Examining the Effect of Host Recruitment Rates on the Transmission of Streptococcus suis in Nursery Swine Populations
Abstract
1. Introduction
2. Results
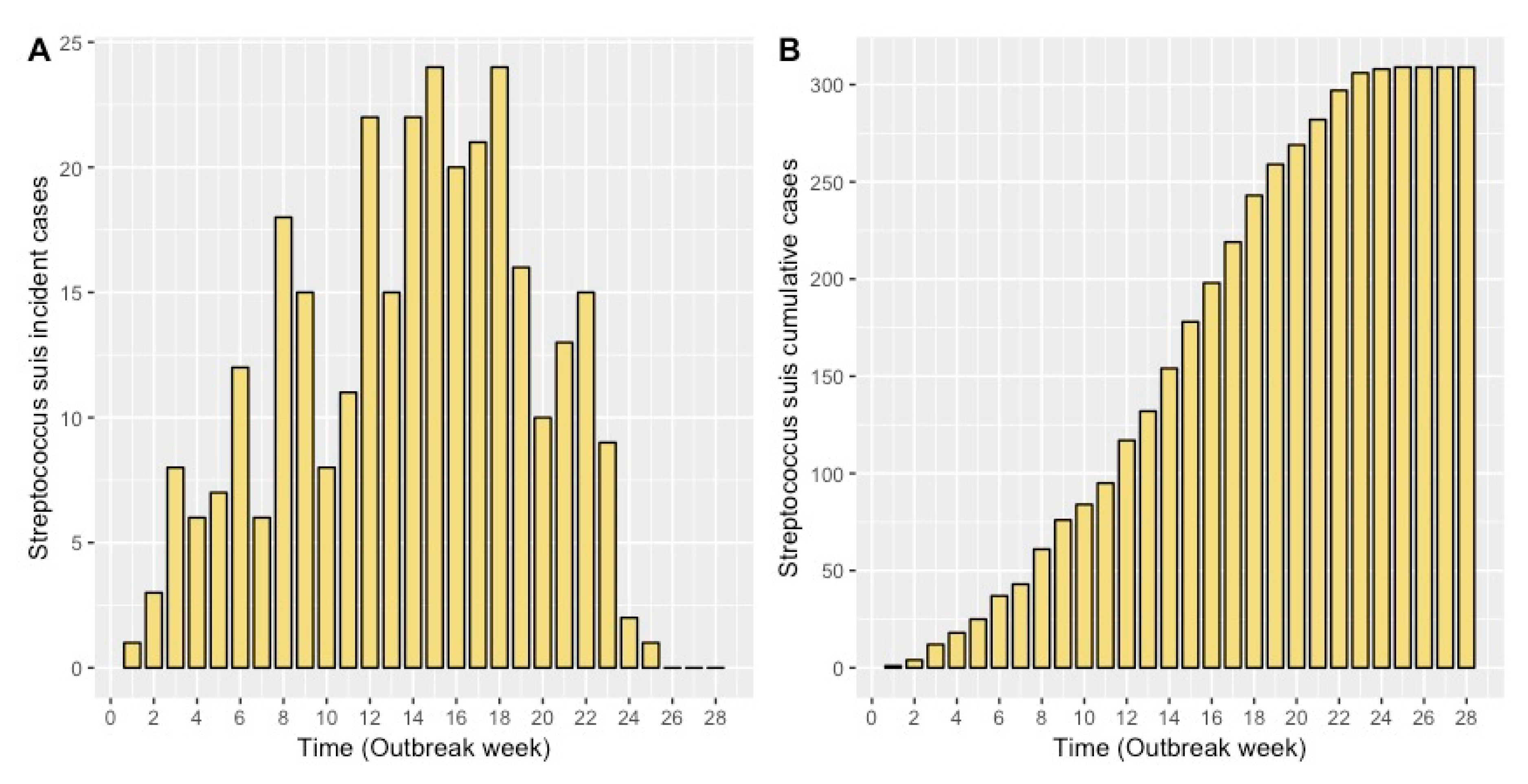
2.1. Model Fit
2.2. Intervention Scenarios
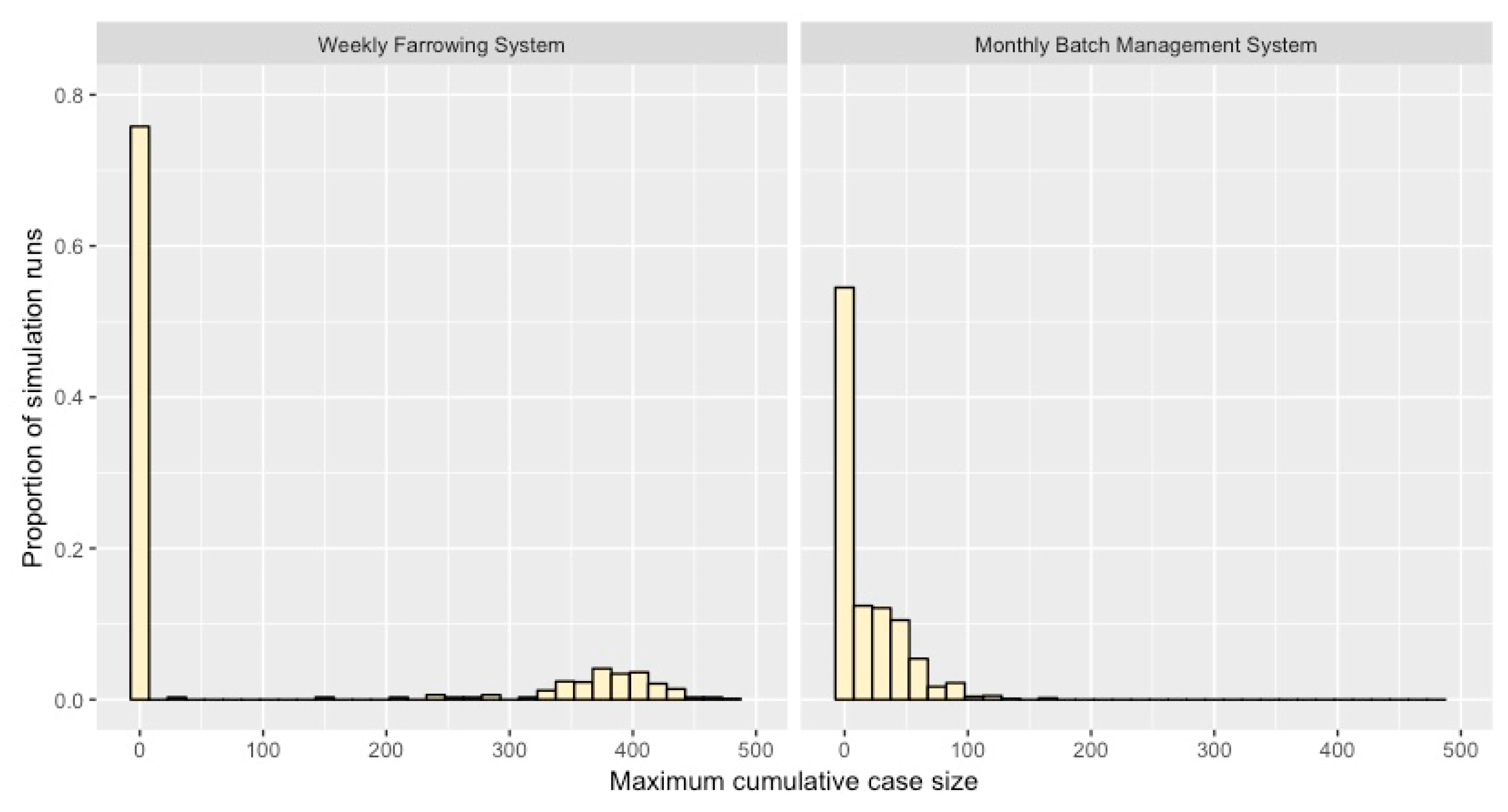
