Recognition of Lipoproteins by Toll-like Receptor 2 and DNA by the AIM2 Inflammasome Is Responsible for Production of Interleukin-1β by Virulent Suilysin-Negative Streptococcus suis Serotype 2
Abstract
1. Introduction
2. Results
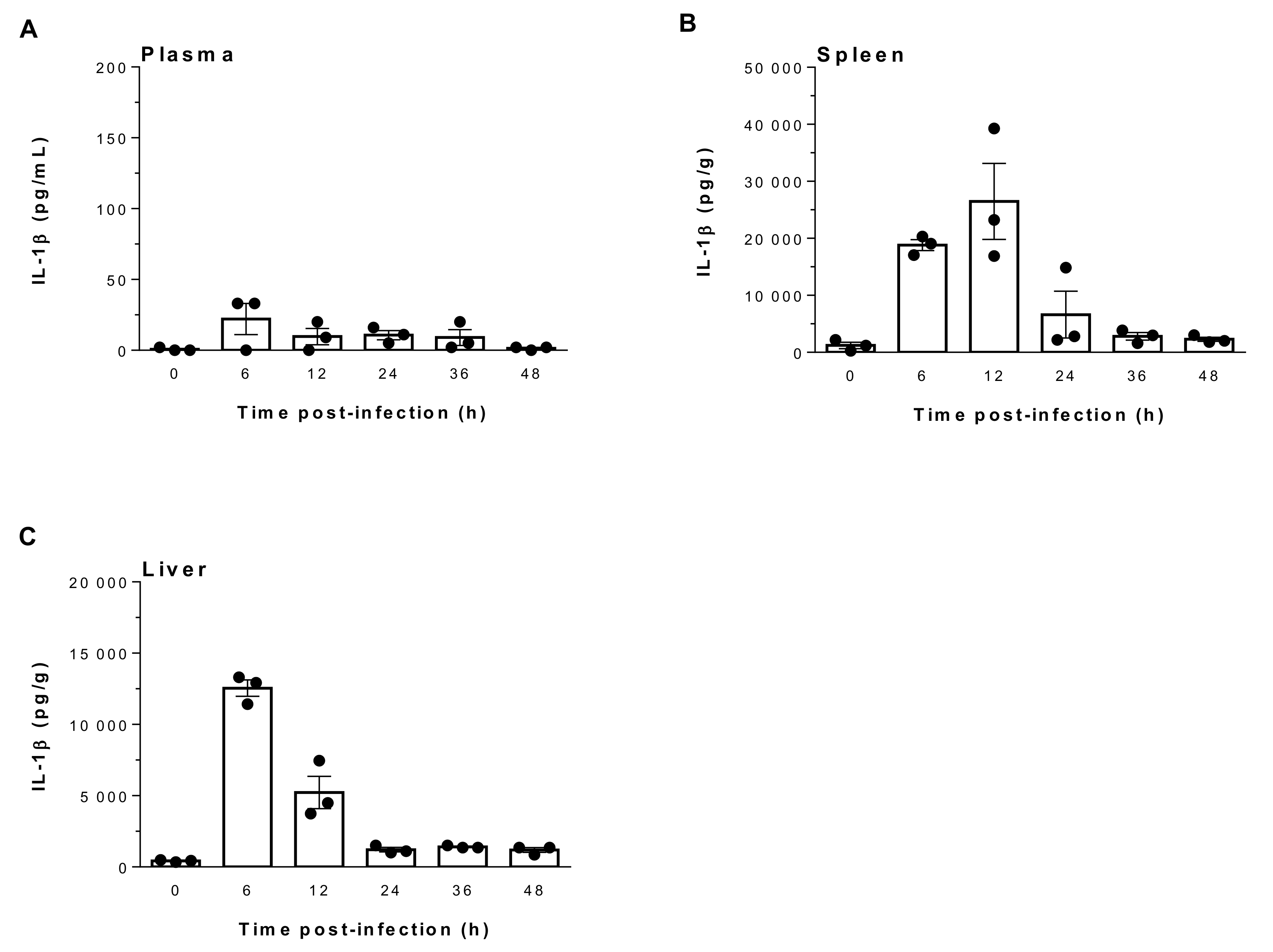
2.1. Suilysin-Negative S. suis Induces Elevated Levels of IL-1β in Spleen and Liver But Not in Plasma
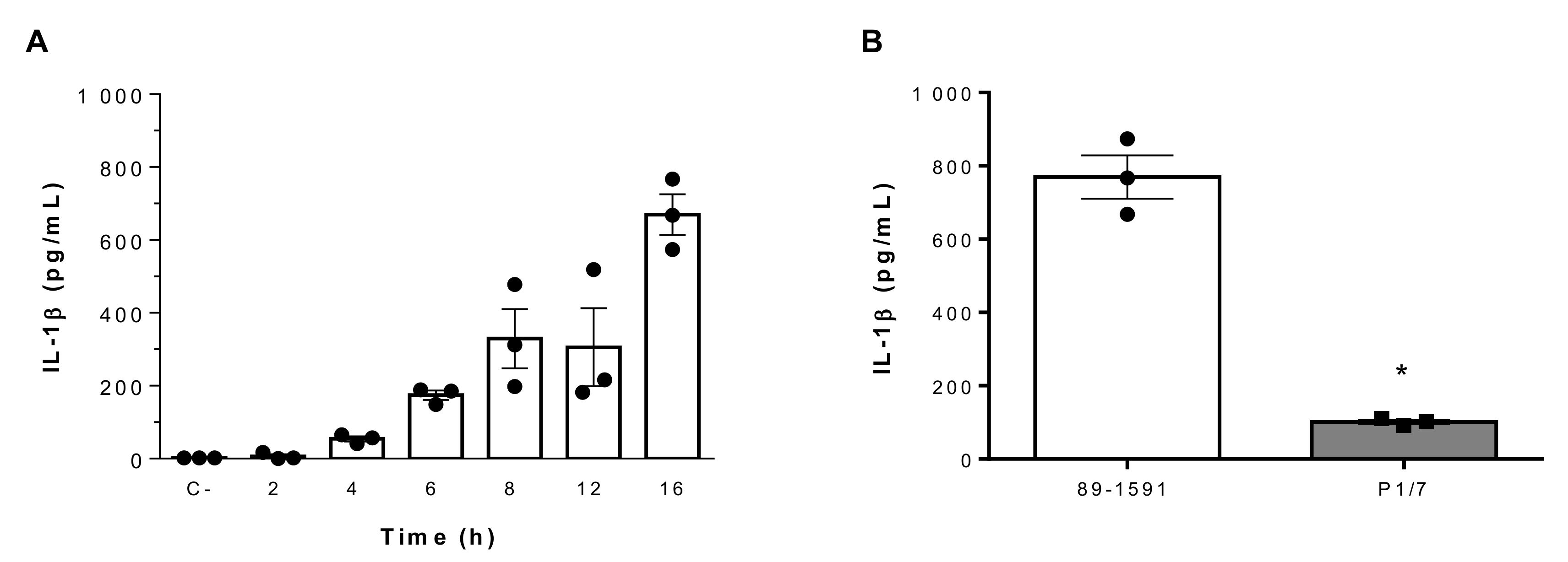
2.2. Suilysin-Negative S. suis Induces Elevated IL-1β Production From Bone-Marrow Dendritic Cells in a Time-Dependent Manner
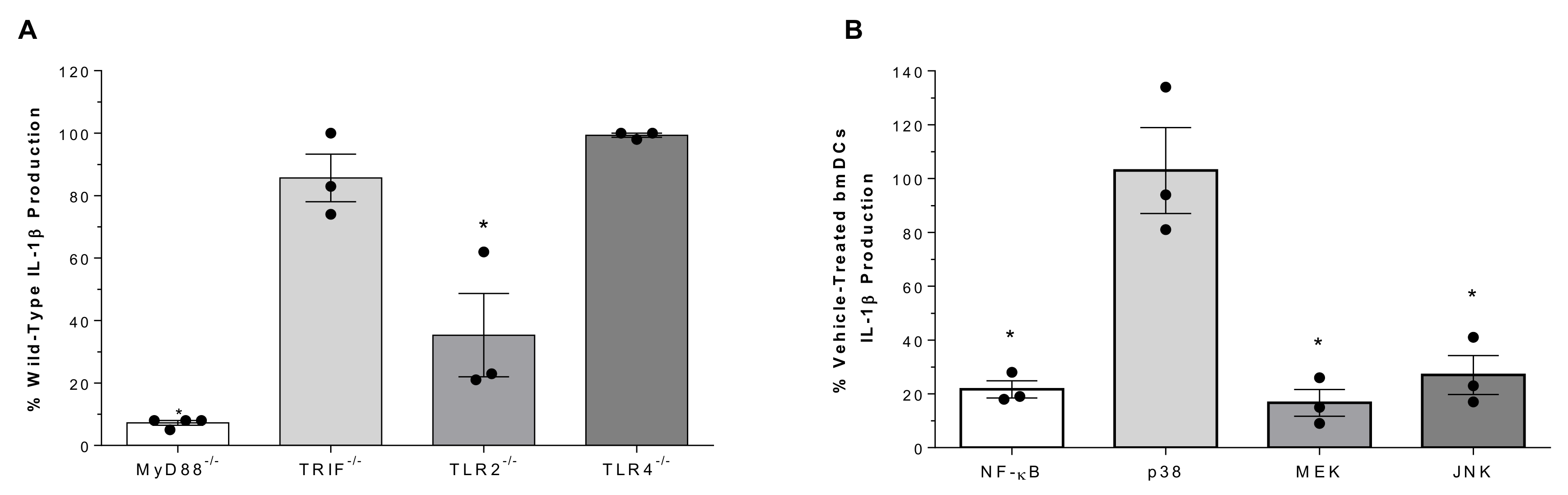
2.3. Role of Toll-Like Receptors and Associated Signaling Pathways in Suilysin-Negative S. suis-Induced IL-1β Production
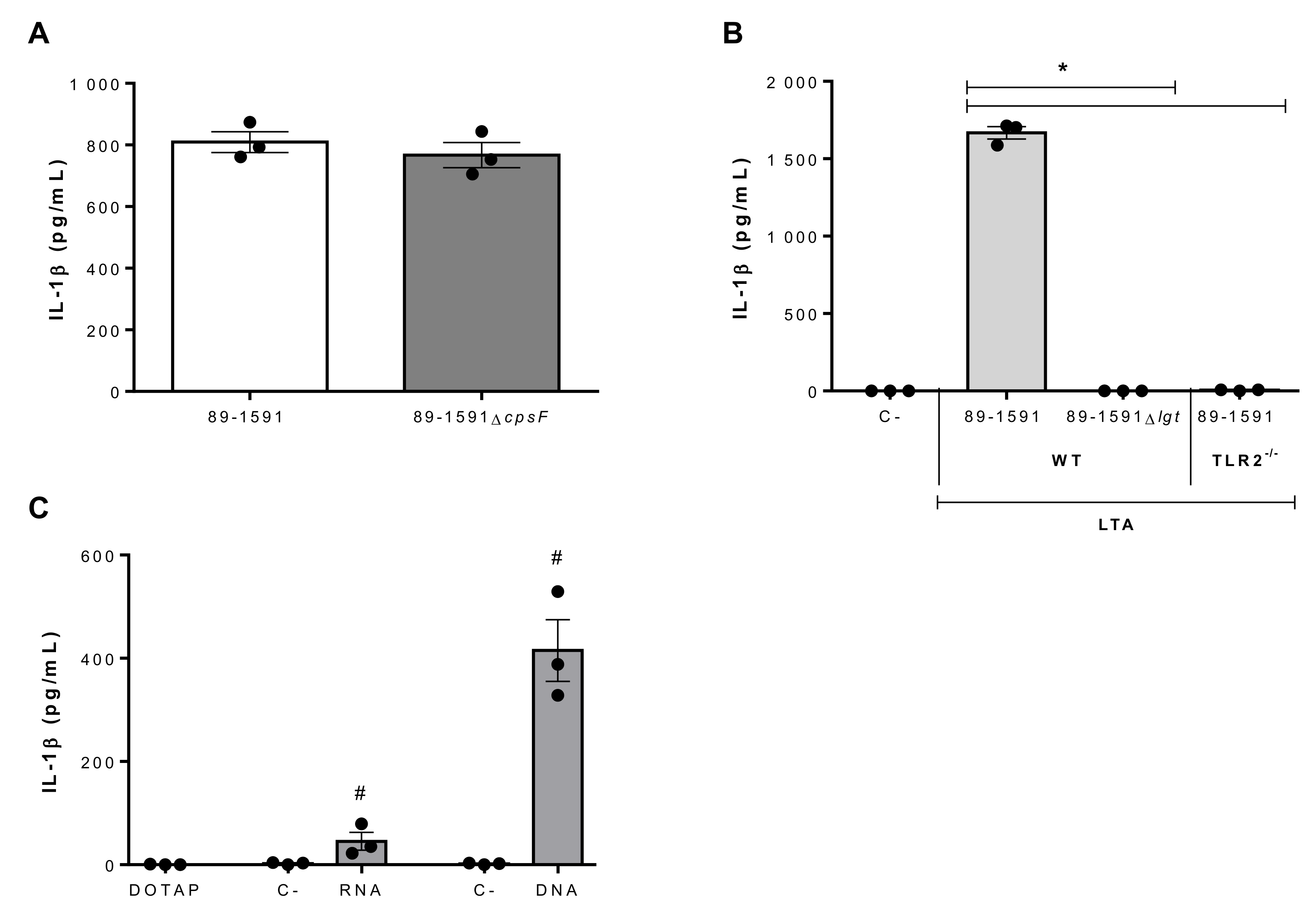
2.4. Suilysin-Negative S. suis Lipoproteins and Nucleic Acids Are Potent Inducers of IL-1β Production
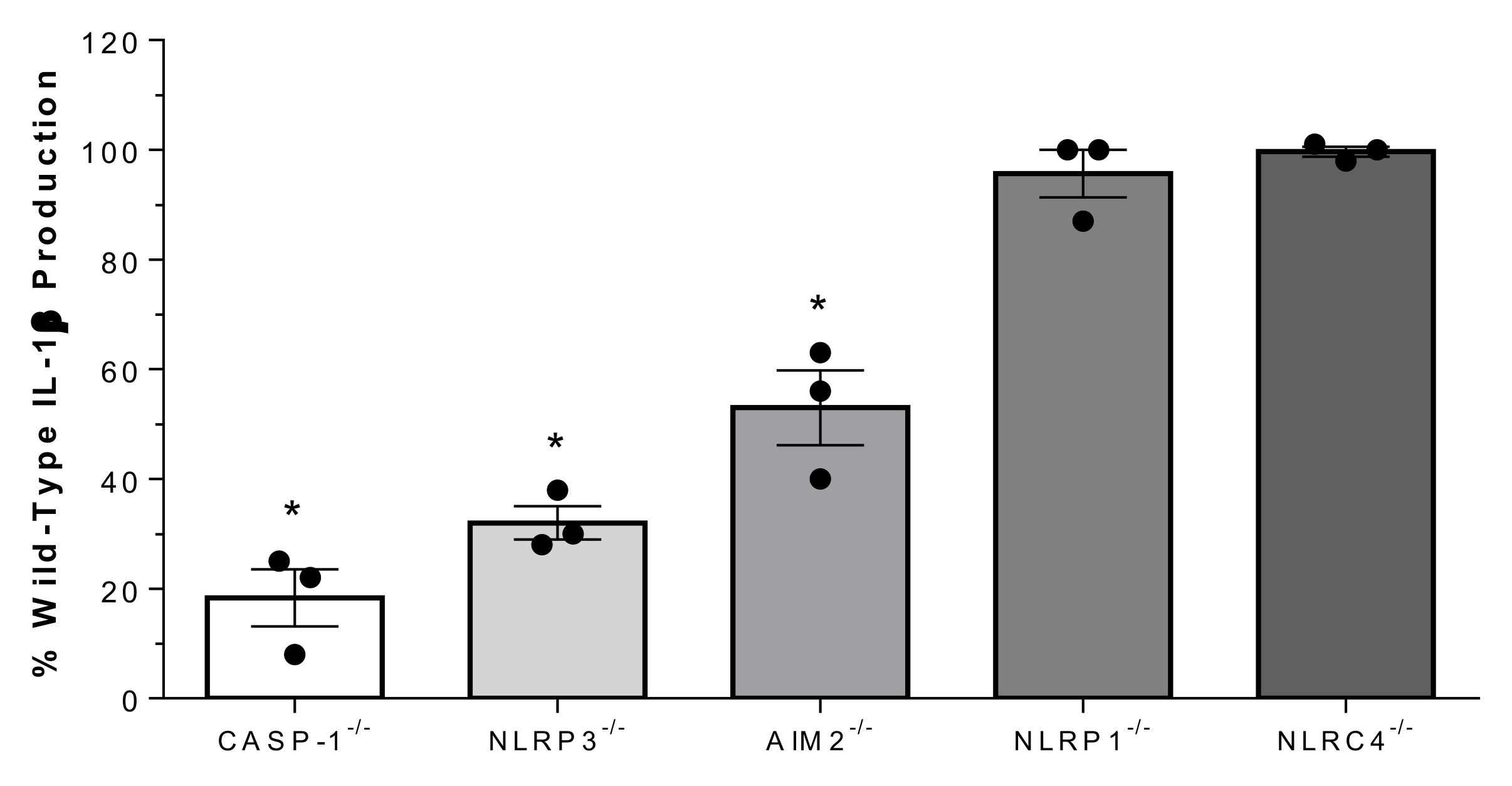
