Host-Parasite Interaction between Parasitic Cymothoid Ceratothoa oestroides and Its Host, Farmed European Sea Bass (Dicentrarchus labrax)
Abstract
1. Introduction
2. Results
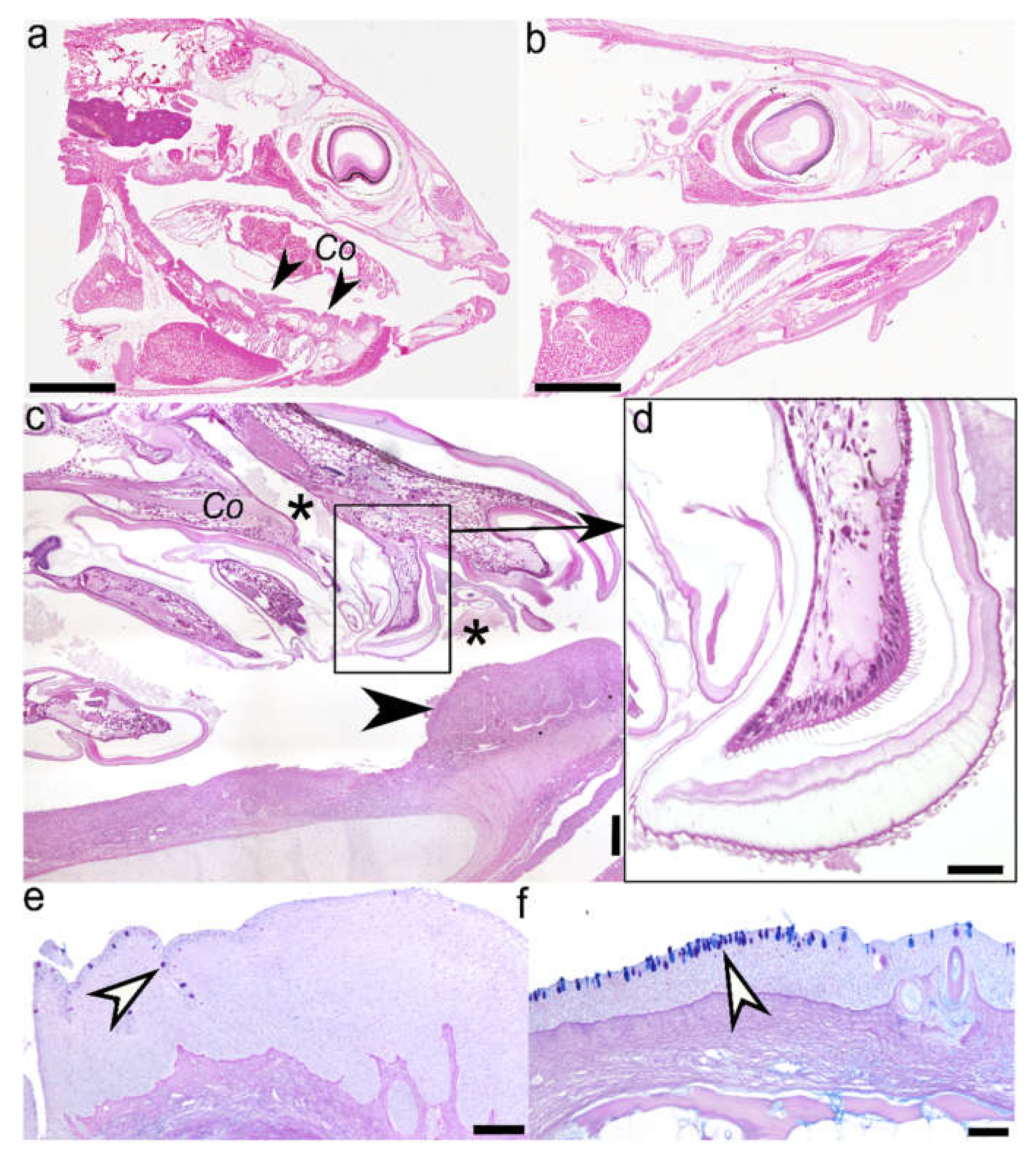
2.1. Histology (Figure 1) and Immunohistochemistry (Figure 2)
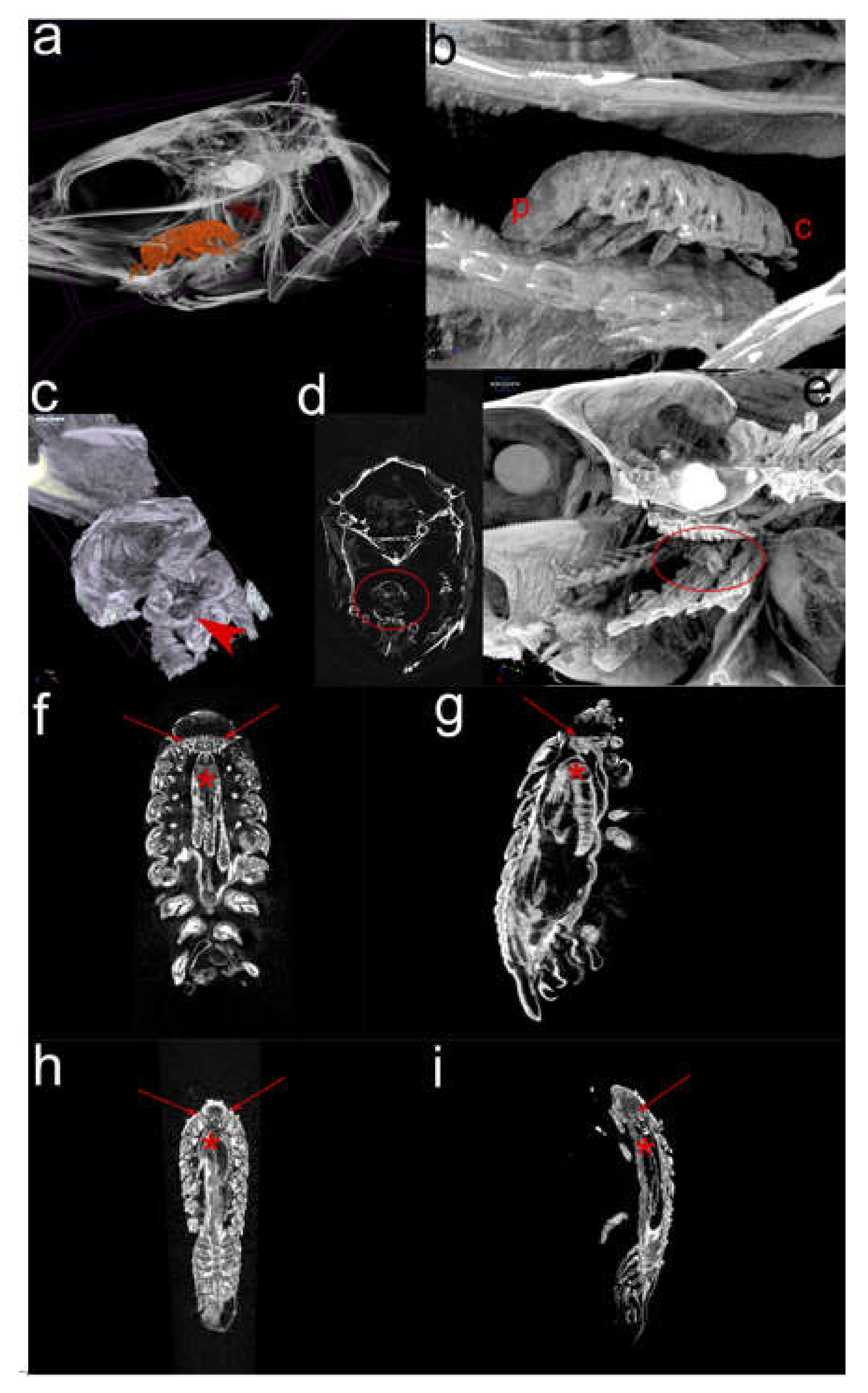
2.2. Micro-computational Tomography (µ-CT) (Figure 3)
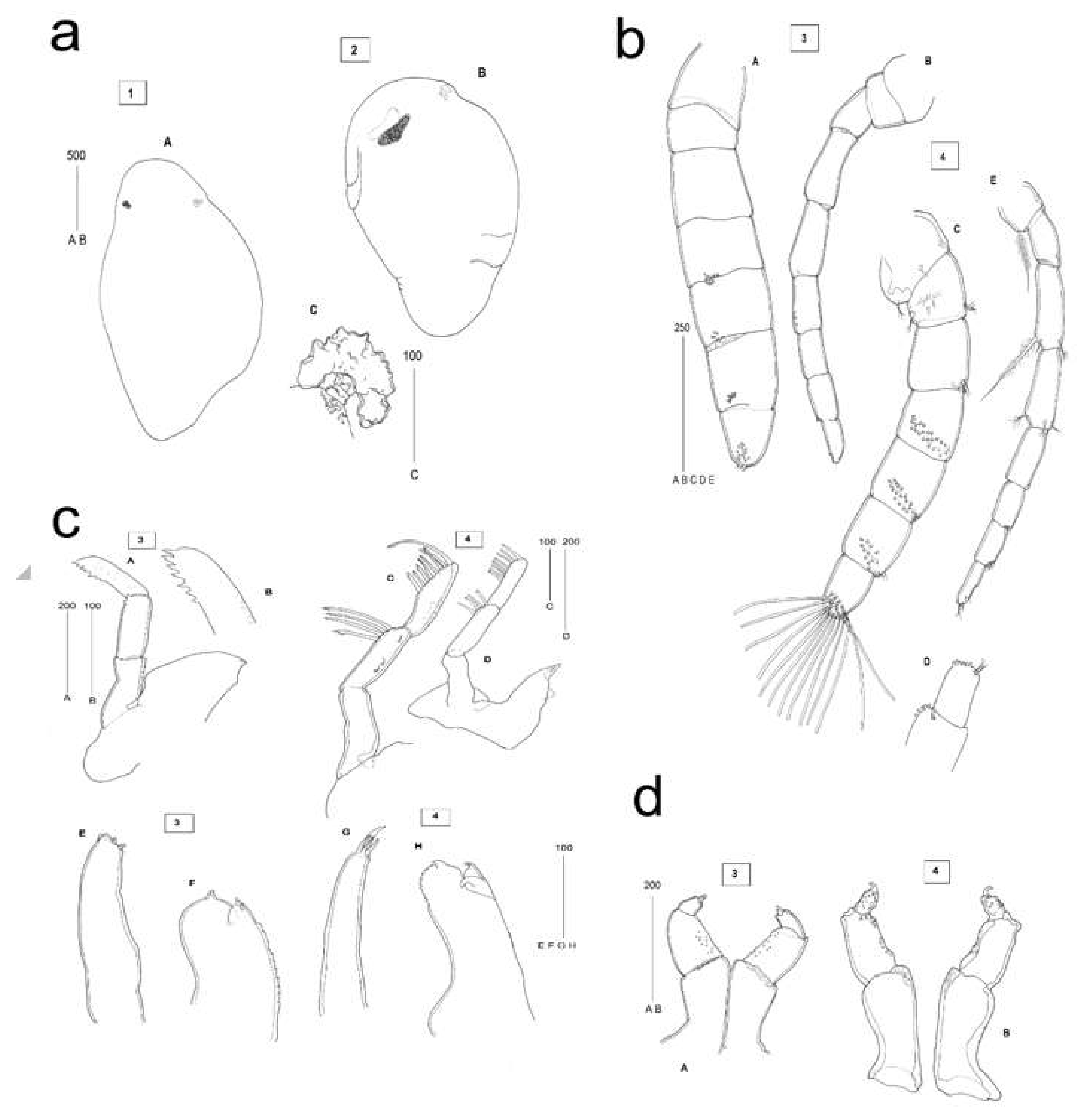
2.3. Description of C. oestroides Intramarsupial Embryonic Stages and Mouthparts (Figure 4)
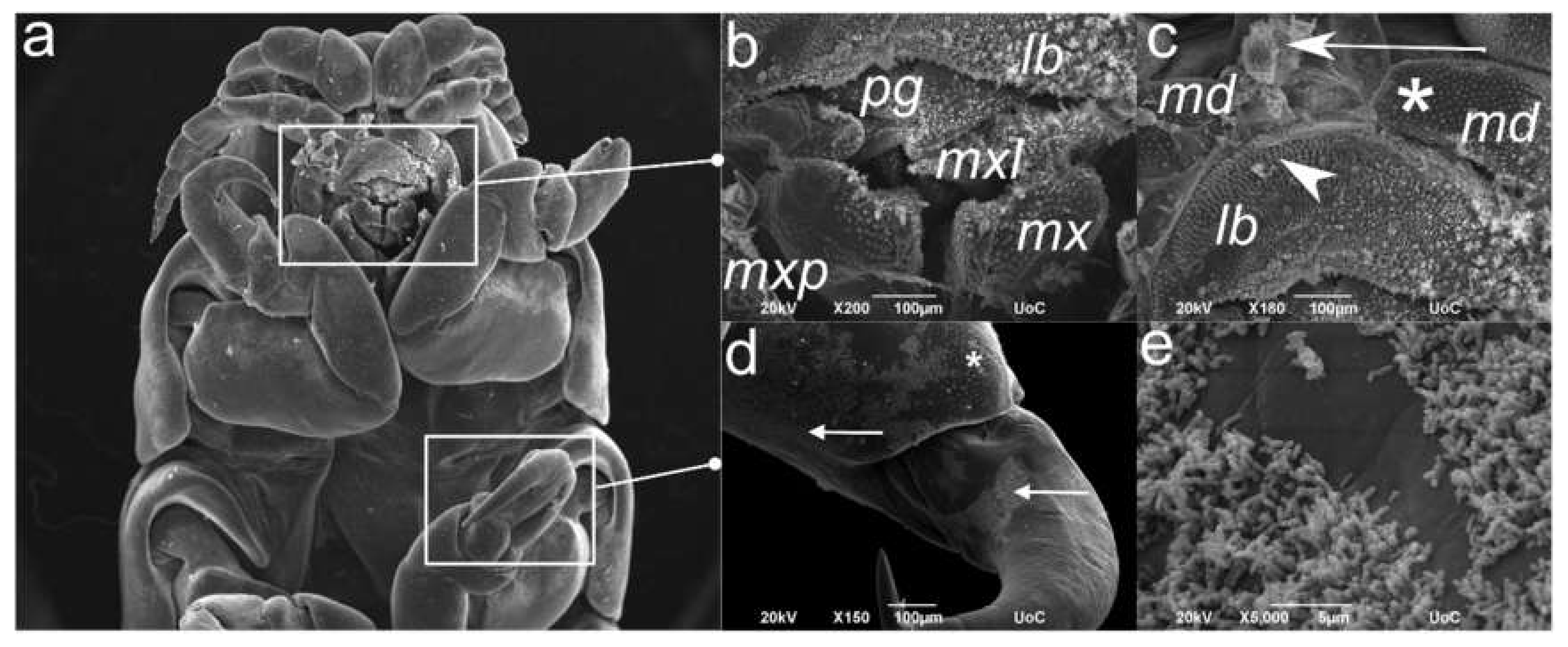
2.4. Scanning Electron Microscopy (SEM) of the C. oestroides Female (Figure 5)
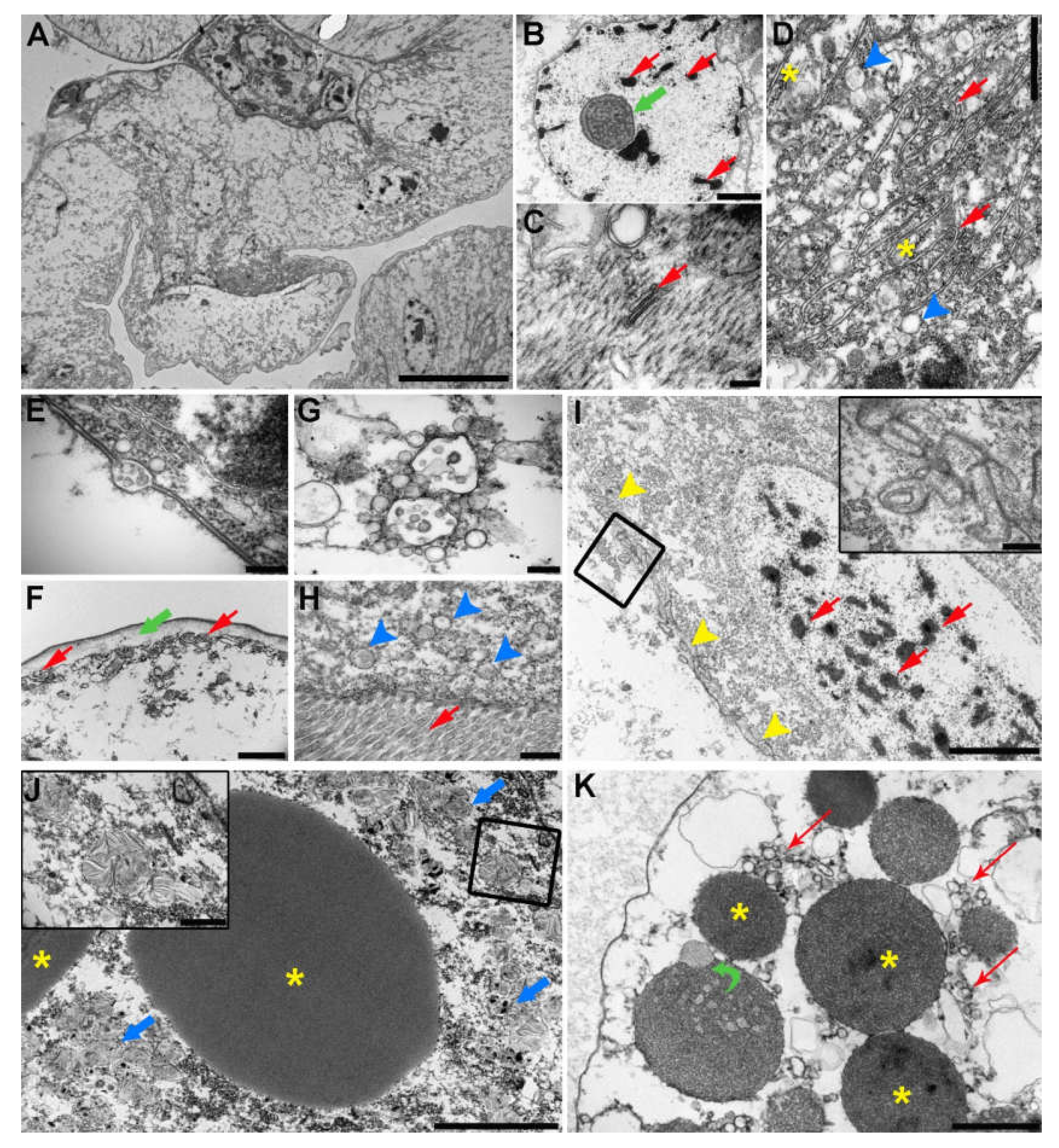
2.5. Transmission Electron Microscopy (TEM) of the C. oestroides Female (Figure 6)
3. Discussion
4. Materials and Methods
4.1. Fish Sampling
4.2. Histology and Immunohistochemistry
4.3. Micro-computational Tomography (µ-CT)
4.4. Descriptions of C. oestroides Intramarsupial Embryonic Stages and Mouthparts
4.5. Scanning Electron Microscopy (SEM)
4.6. Transmission Electron Microscopy (TEM)
4.7. Animal Ethics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smit, N.J.; Bruce, N.L.; Hadfield, K.A. Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. Int. J. Parasitol. Parasites Wildl. 2014, 3, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Poore, G.C.B. Higher classification of the flabelliferan and related Isopoda based on a reappraisal of relationships. Invertebr. Syst. 2003, 17, 893–923. [Google Scholar] [CrossRef][Green Version]
- Trilles, J.P.; Radujković, B. Parasites des poissons marine du Montenegro: Isopodes. Acta Adriat. 1989, 30, 279–306. [Google Scholar]
- Charfi-Cheikhrouha, F.; Zghidi, W.; Ould Yarba, L.; Trilles, J.P. Les Cymothoidae (Isopodes parasites de poissons) des côtes tunisiennes: Écologie et indices parasitologiques. Syst. Parasitol. 2000, 46, 143–150. [Google Scholar] [CrossRef]