2.3. Projected Maximum Cumulative Case Numbers
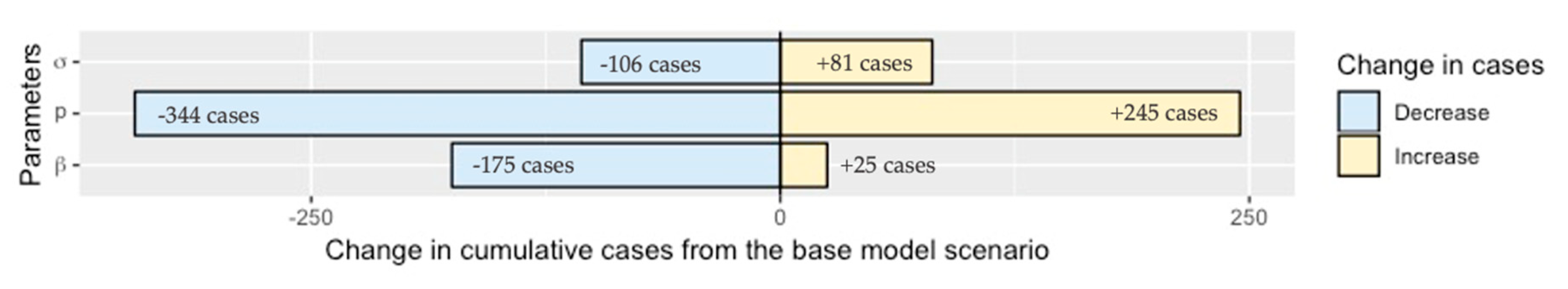
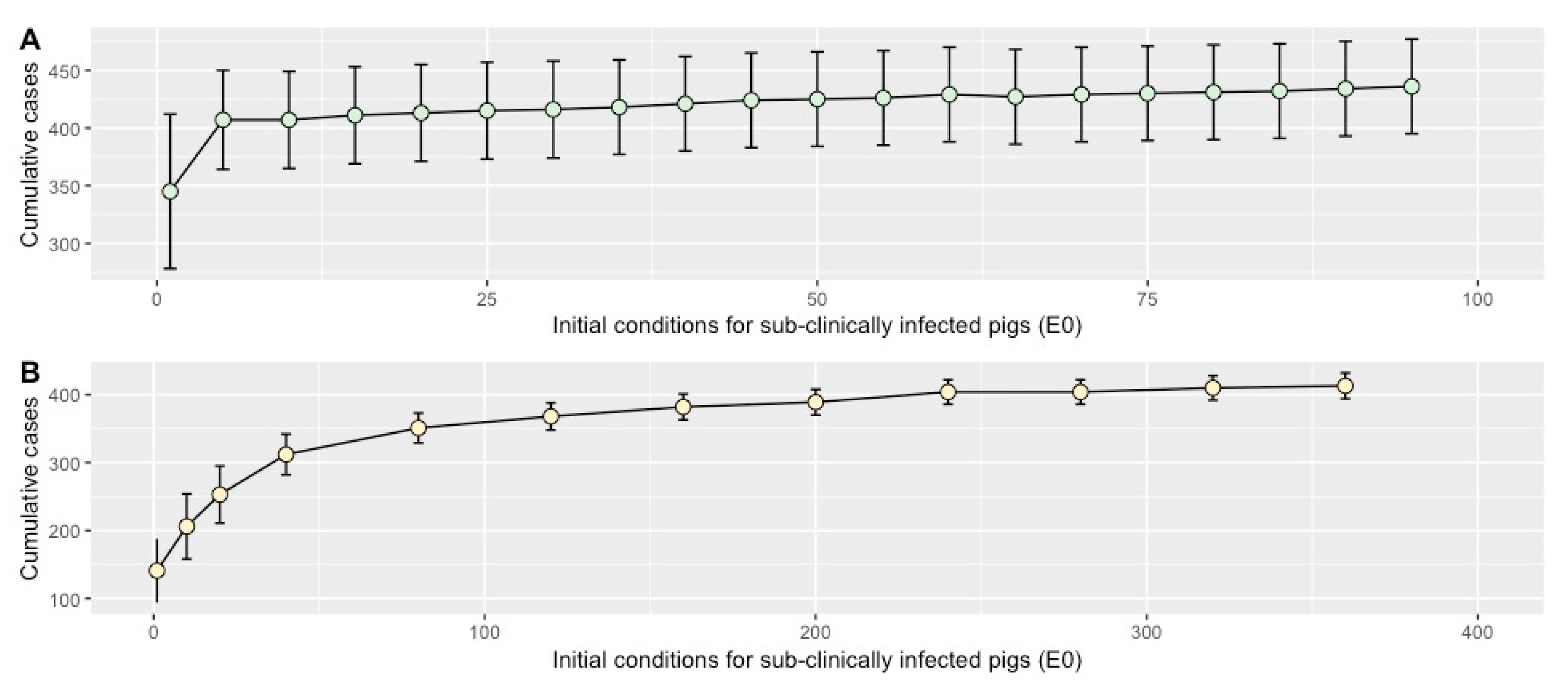
2.4. Sensitivity Analysis
3. Discussion
4. Materials and Methods
4.1. Case Study Data
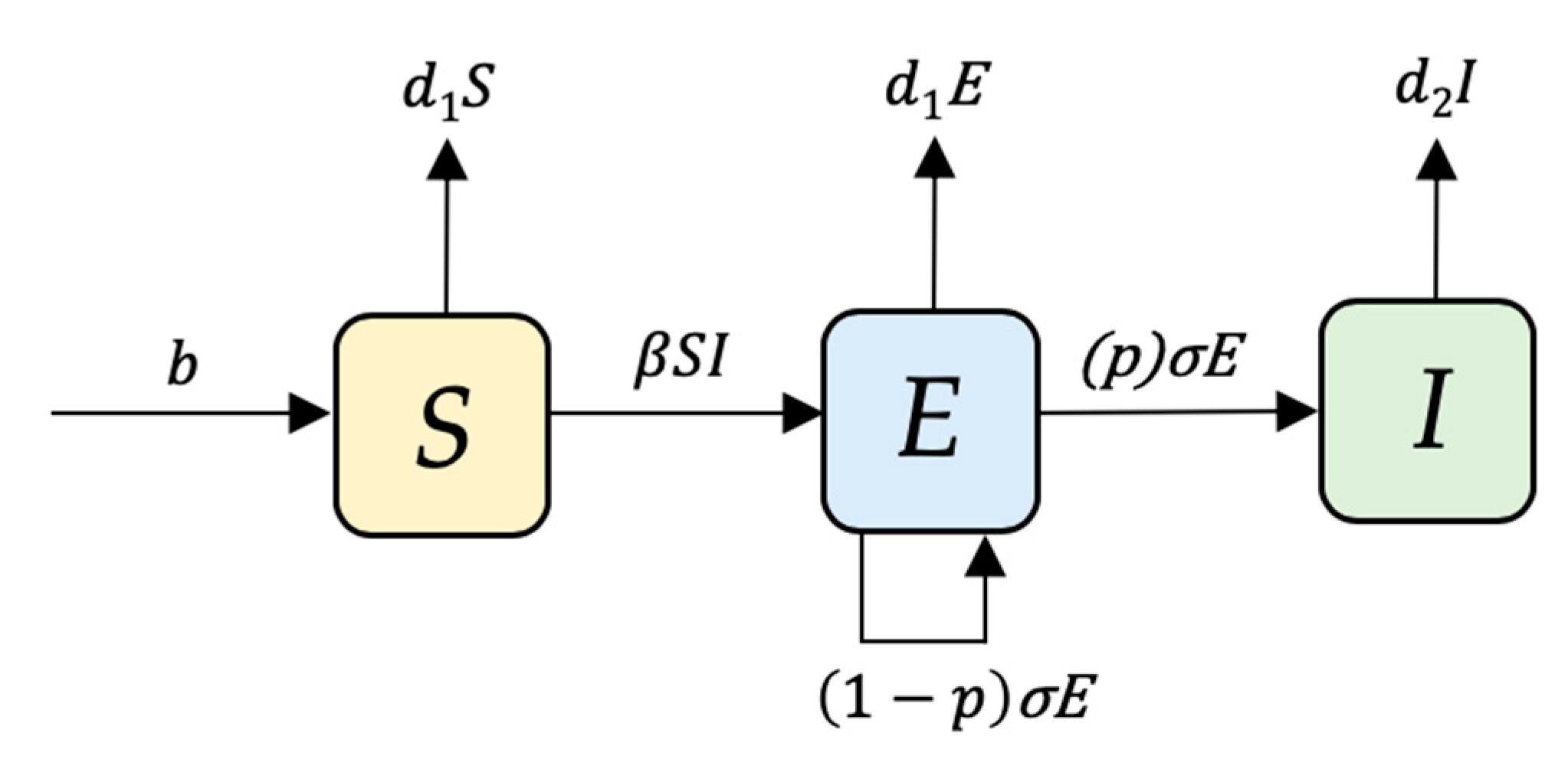
4.2. Model Structure
- S: represents animals that are able to acquire infection from an infectious pig (via direct contact and/or airborne transmission).
- E: represents animals that are sub-clinically infected (non-infectious carriers).
- I: represents animals that are clinically-infected with disease signs (infectious to others).
