Characterization of the Humoral Immune Response to Porcine Epidemic Diarrhea Virus Infection under Experimental and Field Conditions Using an AlphaLISA Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal Study
2.2. Field Animal Study
2.3. Generation of S1 Recombinant Protein
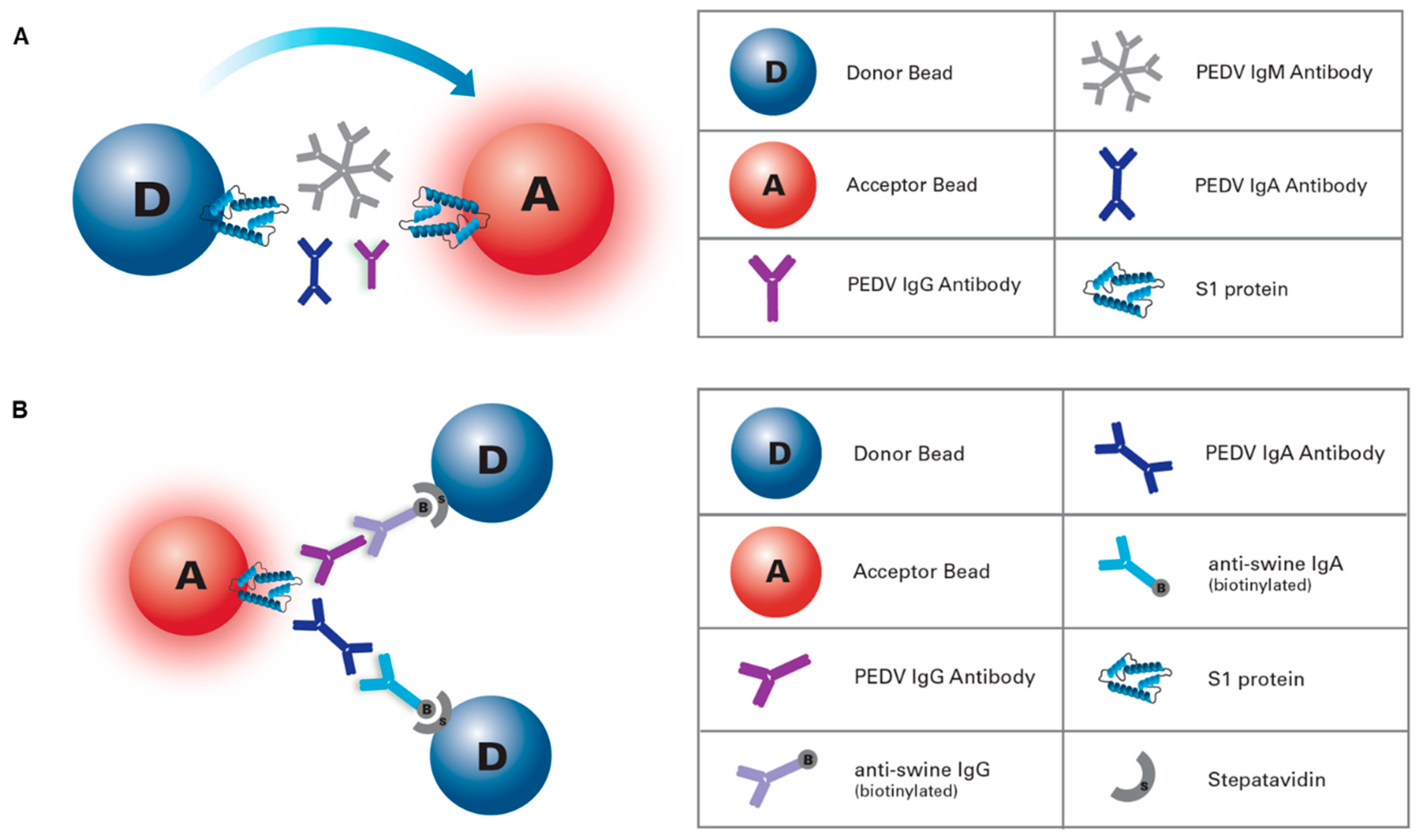
2.4. AlphaLISA Beads Conjugation
2.5. AlphaLISA IgG/IgA Antibody Assay
2.6. AlphaLISA Total Antibody Bridging Assay
2.7. Data Analysis
3. Results
3.1. Diagnostic Performance of AlphaLISA IgG/IgA Antibody Assay
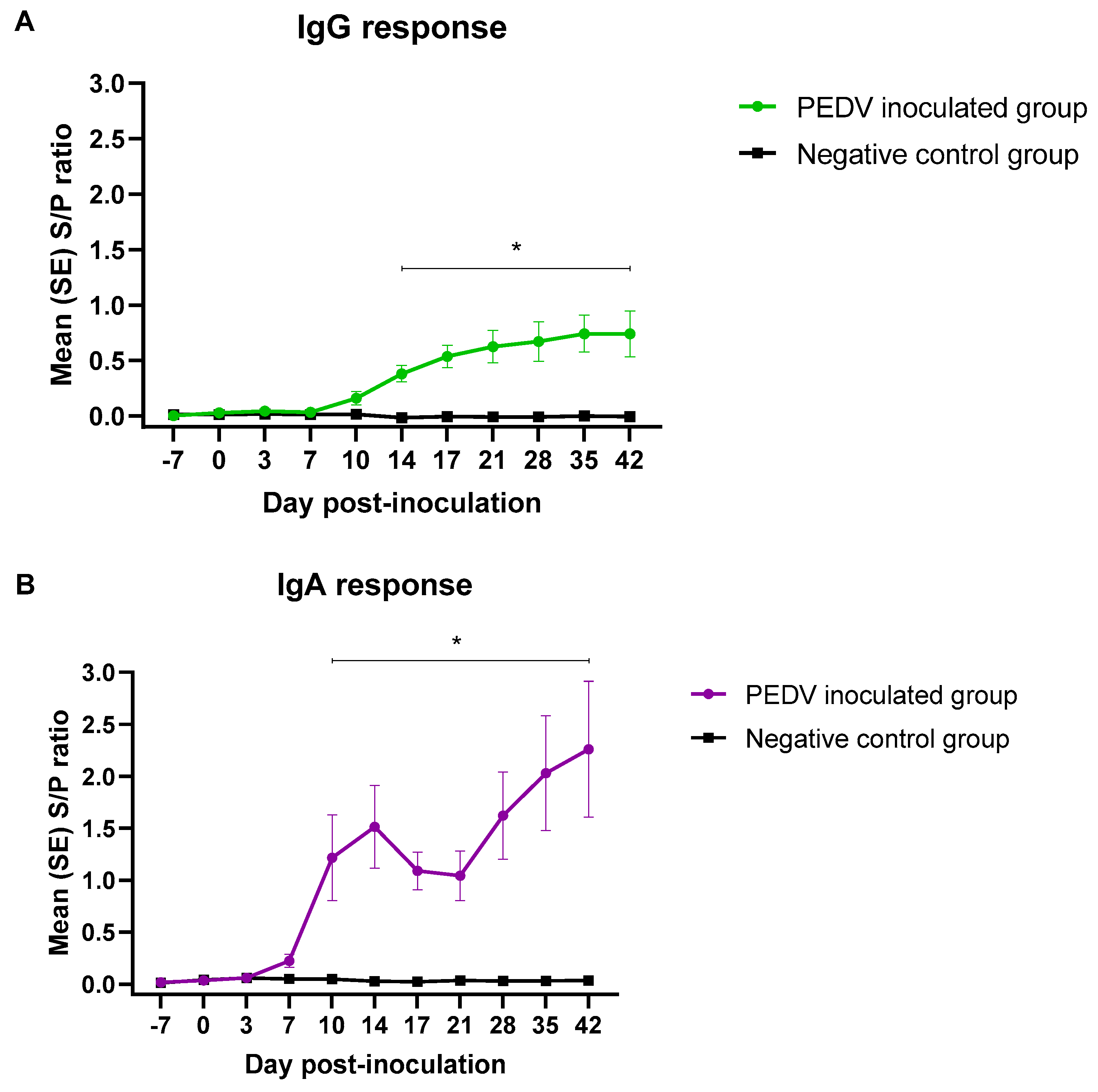
3.2. PEDV Isotype-Specific Antibody Response under Experimental Conditions
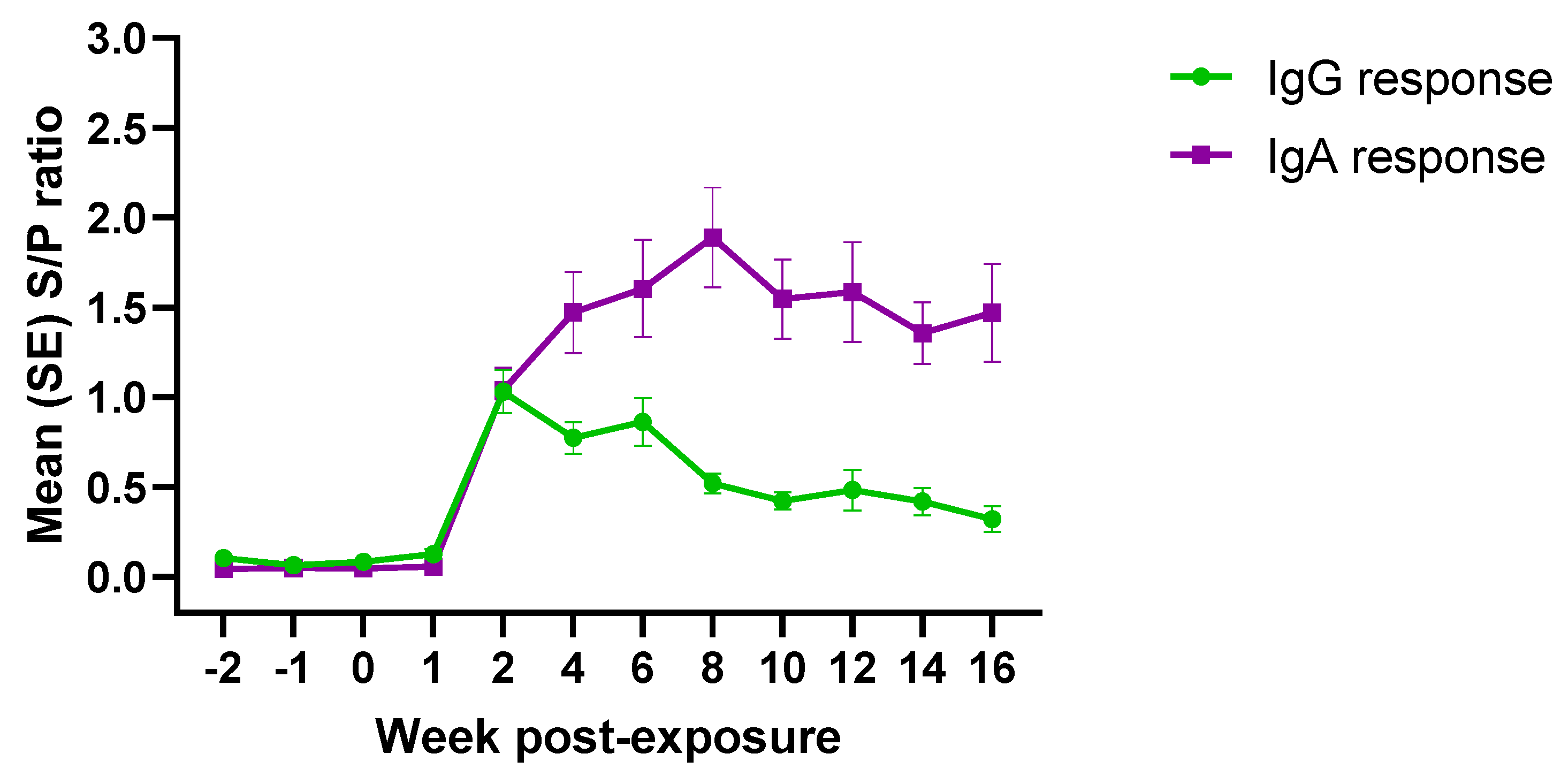
3.3. PEDV Isotype-Specific Antibody Response under Field Conditions
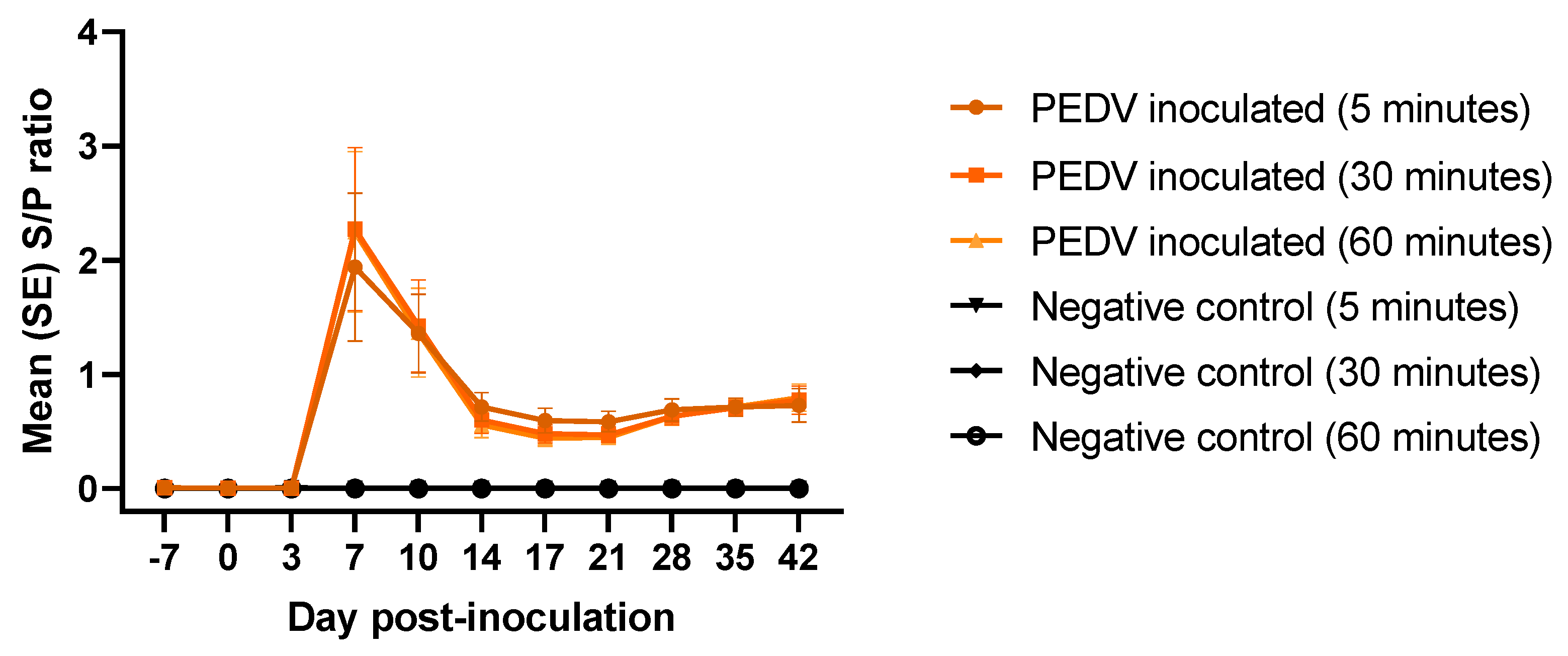
3.4. Rapid detection of PEDV Total Antibodies by AlphaLISA Bridging Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carstens, E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009). Arch. Virol. 2010, 155, 133–146. [Google Scholar] [CrossRef]
- Debouck, P.; Pensaert, M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am. J. Vet. Res. 1980, 41, 219–223. [Google Scholar]
- Madson, D.M.; Magstadt, D.R.; Arruda, P.H.; Hoang, H.; Sun, D.; Bower, L.P.; Bhandari, M.; Burrough, E.R.; Gauger, P.C.; Pillatzki, A.E.; et al. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet. Microbiol. 2014, 174, 60–68. [Google Scholar] [CrossRef]
- Kocherhans, R.; Bridgen, A.; Ackermann, M.; Tobler, K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 2001, 23, 137–144. [Google Scholar] [CrossRef]