- Mladineo, I.; Valić, D. The mechanisms of infection of the buccal isopod Ceratothoa oestroides (Risso, 1836), under experimental conditions. Bull. Eur. Assoc. Fish Pathol. 2002, 22, 304–310. [Google Scholar]
- Mladineo, I. Life cycle of Ceratothoa oestroides, a cymothoid isopod parasite from sea bass Dicentrarchus labrax and sea bream Sparus aurata. Dis. Aquat. Organ. 2003, 57, 97–101. [Google Scholar] [CrossRef]
- Mladineo, I. Prevalence of Ceratothoa oestroides (Risso, 1826), a cymothoid isopode parasite, in cultured sea bass Dicentrarchus labrax L. on two farms in middle Adriatic Sea. Acta Adriat. 2002, 43, 97–102. [Google Scholar]
- Papapanagiotou, E.P.; Trilles, J.P. Cymothoid parasite Ceratothoa parallela inflicts great losses on cultured gilthead sea bream Sparus aurata in Greece. Dis. Aquat. Organ. 2001, 45, 237–239. [Google Scholar] [CrossRef]
- Čolak, S.; Kolega, M.; Mejdandžić, D.; Župan, I.; Šarić, T.; Piplović, E.; Mustać, B. Prevalence and effects of the cymothoid isopod (Ceratothoa oestroides, Risso 1816) on cultured meagre (Argyrosomus regius, Asso 1801) in the Eastern Adriatic Sea. Aquac. Res. 2018, 49, 1001–1007. [Google Scholar] [CrossRef]
- Vagianou, S.; Athanassopoulou, F.; Ragias, V.; Di Cave, D.; Leontides, L.; Golomazou, E. Prevalence and pathology of ectoparasites of mediterranean sea bream and sea bass reared under different environmental and aquaculture conditions. Isr. J. Aquac. 2006, 58, 78–88. [Google Scholar]
- Comission, E. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for on the Hygiene of Foodstuffs; European Parliament: Brussels, Belgium, 2004. [Google Scholar]
- Šarušić, G. Preliminary report by isopod Ceratothoa oestroides (Risso, 1826), in marine cultured fish. Bull. Eur. Assoc. Fish Pathol. 1999, 19, 110–112. [Google Scholar]