4.3. Model Transitions and Initial Conditions
4.4. Model Assumptions
4.5. Model Fitting
4.6. Base Model and Intervention Scenarios
4.7. Simulations of Maximum Cumulative Case Numbers
4.8. Sensitivity Analysis
4.8.1. Parameter Estimates
4.8.2. Initial Conditions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Staats, J.; Feder, I.; Okwumabua, O.; Chengappa, M. Streptococcus Suis: Past and Present. Vet. Res. Commun. 1997, 21, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.; Gottschalk, M. An Update on Streptococcus suis Identification. J. Vet. Diagn. Investig. 1990, 2, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Arai, S.; Osawa, R.; Osaki, M.; Nomoto, R.; Takamatsu, D.; Sekizaki, T. Current Taxonomical Situation of Streptococcus suis. Pathogens 2016, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Vecht, U.; Arends, J.; van der Molen, E.; van Leengoed, L. Differences in Virulence between Two Strains of Streptococcus suis Type II after Experimentally Induced Infection of Newborn Germ-Free Pig. Am. J. Vet. Res. 1989, 50, 1037–1043. [Google Scholar]
- Clifton-Hadley, F.; Alexander, T.; Upton, I.; Duffus, W. Further Studies on Subclinical Carrier State of Streptococcus suis Type 2 in Pigs. Vet. Rec. 1984, 114, 513–518. [Google Scholar] [CrossRef]
- Arends, J.; Harwig, N.; Rudolphy, M.; Zanen, H. Carrier Rate of Streptococcus suis Capsular Type 2 in Palatine Tonsils of Slaughtered Pigs. J. Clin. Microbiol. 1984, 20, 945–947. [Google Scholar] [CrossRef]
- Mwaniki, C.; Robertson, I.; Trott, D.; Atyeo, R.; Lee, B.; Hampson, D. Clonal Analysis and Virulence of Australian Isolates of Streptococcus suis Type 2. Epidemiol. Infect. 1994, 113, 321–334. [Google Scholar] [CrossRef]
- Bonifait, L.; Veillette, M.; Létourneau, V.; Grenier, D.; Duchainea, C. Detection of Streptococcus suis in Bioaerosols of Swine Confinement Buildings. Appl. Environ. Microbiol. 2014, 80, 3296–3304. [Google Scholar] [CrossRef]
- Dekker, N.; Bouma, A.; Daemen, I.; Klinkenberg, D.; van Leengoed, L.; Wagenaar, J.; Stegeman, A. Effect of Spatial Separation of Pigs on Spread of Streptococcus suis Serotype 9. PLoS ONE 2013, 8, e61339. [Google Scholar] [CrossRef]
- Madsen, L.; Nielson, B.; Aalbaek, B.; Jensen, H.; Nielsen, J.; Riising, H. Experiemental Infection of Conventional Pigs with Streptococcus suis Serotype 2 by Aerosolic Exposure. Acta Vet. Scand. 2001, 42, 303–306. [Google Scholar] [CrossRef]
- Madsen, L.; Bak, H.; Nielsen, B.; Jensen, H.; Aalbaek, B.; Riising, H. Bacterial Colonization and Invasion in Pigs Experimentally Exposed to Streptococcus suis Serotype 2 in Aerosol. J. Vet. Med. B Infect. Dis. Vet. Public Health 2002, 49, 211–215. [Google Scholar] [CrossRef]
- Berthelot-Herault, F.; Gottschalk, M.; Labbe, A.; Cariolet, R.; Kobisch, M. Experimental Airborne Transmission of Streptococcus suis Capsular Type 2 in Pigs. Vet. Microbiol. 2001, 82, 69–80. [Google Scholar] [CrossRef]
- Clifton-Hadley, F.; Alexander, T. The Carrier Site and Carrier Rate of Streptococcus suis Type II in Pigs. Vet. Rec. 1980, 107, 40–41. [Google Scholar] [CrossRef] [PubMed]
- van Leengoed, L.; Vecht, U.; Verheyen, E. Streptococcus Type 2 Infections in Pigs in the Netherlands (Part Two). Vet. Q. 1987, 9, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Ossowicz, C. Evaluation of Methods Used for Detecting Streptococcus suis Type 2 in Tonsils, and Investigation of the Carrier State in Pigs. Res. Vet. Sci. 1991, 50, 190–194. [Google Scholar] [CrossRef]
- Schafzahl, W.; Lillie-Jaschniski, K. Eradication of Actinobacillus pleuropneumoniae from a Swine Breeding Herd. Prakt. Tierarzt 2010, 91, 334–339. [Google Scholar]
- Angen, O.; Andreasen, M.; Nielsen, E.; Stockmarr, A.; Baekbo, P. Effect of Tulathromycin on the Carrier Status of Actinobacillus pleuropneumoniae Serotype 2 in the Tonsils of Pigs. Vet. Rec. 2008, 163, 445–447. [Google Scholar] [CrossRef]
- Larsen, I.; Nielsen, S.; Olsen, J.; Nielsen, J. The Efficacy of Oxytetracycline Treatment at Batch, Pen and Individual Level on Lawsonia intracellularis Infection in Nursery Pigs in a Randomised Clinical Trial. Prev. Vet. Med. 2016, 124, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Amass, S.; Clark, L.; Knox, K.; Wu, C.; Hill, M. Streptococcus suis Colonization of Piglets during Parturition. Swine Health Prod. 1996, 4, 4–7. [Google Scholar]
- MacInnes, J.I.; Desrosiers, R. Agents of the “Suis-ide Diseases” of Swine: Actinobacillus suis, Haemophilus parasuis, and Streptococcus suis. Can. J. Vet. Res. 1999, 63, 83–89. [Google Scholar]
- National Animal Health Monitoring System. Swine 2006 Part II: Reference of Swine Health and Health Management Practices in the United States. Available online: http://www.aphis.usda.gov/animal_health/nahms/swine/downloads/swine2006/Swine2006_dr_PartII.PDF (accessed on 10 May 2019).