- Oldham, J. Leter to the editor. Pig Farm. 1972, 10, 72–73. [Google Scholar]
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef]
- Chasey, D.; Cartwright, S.F. Virus-like particles associated with porcine epidemic diarrhoea. Res. Vet. Sci. 1978, 25, 255–256. [Google Scholar] [CrossRef]
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef]
- Takahashi, K.; Okada, K.; Ohshima, K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Nihon Juigaku Zasshi 1983, 45, 829–832. [Google Scholar] [CrossRef]
- Xuan, H.; Xing, D.; Wang, D.; Zhu, W.; Zhao, F.; Gong, H. Study on the culture of porcine epidemic diarrhea virus adapted to fetal porcine intestine primary cell monolayer. Chin. J. Vet. Sci. 1984, 4, 202–208. [Google Scholar]
- Kweon, C.H.; Kwon, B.J.; Jung, T.S.; Kee, Y.J.; Hur, D.H.; Hwang, E.K.; Rhee, J.C.; An, S.H. Isolation of porcine epidemic diarrhea virus (PEDV) infection in Korea. Korean J. Vet. Res. 1993, 33, 249–254. [Google Scholar]
- Cheng, Q. Investigation on epidemic diarrhea in pigs in Qinghai region. Qinghai Xumu Shaoyi Zazhi 1992, 22, 22–23. [Google Scholar]
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012, 18, 161–163. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 2013, 25, 649–654. [Google Scholar] [CrossRef]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare). Scientific Opinion on porcine epidemic diarrhoea and emerging pig deltacoronavirus. EFSA J. 2014, 12, 3877. [Google Scholar] [CrossRef]