- Horton, T.; Okamura, B. Cymothoid isopod parasites in aquaculture: A review and case study of a Turkish sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus) farm. Dis. Aquat. Organ. 2001, 46, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Horton, T.; Okamura, B. Post-haemorrhagic anaemia in sea bass, Dicentrarchus labrax (L.), caused by blood feeding of Ceratothoa oestroides (Isopoda: Cymothoidae). J. Fish Dis. 2003, 26, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Günther, K. Bau und funktion der mundwerkzeuge bei crustaceen aus der familie der cymothoidae (Isopoda). Zoomorphology 1931, 23, 1–79. [Google Scholar]
- Nagler, C.; Haug, J.T. Functional morphology of parasitic isopods: Understanding morphological adaptations of attachment and feeding structures in Nerocila as a pre-requisite for reconstructing the evolution of Cymothoidae. PeerJ 2016, 4, e2188. [Google Scholar] [CrossRef]
- Al-Zubaidy, A.B.; Mhaisen, F.T. The first record of three cymothoid ispods from Red Sea fishes, Yemeni coastal waters. Int. J. Mar. Sci. 2013, 3, 166–172. [Google Scholar]
- Romestand, B.; Thuet, P.; Trilles, J.P. Quelques aspects des mécanismes nutritionnels chez l’isopode Cymothoidae: Ceratothoa oestroides (Risso, 1826). Ann. Parasitol. 1982, 57, 79–89. [Google Scholar] [CrossRef]
- Romestand, B.; Trilles, J.P. Au sujet d’une substance à activité antithrombinique mise en évidence dans les glandes latéro-œsophagiennes de Meinertia oestroides (Risso, 1826) (Isopoda, Flabellifera, Cymothoidae); parasite de poissons. Z. Parasitenkd. 1976, 40, 87–92. [Google Scholar] [CrossRef]
- Romestand, B. Étude écophysiologique des parasitoses à Cymothoadiens. Ann. Parasitol. 1979, 54, 423–448. [Google Scholar] [CrossRef]
- Paiva-Silva, G.O.; Cruz-Oliveira, C.; Nakayasu, E.S.; Maya-Monteiro, C.M.; Dunkov, B.C.; Masuda, H.; Almeida, I.C.; Oliveira, P.L. A heme-degradation pathway in a blood-sucking insect. Proc. Natl. Acad. Sci. USA 2006, 103, 8030–8035. [Google Scholar] [CrossRef]