- Holt, M.; Enright, M.; Alexander, T. Immunisation of Pigs with Live Cultures of Streptococcus suis Type 2. Res. Vet. Sci. 1988, 45, 349–352. [Google Scholar] [CrossRef]
- Busque, P.; Higgins, R.; Caya, F.; Quessy, S. Immunization of Pigs Against Streptococcus suis Serotype 2 Infection Using a Live Avirulent Strain. Can. J. Vet. Res. 1997, 61, 275–279. [Google Scholar] [PubMed]
- Wisselink, H.; Stockhofe-Zurwieden, N.; Hilgers, L.; HE, S. Assessment of Protective Efficacy of Live and Killed Vaccines Based on a Non-Encapsulated Mutant of Streptococcus suis Serotype 2. Vet. Microbiol. 2002, 3, 155–168. [Google Scholar] [CrossRef]
- Segura, M. Streptococcus suis Vaccines: Candidate Antigens and Progress. Expert Rev. Vaccines 2015, 14, 1587–1608. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.; Rohani, P. Modeling Infectious Diseases in Humans and Animals; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Halloran, M. Concepts of Transmission and Dynamics. In Epidemiologic Methods for the Study of Infectious Diseases; Thomas, J., Weber, D., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 63–64. [Google Scholar]
- Black, F.L. Measles Endemicity in Insular Populations: Critical Community Size and Its Evolutionary Implication. J. Theor. Biol. 1966, 11, 207–211. [Google Scholar] [CrossRef]
- Barlett, M. Measles Periodicity and Community Size. J. R. Stat. Soc. 1957, 120, 48–70. [Google Scholar]
- Grenfell, B.T.; Bjùrnstad, O.N.; Kappey, J. Travelling Waves and Spatial Hierarchies in Measles Epidemics. Nature 2001, 414, 716–723. [Google Scholar] [CrossRef]
- Brown, P. Advantages and Disadvantages of Batch Farrowing. Practice 2006, 28, 94–96. [Google Scholar]
- Vangroenweghe, F.; Suls, L.; Van Driessche, E.; Maes, D.; De Graef, E. Health Advantages of Transition to Batch Management System in Farrow-to-Finish Pig Herds. Vet. Med. 2012, 52, 83–91. [Google Scholar] [CrossRef]
- Hernandez-Garcia, J.; Wang, J.; Restif, O.; Holmes, M.A.; Mather, A.E.; Weinert, L.A.; Wileman, T.M.; Thomson, J.R.; Langford, P.R.; Wren, B.W.; et al. Patterns of Antimicrobial Resistance in Streptococcus suis Isolates from Pigs with or without Streptococcal Disease in England between 2009 and 2014. Vet. Microbiol. 2017, 207 (Suppl. C), 117–124. [Google Scholar] [CrossRef]
- Pitzer, V.E.; Aguas, R.; Riley, S.; Loeffen, W.L.A.; Wood, J.L.N.; Grenfell, B.T.; Pitzer, V.E. High Turnover Drives Prolonged Persistence of Influenza in Managed Pig Herds. J. R. Soc. Interface 2016, 13, 20160138. [Google Scholar] [CrossRef] [PubMed]
- Clayton, T.; Duke-Sylvester, S.; Gross, L.J.; Lenhart, S.; Real, L. Optimal Control of a Rabies Epidemic Model with a Birth Pulse. J. Biol. Dyn. 2010, 4, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Auger, J.-P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an Important Pig Pathogen and Emerging Zoonotic Agent—an Update on the Worldwide Distribution Based on Serotyping and Sequence Typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Vötsch, D.; Willenborg, M.; Weldearegay, Y.B.; Valentin-weigand, P. Streptococcus suis—The “Two Faces ” of a Pathobiont in the Porcine Respiratory Tract. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lamont, M.; Edwards, P.; Windsor, R. Streptococcal Meningitis in Pigs: Results of a Five-Year Survey. Vet. Rec. 1980, 107, 467–469. [Google Scholar] [CrossRef]
- Robertson, I.; Blackmore, D. Prevalence of Streptococcus suis Types 1 and 2 in Domestic Pigs in Australia and New Zealand. Vet. Rec. 1989, 124, 391–394. [Google Scholar] [CrossRef]
- Clifton-Hadley, F.A.; Alexander, T.; Enright, M.R. The epidemiology, diagnosis, treatment and control of Streptococcus suis Type-2 Infection. In Proceedings of the AASV Annual Meeting, Minneapolis, MN, USA, 16–18 March 1986; pp. 473–491. [Google Scholar]
- Brisebois, L.M.; Charlebois, R.; Higgins, R.; Nadeau, M. Prevalence of Streptococcus suis in Four to Eight Week Old Clinically Healthy Piglets. Can. J. Vet. Res. 1990, 54, 174–177. [Google Scholar]
- Dee, C.; Corey, M. The Survival of Streptococcus suis on Farm and Veterinary Equipment. J. Swine Health Prod. 1993, 1, 17–20. [Google Scholar]
- Casanova-Higes, A.; Marín-Alcalá, C.; Andrés-Barranco, S.; Cebollada-Solanas, A.; Alvarez, J.; Mainar-Jaime, R.C. Weaned Piglets: Another Factor to Be Considered for the Control of Salmonella Infection in Breeding Pig Farms. Vet. Res. 2019, 50. [Google Scholar] [CrossRef]
- Cador, C.; Rose, N.; Willem, L.; Andraud, M. Influenza A Virus Persistence within Farrow- to-Finish Pig Farms: Insights from a Stochastic Event-Driven Metapopulation Model. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Hopkins, D.; Poljak, Z.; Farzan, A.; Friendship, R. Factors Contributing to Mortality during a Streptococcus suis Outbreak in Nursery Pigs. Can. Vet. J. 2018, 59, 623–630. [Google Scholar] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Giang, E. Using Infectious Disease Models to Identify Management Approaches for Streptococcus suis Disease in Swine Populations. MSc Thesis, University of Guelph, Guelph, ON, Canada, December 2019. [Google Scholar]
- Johnson, P. Adaptivetau: Efficient Stochastic Simulations. R Package Version 2.2-3. 2019. Available online: https://cran.r-project.org/web/packages/adaptivetau/index.html (accessed on 3 April 2019).