- Shibata, I.; Tsuda, T.; Mori, M.; Ono, M.; Sueyoshi, M.; Uruno, K. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet. Microbiol. 2000, 72, 173–182. [Google Scholar] [CrossRef]
- Choudhury, B.; Dastjerdi, A.; Doyle, N.; Frossard, J.P.; Steinbach, F. From the field to the lab—An European view on the global spread of PEDV. Virus Res. 2016, 226, 40–49. [Google Scholar] [CrossRef]
- Lin, C.M.; Saif, L.J.; Marthaler, D.; Wang, Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016, 226, 20–39. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Marthaler, D.; Wang, Q.; Culhane, M.R.; Rossow, K.D.; Rovira, A.; Collins, J.; Saif, L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013-February 2014. Emerg. Infect. Dis. 2014, 20, 1620–1628. [Google Scholar] [CrossRef]
- Chen, Q.; Gauger, P.C.; Stafne, M.R.; Thomas, J.T.; Madson, D.M.; Huang, H.; Zheng, Y.; Li, G.; Zhang, J. Pathogenesis comparison between the United States porcine epidemic diarrhoea virus prototype and S-INDEL-variant strains in conventional neonatal piglets. J. Gen. Virol. 2016, 97, 1107–1121. [Google Scholar] [CrossRef]
- Diel, D.G.; Lawson, S.; Okda, F.; Singrey, A.; Clement, T.; Fernandes, M.H.V.; Christopher-Hennings, J.; Nelson, E.A. Porcine epidemic diarrhea virus: An overview of current virological and serological diagnostic methods. Virus Res. 2016, 226, 60–70. [Google Scholar] [CrossRef]
- Ullman, E.F.; Kirakossian, H.; Switchenko, A.C.; Ishkanian, J.; Ericson, M.; Wartchow, C.A.; Pirio, M.; Pease, J.; Irvin, B.R.; Singh, S.; et al. Luminescent oxygen channeling assay (LOCI): Sensitive, broadly applicable homogeneous immunoassay method. Clin. Chem. 1996, 42, 1518–1526. [Google Scholar] [CrossRef]