- Pereira, L.O.R.; Oliveira, P.L.; Almeida, I.C.; Paiva-Silva, G.O. Biglutaminyl-biliverdin IX alpha as a heme degradation product in the dengue fever insect-vector Aedes aegypti. Biochemistry 2007, 46, 6822–6829. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Silva, J.R.; Dansa-Petretski, M.; De Souza, W.; Lins, U.; Braga, C.M.S.; Masuda, H.; Oliveira, P.L. Haem detoxification by an insect. Nature 1999, 400, 517–518. [Google Scholar] [CrossRef]
- Oliveira, M.F.; D’Avila, J.C.; Torres, C.R.; Oliveria, P.L.; Tempore, A.J.; Rumjanek, F.D.; Braga, C.M.; Silva, J.R.; Dansa-Petretski, M.; Oliveira, M.A.; et al. Haemozoin in Schistosoma mansoni. Mol. Biochem. Parasitol. 2000, 111, 217–221. [Google Scholar] [CrossRef]
- Lvova, M.; Zhukova, M.; Kiseleva, E.; Mayboroda, O.; Hensbergen, P.; Kizilova, E.; Ogienko, A.; Besprozvannykh, V.; Sripa, B.; Katokhin, A.; et al. Hemozoin is a product of heme detoxification in the gut of the most medically important species of the family Opisthorchiidae. Int. J. Parasitol. 2016, 46, 147–156. [Google Scholar] [CrossRef]
- Štrus, J.; Žnidaršič, N.; Mrak, P.; Bogataj, U.; Vogt, G. Structure, function and development of the digestive system in malacostracan crustaceans and adaptation to different lifestyles. Cell Tissue Res. 2019, 377, 415–443. [Google Scholar] [CrossRef]
- Storch, V.; Strus, J.; Brandt, A. Microscopic anatomy and ultrastructure of the digestive system of Natatolana obtusata (Vanhöffen, 1914) (Crustacea, Isopoda). Acta Zool. 2002, 83, 1–14. [Google Scholar] [CrossRef]
- Guarino, S.M.; Gambardella, C.; Gragnaniello, G.; De Nicola, M. A preliminary study of hepatopancreas ultrastructure in Idotea balthica (Isopoda). Crustaceana 1994, 66, 153–162. [Google Scholar]
