988. [Google Scholar] [CrossRef] [PubMed]
- van Gerwe, T.; Miflin, J.K.; Templeton, J.M.; Bouma, A.; Wagenaar, J.A.; Jacobs-Reitsma, W.F.; Stegeman, A.; Klinkenberg, D. Quantifying transmission of Campylobacter jejuni in commercial broiler flocks. Appl. Environ. Microbiol. 2009, 75, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Awad, W.A.; Mann, E.; Dzieciol, M.; Hess, C.; Schmitz-Esser, S.; Wagner, M.; Hess, M. Age-Related Differences in the Luminal and Mucosa-Associated Gut Microbiome of Broiler Chickens and Shifts Associated with Campylobacter jejuni Infection. Front. Cell. Infect. Microbiol. 2016, 6, 154. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Lovanh, N.; Cook, K.L.; Rothrock, M.J.; Miles, D.M.; Sistani, K. Spatial shifts in microbial population structure within poultry litter associated with physicochemical properties. Poult. Sci. 2007, 86, 1840–1849. [Google Scholar] [CrossRef]
- Wang, L.; Lilburn, M.; Yu, Z. Intestinal Microbiota of Broiler Chickens as Affected by Litter Management Regimens. Front. Microbiol. 2016, 7, 593. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, A.; Fravalo, P.; Yergeau, E.; Arsenault, J.; Lahaye, L.; Letellier, A. Chicken Caecal Microbiome Modifications Induced by Campylobacter jejuni Colonization and by a Non-Antibiotic Feed Additive. PLoS ONE 2015, 10, e0131978. [Google Scholar] [CrossRef] [PubMed]
- Connerton, P.L.; Richards, P.J.; Lafontaine, G.M.; O’Kane, P.M.; Ghaffar, N.; Cummings, N.J.; Smith, D.L.; Fish, N.M.; Connerton, I.F. The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens. Microbiome 2018, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Dingle, K.E.; Colles, F.M.; Wareing, D.R.; Ure, R.; Fox, A.J.; Bolton, F.E.; Bootsma, H.J.; Willems, R.J.; Urwin, R.; Maiden, M.C. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef]
- Federation_of_Animal_Science_Societies. Guide for the Care and Use of Agricultural Animals in Research and Teaching. Available online: www.fass.org/docs/agguide3rd/Ag_Guide_3rd_ed.pdf (accessed on 20 April 2020).
- International_Organization_for_Standardization. Microbiology of the food chain—Horizontal method for detection and enumeration of Campylobacter spp.—Part 1: Detection method (ISO 10272-1:2017). 2017. Available online: https://www.iso.org/standard/63225.html (accessed on 20 April 2020).
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Escudie, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef]
- Mahe, F.; Rognes, T.; Quince, C.; de Vargas, C.; Dunthorn, M. Swarm: Robust and fast clustering method for amplicon-based studies. PeerJ 2014, 2, e593. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| Day 20 | Day 35 | Day 21 | Day 35 | |
| Control | 824 ± 72 (n = 23) | 2444 ± 286 (n = 14) | 878 ± 145 (n = 29) | 2350 ± 286 (n = 15) |
| Litter treatment | 856 ± 71 (n = 30) | 2497 ± 229 (n = 14) | 880 ± 160 (n = 25) | 2341 ± 345 (n = 15) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thépault, A.; Roulleau, X.; Loiseau, P.; Cauquil, L.; Poezevara, T.; Hyronimus, B.; Quesne, S.; Souchaud, F.; Keita, A.; Chemaly, M.; et al. Effect of Litter Treatment on Campylobacter jejuni in Broilers and on Cecal Microbiota. Pathogens 2020, 9, 333. https://doi.org/10.3390/pathogens9050333
Thépault A, Roulleau X, Loiseau P, Cauquil L, Poezevara T, Hyronimus B, Quesne S, Souchaud F, Keita A, Chemaly M, et al. Effect of Litter Treatment on Campylobacter jejuni in Broilers and on Cecal Microbiota. Pathogens. 2020; 9(5):333. https://doi.org/10.3390/pathogens9050333
Chicago/Turabian StyleThépault, Amandine, Xavier Roulleau, Pauline Loiseau, Laurent Cauquil, Typhaine Poezevara, Bertrand Hyronimus, Ségolène Quesne, Florent Souchaud, Alassane Keita, Marianne Chemaly, and et al. 2020. "Effect of Litter Treatment on Campylobacter jejuni in Broilers and on Cecal Microbiota" Pathogens 9, no. 5: 333. https://doi.org/10.3390/pathogens9050333
APA StyleThépault, A., Roulleau, X., Loiseau, P., Cauquil, L., Poezevara, T., Hyronimus, B., Quesne, S., Souchaud, F., Keita, A., Chemaly, M., & Guyard-Nicodème, M. (2020). Effect of Litter Treatment on Campylobacter jejuni in Broilers and on Cecal Microbiota. Pathogens, 9(5), 333. https://doi.org/10.3390/pathogens9050333