2.5. Suilysin-Negative S. suis-Induced IL-1β Production Depends on Caspase-1 and the NLRP3 and AIM2 Inflammasomes
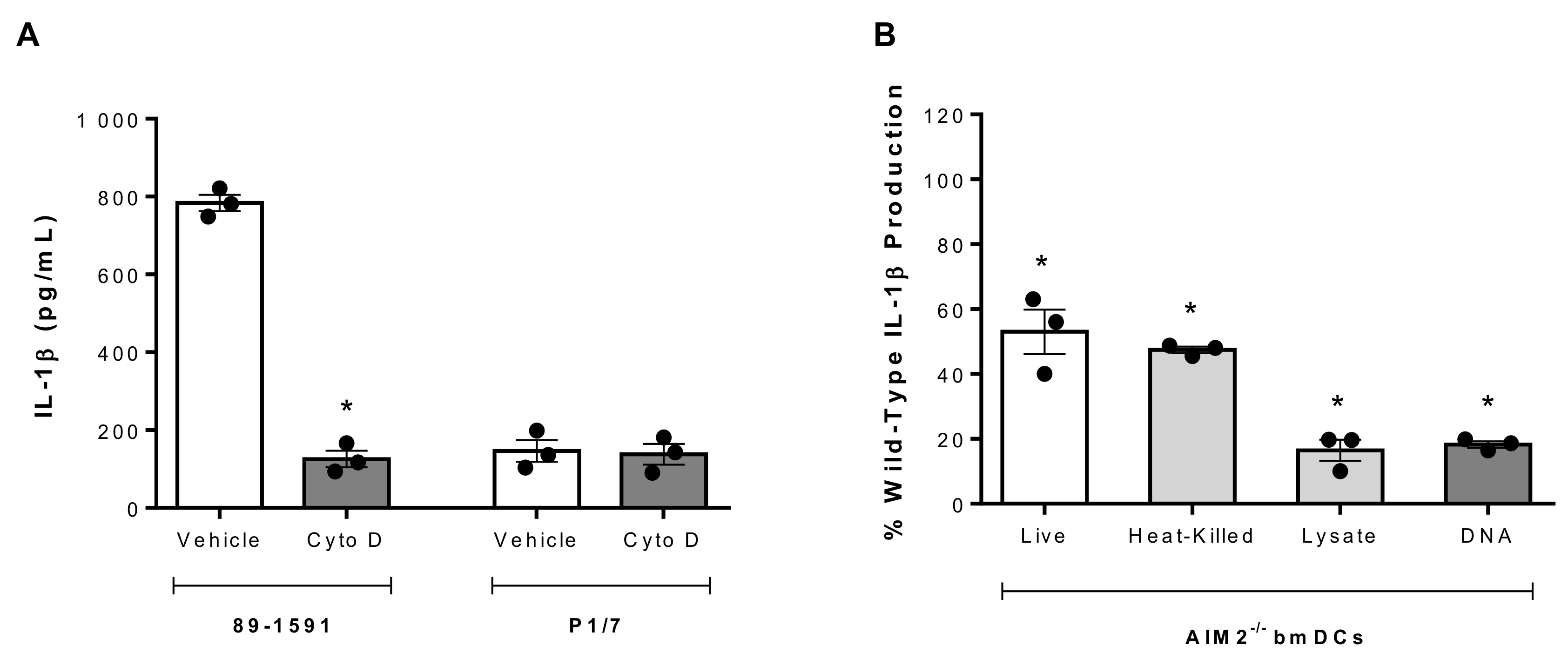
2.6. S. suis Strain 89-1591-Induced IL-1β Production Requires Internalization and Intracellular DNA Sensing by the AIM2 Inflammasome
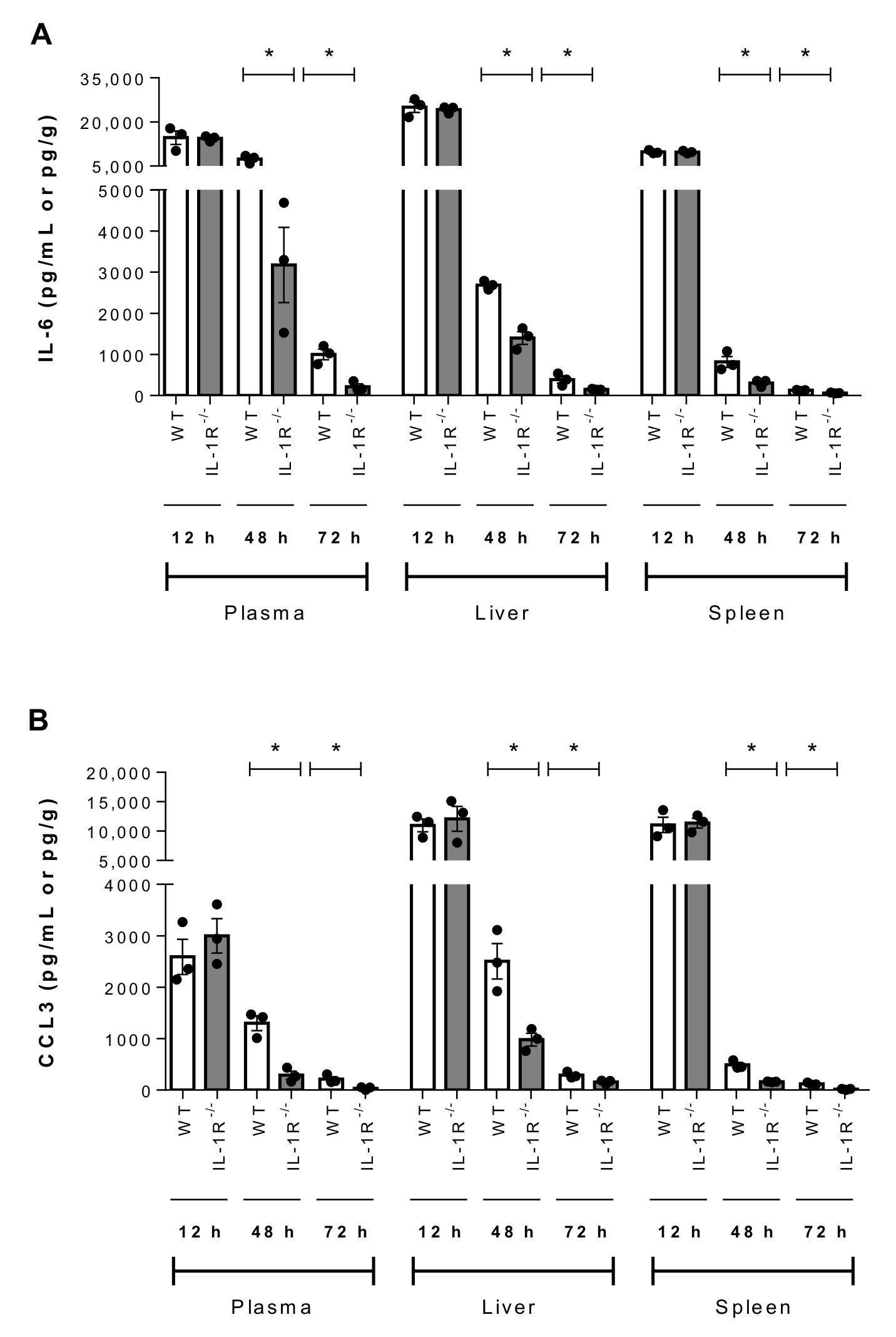
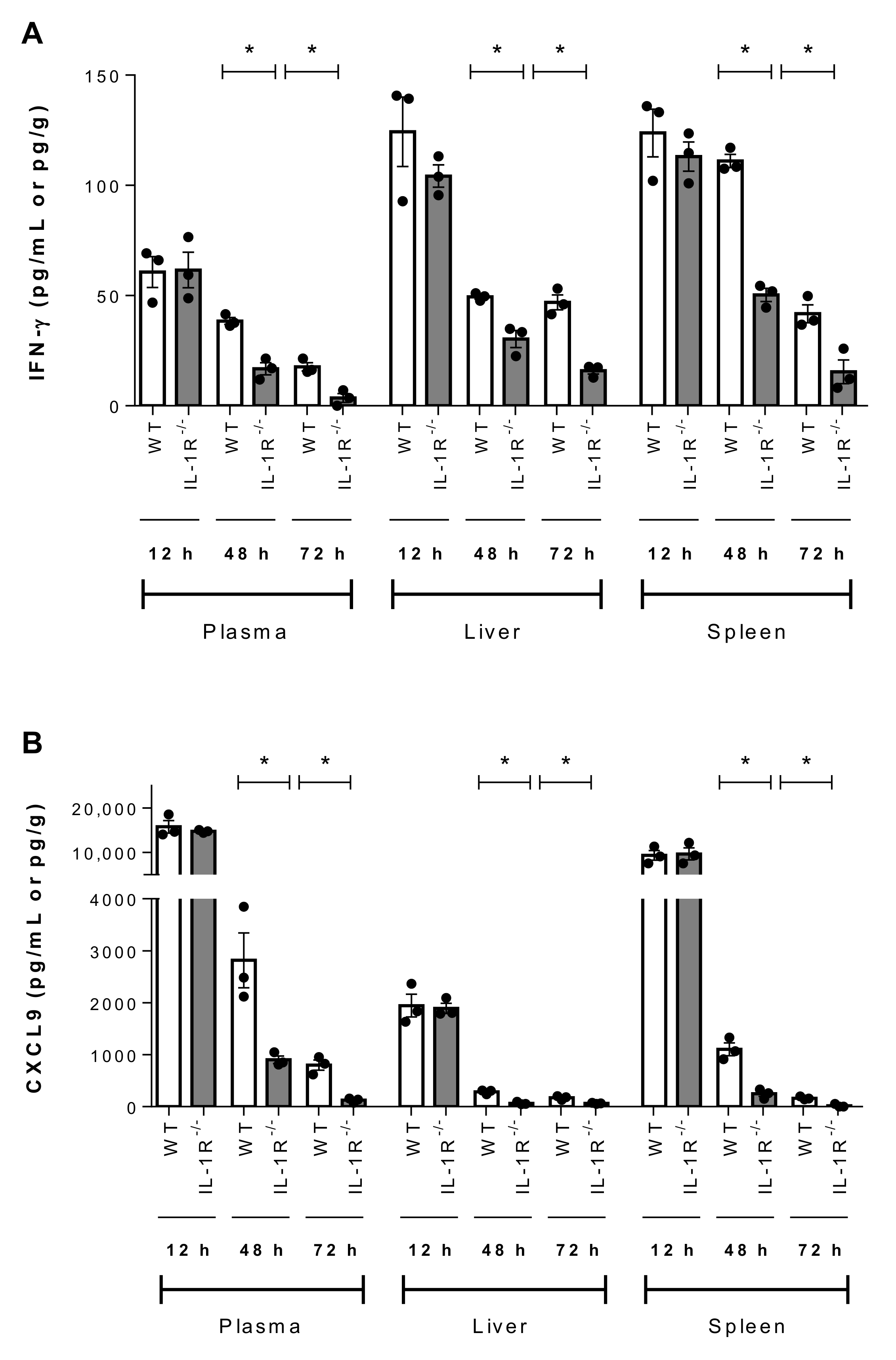
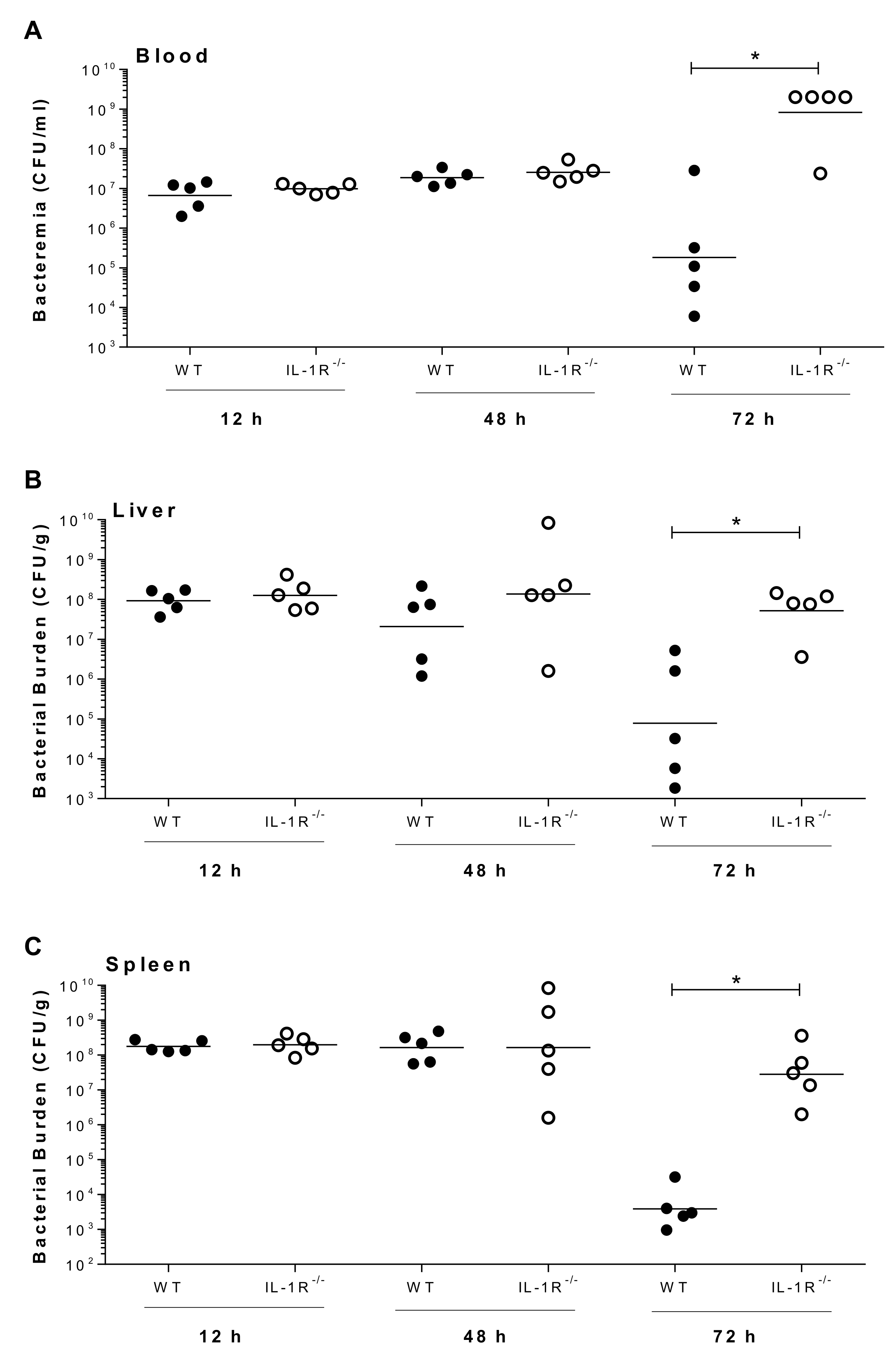
2.7. IL-1 Signaling Plays a Beneficial Role in Host Survival During the Systemic Infection Induced by Suilysin-Negative S. suis
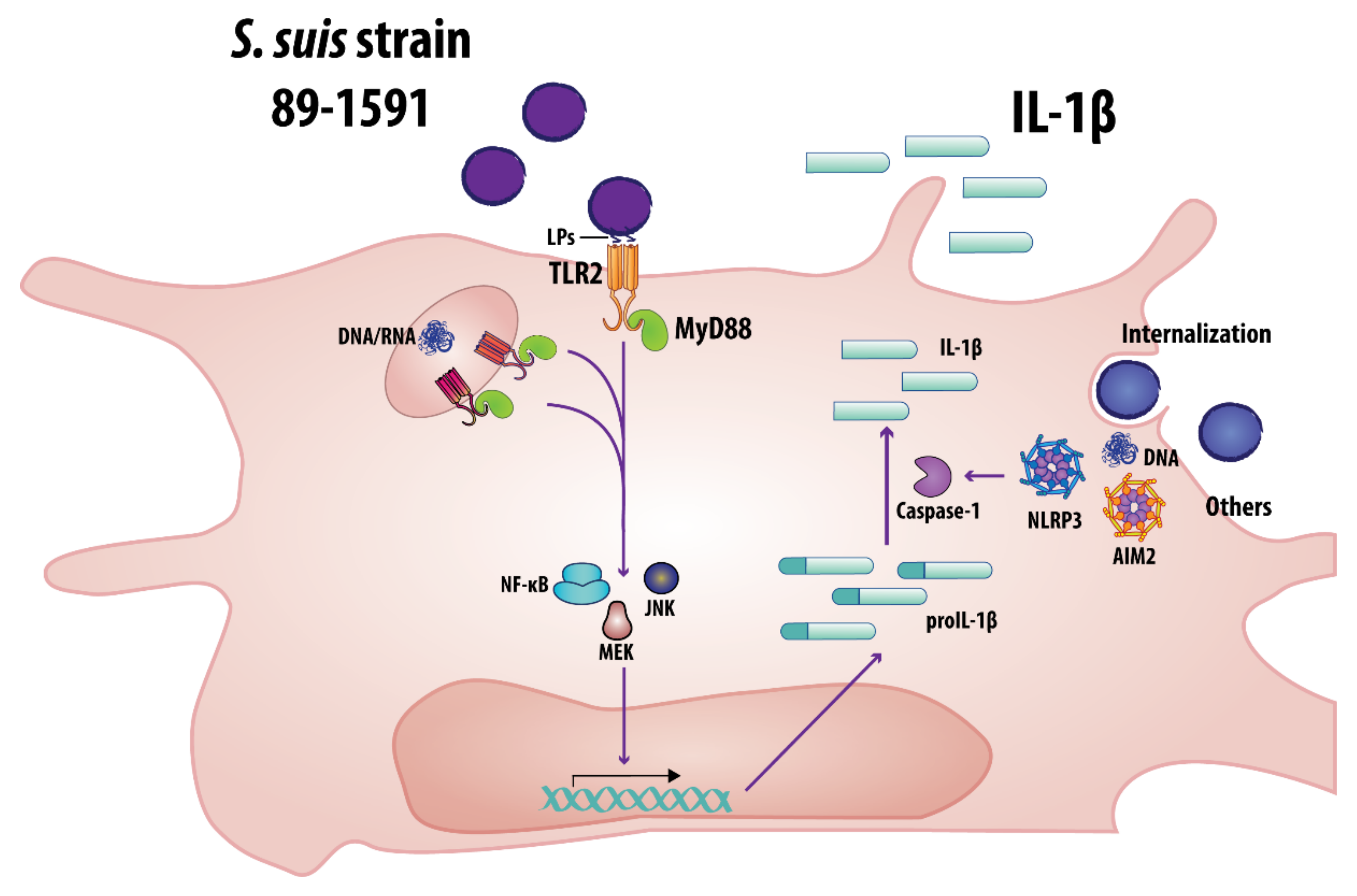
3. Discussion
4. Material and Methods
4.1. Ethics Statement
4.2. S. suis Strains and Growth Conditions
4.3. Lipoteichoic Acid Preparation
4.4. Mice
4.5. Generation of Bone Marrow-Derived Dendritic Cells
4.6. S. suis Infection of Bone Marrow-Derived Dendritic Cells
4.7. S. suis DNA and RNA Preparation and Transfection of Cells
4.8. Preparation of Heat-Killed S. suis and Bacterial Lysates