- Longo, G.; Trovato, M.; Mazzei, V.; Ferrante, M.; Conti, G.O. Ligia italica (Isopoda, Oniscidea) as Bioindicator of Mercury Pollution of Marine Rocky Coasts. PLoS ONE 2013, 8, e58548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Štrus, J.; Burkhardt, P.; Storch, V. The ultrastructure of the midgut glands in Ligia italica (Isopoda) under different nutritional conditions. Helgoländer Meeresunters. 1985, 39, 367–374. [Google Scholar]
- Behm, C.A. Metabolism. In The Biology of Nematodes; Lee, D.L., Ed.; Taylor & Francis: London, UK, 2002; pp. 514–572. [Google Scholar]
- Mladineo, I.; Hrabar, J.; Vrbatović, A.; Duvnjak, S.; Gomerčić, T.; Đuras, M. Microbiota and gut ultrastructure of Anisakis pegreffii isolated from stranded cetaceans in the Adriatic Sea. Parasit. Vectors 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Giari, L.; Konecny, R.; Jaeger, P.; Manera, M. Immunohistochemistry, ultrastructure and pathology of gills of Abramis brama from Lake Mondsee, Austria, infected with Ergasilus sieboldi (Copepoda). Dis. Aquat. Organ. 2003, 53, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Pleić, I.L.; Bušelić, I.; Trumbić, Ž.; Bočina, I.; Šprung, M.; Mladineo, I. Expression analysis of the Atlantic bluefin tuna (Thunnus thynnus) pro-inflammatory cytokines, IL-1β, TNFα1 and TNFα2 in response to parasites Pseudocycnus appendiculatus (Copepoda) and Didymosulcus katsuwonicola (Digenea). Fish Shellfish. Imunol. 2015, 45, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Das, S.K.; Samal, J.; Thatoi, H.N. Epidermal mucus, a major determinant in fish health: A review. Iran. J. Vet. Res. 2018, 19, 72–81. [Google Scholar] [PubMed]
- Nolan, D.T.; Riley, P.; Bonga Wendelaar, S.E. Infection with low numbers of the sea louse Lepeophtheirus salmonis induces stress-related effects in postsmolt Atlantic salmon (Salmo salar). Candaian J. Fish. Aquat. Sci. 1999, 56, 947–959. [Google Scholar] [CrossRef]