- Gillespie, D.T. Exact Stochastic Simulation of Coupled Chemical Reactions. J. Phys. Chem. 1977, 81, 2340–2361. [Google Scholar] [CrossRef]
- Unterweger, C.; Ruczizka, U.; Spergser, J.; Baums, C.; Hennig-Pauka, I. Effect of Early-Life Treatment of Piglets with Long-Acting Ceftiofur on Colonization of Streptococcus suis Serotype 7 and Elicitation of Specific Humoral Immunity in a Farm Dealing with Streptococcal Diseases. Pathogens 2018, 29, E34. [Google Scholar] [CrossRef] [PubMed]
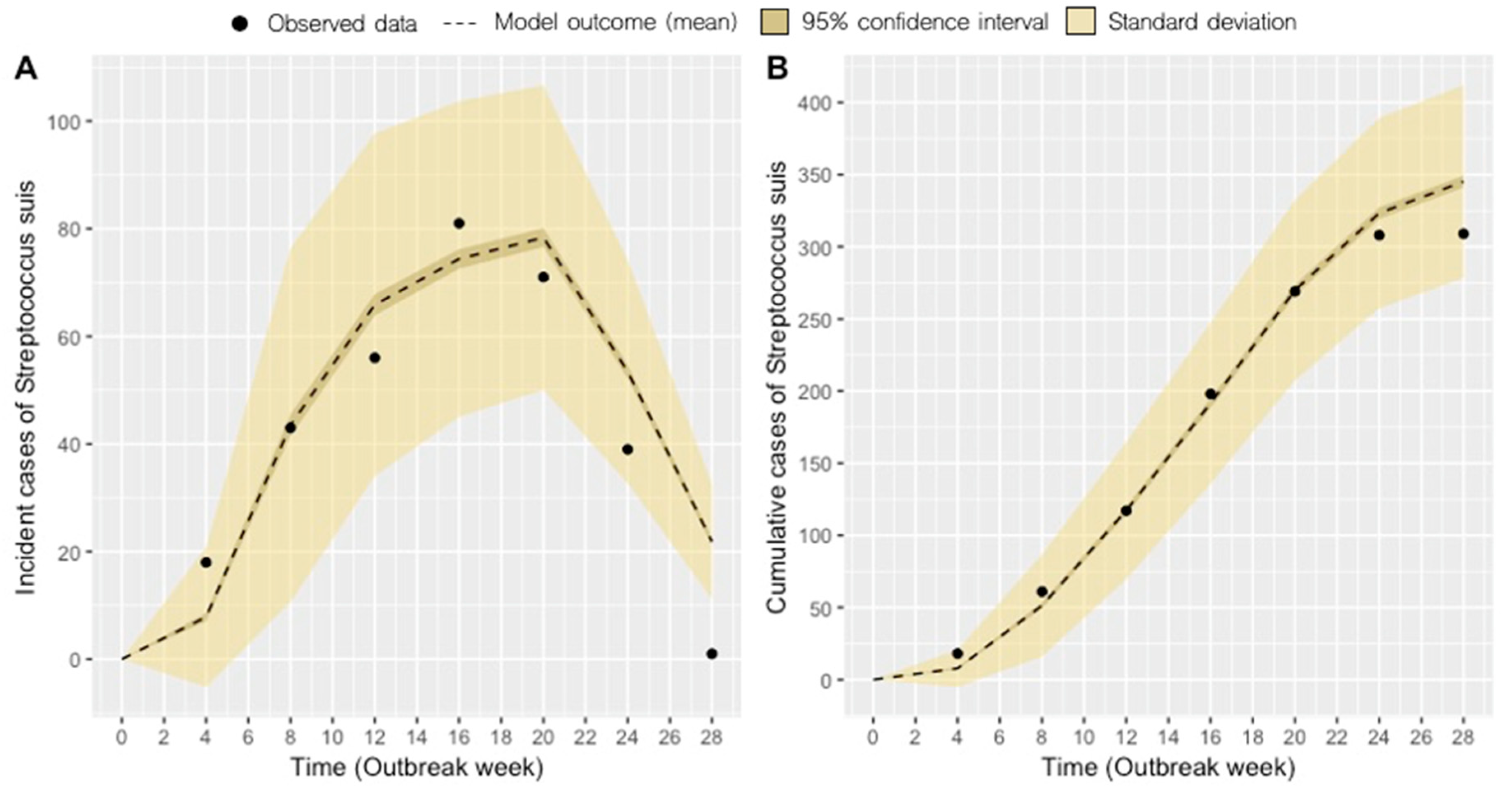
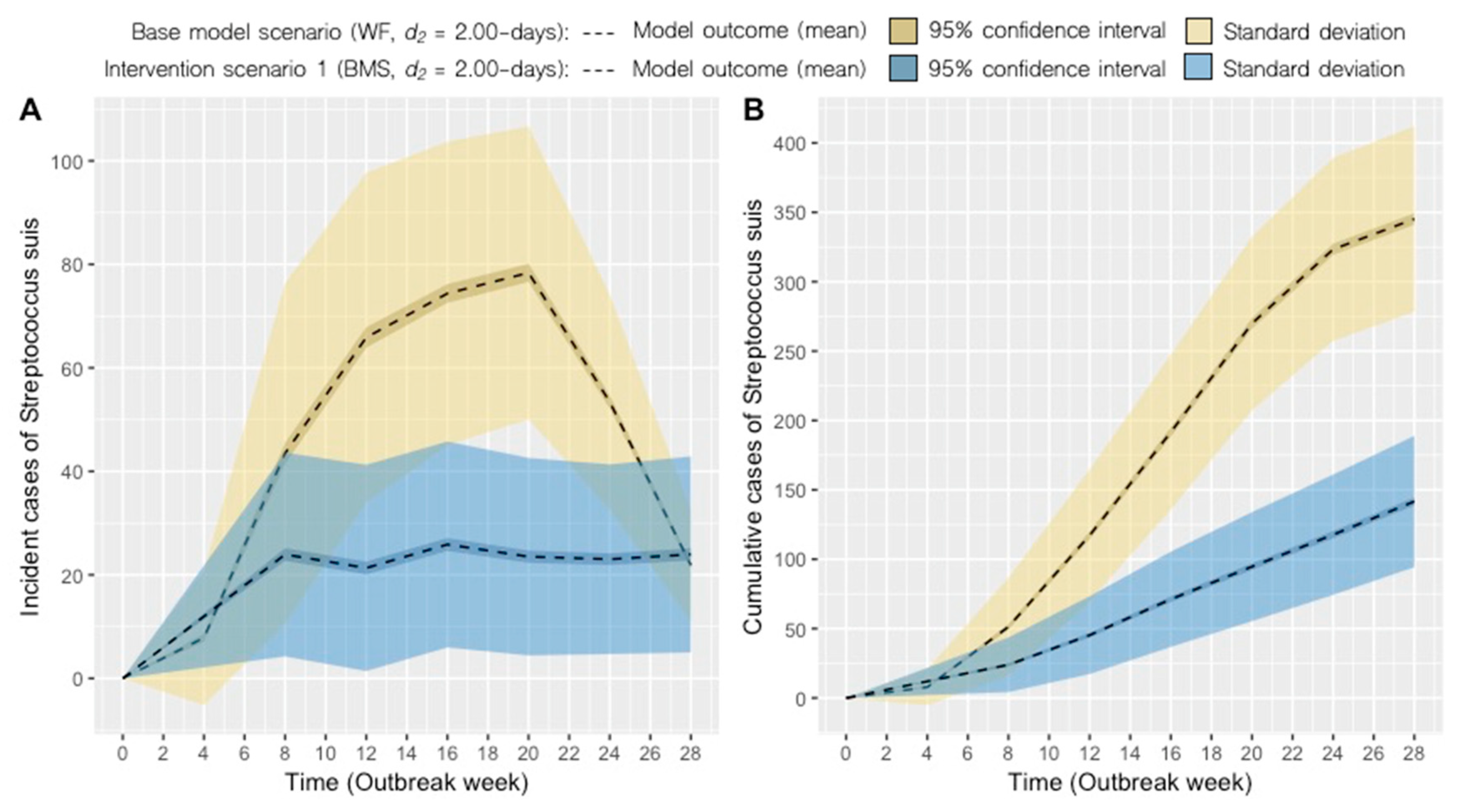
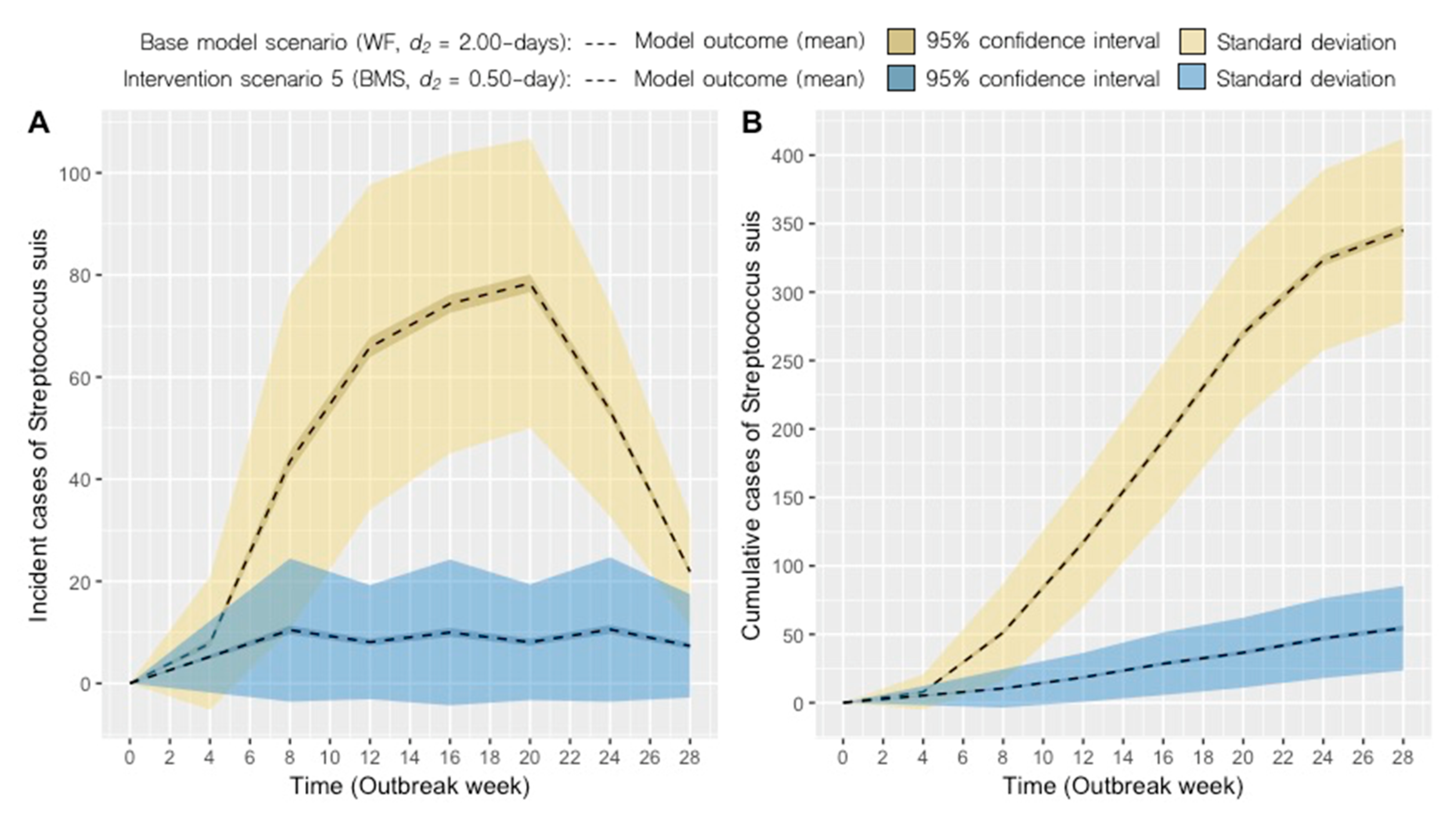
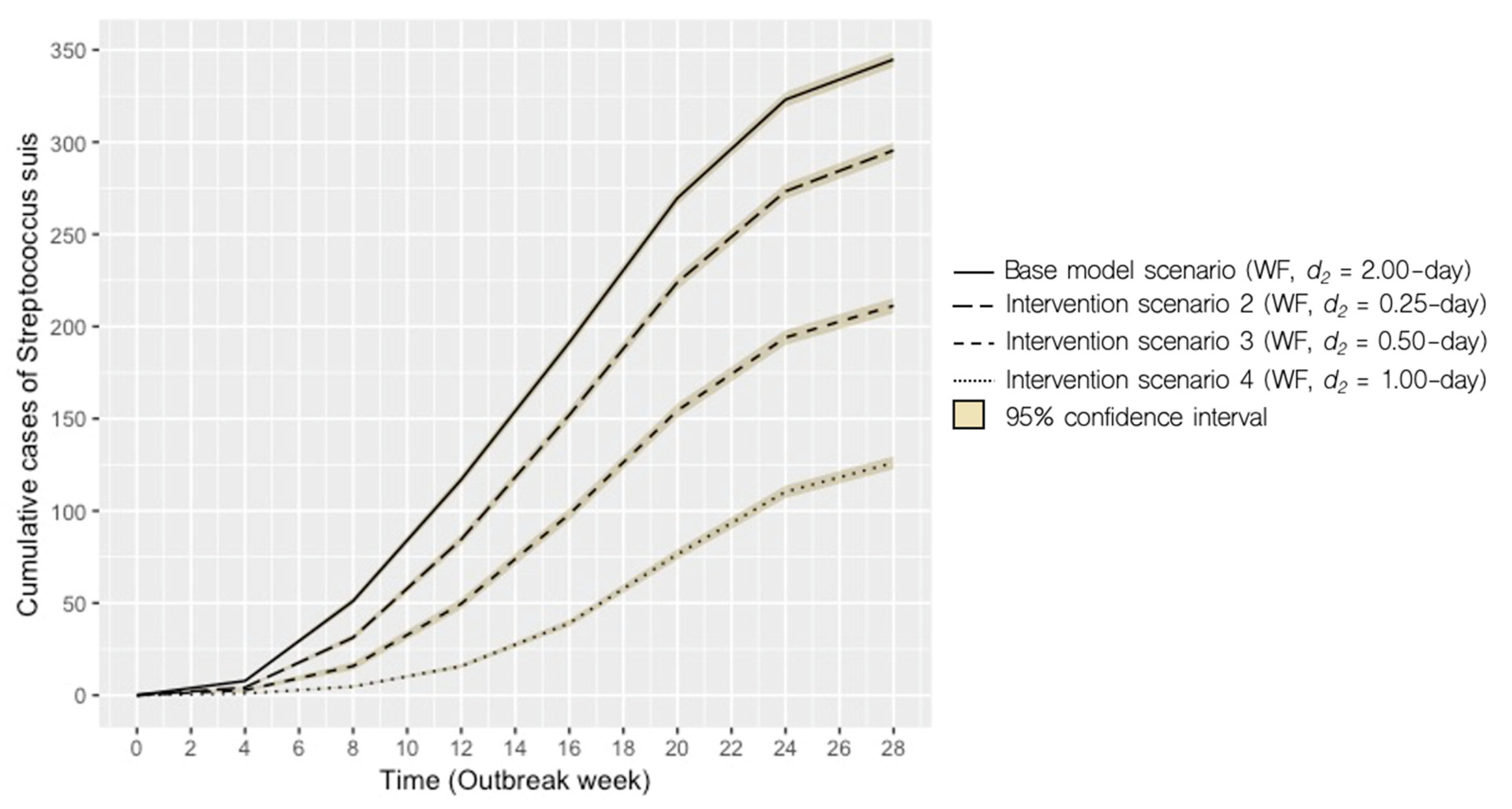
| Scenario | System 1 | Disease-removal Rate, d2 (days)2 | Mean Monthly Incidence, Range | Mean Cumulative Incidence ± SD3 | ∆ cumulative cases (%) |
|---|---|---|---|---|---|
| Base model | WF | 2.00 | 49 (7–78) | 345 ± 67 | – |
| 1 | BMS | 2.00 | 24 (21–26) | 141 ± 47 | −59 |
| 2 | WF | 0.25 | 18 (1–37) | 125 ± 57 | -64 |
| 3 | WF | 0.50 | 30 (2–57) | 212 ± 73 | −39 |
| 4 | WF | 1.00 | 42 (4–71) | 295 ± 70 | −14 |
| 5 | BMS | 0.50 | 8 (7–11) | 54 ± 31 | −84 |
| Transition | Event | Transition Rate |
|---|---|---|
| (S, E, I) → (S+1, E, I) | Recruitment of susceptible pigs | b |