- Gimenez-Lirola, L.G.; Zhang, J.; Carrillo-Avila, J.A.; Chen, Q.; Magtoto, R.; Poonsuk, K.; Baum, D.H.; Pineyro, P.; Zimmerman, J. Reactivity of Porcine Epidemic Diarrhea Virus Structural Proteins to Antibodies against Porcine Enteric Coronaviruses: Diagnostic Implications. J. Clin. Microbiol. 2017, 55, 1426–1436. [Google Scholar] [CrossRef]
- Bjustrom-Kraft, J.; Woodard, K.; Gimenez-Lirola, L.; Rotolo, M.; Wang, C.; Sun, Y.; Lasley, P.; Zhang, J.; Baum, D.; Gauger, P.; et al. Porcine epidemic diarrhea virus (PEDV) detection and antibody response in commercial growing pigs. BMC Vet. Res. 2016, 12, 99. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Lucio de Esesarte, E.; Guo, H.; van den Elzen, P.; Aarts, E.; van den Born, E.; Rottier, P.J.M.; Bosch, B.J. Cell Attachment Domains of the Porcine Epidemic Diarrhea Virus Spike Protein Are Key Targets of Neutralizing Antibodies. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Subramaniam, S.; Cao, D.; Tian, D.; Cao, Q.M.; Overend, C.; Yugo, D.M.; Matzinger, S.R.; Rogers, A.J.; Heffron, C.L.; Catanzaro, N.; et al. Efficient priming of CD4 T cells by Langerin-expressing dendritic cells targeted with porcine epidemic diarrhea virus spike protein domains in pigs. Virus Res. 2017, 227, 212–219. [Google Scholar] [CrossRef]