- Nolan, D.T.; van der Salm, A.L.; Bonga Wendelaar, S.E. The host-parasite relationship between the rainbow trout (Oncorhynchus mykiss) and the ectoparasite Argulus foliaceus (Crustacea: Branchiura): Epithelial mucous cell response, cortisol and factors which may influence parasite establishment. Contrib. Zool. 2000, 69, 57–63. [Google Scholar] [CrossRef]
- Wang, T.; Mai, K.; Ai, Q. A review of cyclooxygenase-2 role in fish. Austin J. Nutr. Metab. 2016, 3, 1037. [Google Scholar]
- Choe, K.P.; Havird, J.; Rose, R.; Hyndman, K.; Piermarini, P.; Evans, D.H. COX2 in a euryhaline teleost, Fundulus heteroclitus: Primary sequence, distribution, localization, and potential function in gills during salinity acclimation. J. Exp. Biol. 2006, 209, 1696–1708. [Google Scholar] [CrossRef]
- Mathews, M.B.; Bernstein, R.M.; Franza, B.R.; Garrels, J.I. Identity of the proliferating cell nuclear antigen and cyclin. Nature 1984, 309, 374–376. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Giari, L.; Lui, A.; Squerzanti, S.; Castaldelli, G.; Shinn, A.P.; Manera, M.; Lorenzoni, M. Proliferative cell nuclear antigen (PCNA) expression in the intestine of Salmo trutta trutta naturally infected with an acanthocephalan. Parasites Vectors 2012, 5, 198. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Mladineo, I.; Naya-Català, F.; Dirks, R.P.; Jong-Raadsen, S.; Vrbatović, A.; Hrabar, J.; Pérez-Sánchez, J.; Sitjà-Bobadilla, A. Acting locally—Affecting globally: RNA sequencing of gilthead sea bream with a mild Sparicotyle chrysophrii infection reveals effects on apoptosis, immune and hypoxia related genes. BMC Genom. 2019, 20, 200. [Google Scholar] [CrossRef]