4.9. IL-1β Quantification in Cell Culture Supernatants
4.10. Determination of Cell mRNA Expression by RT-qPCR
4.11. S. suis Mouse Model of Infection
4.12. Measurement of Plasma, Liver and Spleen Pro-Inflammatory Mediators
4.13. Measurement of Blood, Spleen, and Liver Bacterial Burden
4.14. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIM2 | absent in melanoma 2 |
| bmDC | bone marrow-derived dendritic cell |
| CCL | C-C motif chemokine ligand |
| CFU | colony-forming unit |
| CXCL | C-X-C motif chemokine ligand |
| DC | dendritic cell |
| DMSO | dimethylsulfoxide |
| IFN | interferon |
| IL | interleukin |
| IL-1R | interleukin-1 receptor |
| JNK | c-Jun N-terminal kinase |
| LP | lipoprotein |
| LTA | lipoteichoic acid |
| MAPK | mitogen-activated protein kinase |
| MEK1/2 | mitogen-activated protein kinase 1/2 |
| MyD88 | myeloid differentiation primary response 88 |
| NF-κB | nuclear factor kappa B |
| NLR | NOD-like receptor |
| NLRC | NLR family CARD domain-containing protein |
| NLRP | NLR family pyrin domain-containing |
| NOD | nucleotide oligomerization domain |
| PCR | polymerase chain reaction |
| p.i. | post-infection |
| PRR | pattern recognition receptor |
| p38 | p38 mitogen-activated protein kinase |
| SLY | suilysin |
| ST | sequence type |
| THA | Todd Hewitt broth agar |
| THB | Todd Hewitt broth |
| TLR | Toll-like receptor |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
References
- Gottschalk, M.; Segura, M. Streptococcosis. In Diseases of Swine; Wiley: Hoboken, NJ, USA, 2019; pp. 934–950. [Google Scholar]
- Gottschalk, M.; Xu, J.; Calzas, C.; Segura, M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Futur. Microbiol. 2010, 5, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.T.H.; Hoa, N.T.; Nga, T.V.T.; Linh, L.D.; Chau, T.T.H.; Sinh, D.X.; Phu, N.H.; Van