- Okda, F.; Liu, X.; Singrey, A.; Clement, T.; Nelson, J.; Christopher-Hennings, J.; Nelson, E.A.; Lawson, S. Development of an indirect ELISA, blocking ELISA, fluorescent microsphere immunoassay and fluorescent focus neutralization assay for serologic evaluation of exposure to North American strains of Porcine Epidemic Diarrhea Virus. BMC Vet. Res. 2015, 11, 180. [Google Scholar] [CrossRef]
- Bosch, B.J.; van der Zee, R.; de Haan, C.A.; Rottier, P.J. The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. J. Virol. 2003, 77, 8801–8811. [Google Scholar] [CrossRef]
- Okda, F.A.; Lawson, S.; Singrey, A.; Nelson, J.; Hain, K.S.; Joshi, L.R.; Christopher-Hennings, J.; Nelson, E.A.; Diel, D.G. The S2 glycoprotein subunit of porcine epidemic diarrhea virus contains immunodominant neutralizing epitopes. Virology 2017, 509, 185–194. [Google Scholar] [CrossRef]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef]
| Response | Group | Day Post-Inoculation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −7 | 0 | 3 | 7 | 10 | 14 | 17 | 21 | 28 | 35 | 42 | ||
| IgG | Negative | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 |
| PEDV | 0/12 | 0/12 | 0/12 | 0/12 | 1/12 | 9/12 | 9/12 | 9/12 | 10/12 | 11/12 | 11/12 | |
| IgA | Negative | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 |
| PEDV | 0/12 | 0/12 | 0/12 | 7/12 | 10/12 | 11/12 | 10/12 | 10/12 | 11/12 | 11/12 | 11/12 | |
| Response | Week Post-Exposure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | |
| IgG | 0/30 | 1/29 | 1/30 | 4/30 | 28/30 | 27/30 | 24/27 | 26/30 | 20/30 | 19/30 | 20/30 | 13/30 |