- Byadgi, O.; Beraldo, P.; Volpatti, D.; Massimo, M.; Bulfon, C.; Galeotti, M. Expression of infection-related immune response in European sea bass (Dicentrarchus labrax) during a natural outbreak from a unique dinoflagellate Amyloodinium ocellatum. Fish Shellfish Immunol. 2019, 84, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.J.; Wang, T. The innate and adaptive immune system of fish. In Infectious Diseases in Aquaculture: Prevention and Control; Austin, B., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 3–68. [Google Scholar]
- Tadiso, T.M.; Krasnov, A.; Skugor, S.; Afanasyev, S.; Hordvik, I.; Nilsen, F. Gene expression analyses of immune responses in Atlantic salmon during early stages of infection by salmon louse (Lepeophtheirus salmonis) revealed bi-phasic responses coinciding with the copepod-chalimus transition. BMC Genom. 2011, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Carman, K.R.; Dobbs, F.C. Epibiotic microorganisms on copepods and other marine crustaceans. Microsc. Res. Tech. 1997, 37, 116–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Liu, Y.; Cao, Y.; He, S.; Huo, F.; Qin, C.; Yao, B.; Ringø, E. High-yield production of a chitinase from Aeromonas veronii B565 as a potential feed supplement for warm-water aquaculture. Appl. Microbiol. Biotechnol. 2014, 98, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Smyrli, M.; Prapas, A.; Rigos, G.; Kokkari, C.; Pavlidis, M.; Katharios, P. Aeromonas veronii infection associated with high morbidity and mortality in farmed European seabass Dicentrarchus labrax in the Aegean Sea, Greece. Fish Pathol. 2017, 52, 68–81. [Google Scholar] [CrossRef]
- Wahl, M.; Goecke, F.; Labes, A.; Dobretsov, S.; Weinberger, F. The second skin: Ecological role of epibiotic biofilms on marine organisms. Front. Microbiol. 2012, 3, 292. [Google Scholar] [CrossRef]