Chuong, L.; Diep, T.S.; Campbell, J.; et al. Streptococcus suis meningitis in adults in Vietnam. Clin. Infect. Dis. 2008, 46, 659–667. [Google Scholar] [PubMed]
- Goyette-Desjardins, G.; Auger, J.-P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Berthelot-Hérault, F.; Gottschalk, M.; Morvan, H.; Kobisch, M. Dilemma of virulence of Streptococcus suis: Canadian isolate 89-1591 characterized as a virulent strain using a standardized experimental model in pigs. Can. J. Vet. Res. 2005, 69, 236–240. [Google Scholar] [PubMed]
- Estrada, A.A.; Gottschalk, M.; Rossow, S.; Rendahl, A.; Gebhart, C.; Marthaler, D.G. Serotype and genotype (multilocus sequence type) of Streptococcus suis isolates from the United States serve as predictors of pathotype. J. Clin. Microbiol. 2019, 57, e00377-19. [Google Scholar] [CrossRef]
- Kerdsin, A.; Akeda, Y.; Takeuchi, D.; Dejsirilert, S.; Gottschalk, M.; Oishi, K. Genotypic diversity of Streptococcus suis strains isolated from humans in Thailand. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 917–925. [Google Scholar] [CrossRef]
- Athey, T.B.T.; Auger, J.-P.; Teatero, S.; Dumesnil, A.; Takamatsu, D.; Wasserscheid, J.; Dewar, K.; Gottschalk, M.; Fittipaldi, N. Complex population structure and virulence differences among serotype 2 Streptococcus suis strains belonging to sequence type 28. PLoS ONE 2015, 10, 48. [Google Scholar] [CrossRef]
- Auger, J.-P.; Fittipaldi, N.; Benoit-Biancamano, M.-O.; Segura, M.; Gottschalk, M. Virulence studies of different sequence types and geographical origins of Streptococcus suis serotype 2 in a mouse model of infection. Pathogens 2016, 5, 48. [Google Scholar] [CrossRef]
- Fittipaldi, N.; Xu, J.; Lacouture, S.; Tharavichitkul, P.; Osaki, M.; Sekizaki, T.; Takamatsu, D.; Gottschalk, M. Lineage and virulence of Streptococcus suis serotype 2 isolates from North America. Emerg. Infect. Dis. 2011, 17, 2239–2244. [Google Scholar] [CrossRef]