| (S, E, I) → (S-1, E+1, I) | Infection of a susceptible pig | β*S*I |
| (S, E, I) → (S, E-1, I+1) | Sub-clinically infected pig develops clinical signs | (p)σ*E |
| (S, E, I) → (S, E+1, I) | Sub-clinically infected pig remains sub-clinical | (1 – p)σ*E |
| (S, E, I) → (S-1, E, I) | Production removal of susceptible pig | d1*S |
| (S, E, I) → (S, E-1, I) | Production removal of sub-clinically infected pig | d1*E |
| (S, E, I) → (S, E, I-1) | Disease-removal of clinically infected pigs | d2*I |
| Parameter | Definition | Value | References |
| β | Transmission coefficient for I | 1.08 | Calculated |
| R0 | Basic reproductive number | 1.4 | [47] |
| D | Duration of infectiousness | 1.8 weeks | Fitted |
| σ | Duration of latent period | 4.8 weeks | Fitted |
| p | Probability of becoming clinically infected | 0.22 | Fitted |
| 1 – p | Probability of remaining sub-clinically infected | 0.78 | Fitted |
| b | Recruitment rate of susceptible pigs | 100 pigs/week | [45] |
| d1 | Production removal rates for S and E | 6 weeks | [45] |
| d2 | Disease-removal rates for I | 2.00 days | Assumed |
| Scenario | Farrowing System 1 | Batch Size (no. of pigs) | Disease-Removal Rate, d2 (days)2 | Entry into Nursery |
|---|---|---|---|---|
| Base model | WF | 100 | 2.00 | Weekly |