| IgA | 0/30 | 0/29 | 0/30 | 2/30 | 29/30 | 28/30 | 25/27 | 30/30 | 28/30 | 29/30 | 30/30 | 28/30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimpston-Burkgren, K.; Mora-Díaz, J.C.; Roby, P.; Bjustrom-Kraft, J.; Main, R.; Bosse, R.; Giménez-Lirola, L.G. Characterization of the Humoral Immune Response to Porcine Epidemic Diarrhea Virus Infection under Experimental and Field Conditions Using an AlphaLISA Platform. Pathogens 2020, 9, 233. https://doi.org/10.3390/pathogens9030233
Kimpston-Burkgren K, Mora-Díaz JC, Roby P, Bjustrom-Kraft J, Main R, Bosse R, Giménez-Lirola LG. Characterization of the Humoral Immune Response to Porcine Epidemic Diarrhea Virus Infection under Experimental and Field Conditions Using an AlphaLISA Platform. Pathogens. 2020; 9(3):233. https://doi.org/10.3390/pathogens9030233
Chicago/Turabian StyleKimpston-Burkgren, Kay, Juan Carlos Mora-Díaz, Philippe Roby, Jordan Bjustrom-Kraft, Rodger Main, Roger Bosse, and Luis Gabriel Giménez-Lirola. 2020. "Characterization of the Humoral Immune Response to Porcine Epidemic Diarrhea Virus Infection under Experimental and Field Conditions Using an AlphaLISA Platform" Pathogens 9, no. 3: 233. https://doi.org/10.3390/pathogens9030233
APA StyleKimpston-Burkgren, K., Mora-Díaz, J. C., Roby, P., Bjustrom-Kraft, J., Main, R., Bosse, R., & Giménez-Lirola, L. G. (2020). Characterization of the Humoral Immune Response to Porcine Epidemic Diarrhea Virus Infection under Experimental and Field Conditions Using an AlphaLISA Platform. Pathogens, 9(3), 233. https://doi.org/10.3390/pathogens9030233