- Bakenhaster, M.D.; McBride, R.S.; Price, W.W. Life history of Glossobius hemiramphi (Isopoda: Cymothoidae): Development, reproduction, and symbiosis with its host Hemiramphus Brasiliensis (Pisces: Hemiramphidae). J. Crustac. Biol. 2006, 26, 283–294. [Google Scholar] [CrossRef][Green Version]
- Hadfield, K.A.; Bruce, N.L.; Smit, N.J. Review of the fish parasitic genus Ceratothoa Dana, 1852 (Crustacea, Isopoda, Cymothoidae) from South Africa, including the description of two new species. Zookeys 2014, 400, 1–42. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladineo, I.; Hrabar, J.; Vidjak, O.; Bočina, I.; Čolak, S.; Katharios, P.; Cascarano, M.C.; Keklikoglou, K.; Volpatti, D.; Beraldo, P. Host-Parasite Interaction between Parasitic Cymothoid Ceratothoa oestroides and Its Host, Farmed European Sea Bass (Dicentrarchus labrax). Pathogens 2020, 9, 230. https://doi.org/10.3390/pathogens9030230
Mladineo I, Hrabar J, Vidjak O, Bočina I, Čolak S, Katharios P, Cascarano MC, Keklikoglou K, Volpatti D, Beraldo P. Host-Parasite Interaction between Parasitic Cymothoid Ceratothoa oestroides and Its Host, Farmed European Sea Bass (Dicentrarchus labrax). Pathogens. 2020; 9(3):230. https://doi.org/10.3390/pathogens9030230
Chicago/Turabian StyleMladineo, Ivona, Jerko Hrabar, Olja Vidjak, Ivana Bočina, Slavica Čolak, Pantelis Katharios, Maria Chiara Cascarano, Kleoniki Keklikoglou, Donatella Volpatti, and Paola Beraldo. 2020. "Host-Parasite Interaction between Parasitic Cymothoid Ceratothoa oestroides and Its Host, Farmed European Sea Bass (Dicentrarchus labrax)" Pathogens 9, no. 3: 230. https://doi.org/10.3390/pathogens9030230
APA StyleMladineo, I., Hrabar, J., Vidjak, O., Bočina, I., Čolak, S., Katharios, P., Cascarano, M. C., Keklikoglou, K., Volpatti, D., & Beraldo, P. (2020). Host-Parasite Interaction between Parasitic Cymothoid Ceratothoa oestroides and Its Host, Farmed European Sea Bass (Dicentrarchus labrax). Pathogens, 9(3), 230. https://doi.org/10.3390/pathogens9030230