| 1 | BMS | 400 | 2.00 | Monthly |
| 2 | WF | 100 | 0.25 | Weekly |
| 3 | WF | 100 | 0.50 | Weekly |
| 4 | WF | 100 | 1.00 | Weekly |
| 5 | BMS | 400 | 0.50 | Monthly |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giang, E.; Hetman, B.M.; Sargeant, J.M.; Poljak, Z.; Greer, A.L. Examining the Effect of Host Recruitment Rates on the Transmission of Streptococcus suis in Nursery Swine Populations. Pathogens 2020, 9, 174. https://doi.org/10.3390/pathogens9030174
Giang E, Hetman BM, Sargeant JM, Poljak Z, Greer AL. Examining the Effect of Host Recruitment Rates on the Transmission of Streptococcus suis in Nursery Swine Populations. Pathogens. 2020; 9(3):174. https://doi.org/10.3390/pathogens9030174
Chicago/Turabian StyleGiang, Elissa, Benjamin M. Hetman, Jan M. Sargeant, Zvonimir Poljak, and Amy L. Greer. 2020. "Examining the Effect of Host Recruitment Rates on the Transmission of Streptococcus suis in Nursery Swine Populations" Pathogens 9, no. 3: 174. https://doi.org/10.3390/pathogens9030174
APA StyleGiang, E., Hetman, B. M., Sargeant, J. M., Poljak, Z., & Greer, A. L. (2020). Examining the Effect of Host Recruitment Rates on the Transmission of Streptococcus suis in Nursery Swine Populations. Pathogens, 9(3), 174. https://doi.org/10.3390/pathogens9030174