- Fittipaldi, N.; Segura, M.; Grenier, D.; Gottschalk, M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Futur. Microbiol. 2012, 7, 259–279. [Google Scholar] [CrossRef]
- Segura, M.; Fittipaldi, N.; Calzas, C.; Gottschalk, M. Critical Streptococcus suis virulence factors: Are they all really critical? Trends Microbiol. 2017, 25, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Lavagna, A.; Auger, J.-P.; Dumesnil, A.; Roy, D.; Girardin, S.E.; Gisch, N.; Segura, M.; Gottschalk, M. Interleukin-1 signaling induced by Streptococcus suis serotype 2 is strain-dependent and contributes to bacterial clearance and inflammation during systemic disease in a mouse model of infection. Vet. Res. 2019, 50, 52. [Google Scholar] [CrossRef] [PubMed]
- Lecours, M.-P.; Segura, M.; Fittipaldi, N.; Rivest, S.; Gottschalk, M. Immune receptors involved in Streptococcus suis recognition by dendritic cells. PLoS ONE 2012, 7, e44746. [Google Scholar] [CrossRef] [PubMed]
- Graveline, R.; Segura, M.; Radzioch, D.; Gottschalk, M. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 2007, 19, 375–389. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Auger, J.-P.; Benoit-Biancamano, M.-O.; Bédard, C.; Segura, M.; Gottschalk, M. Differential role of MyD88 signaling in Streptococcus suis serotype 2-induced systemic and central nervous system diseases. Int. Immunol. 2019, 31, 697–714. [Google Scholar] [CrossRef]
- Lecours, M.-P.; Gottschalk, M.; Houde, M.; Lemire, P.; Fittipaldi, N.; Segura, M. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J. Infect. Dis. 2011, 204, 919–929. [Google Scholar] [CrossRef]
- Auger, J.-P.; Santinón, A.; Roy, D.; Mossman, K.; Xu, J.; Segura, M.; Gottschalk, M. Type I interferon induced by Streptococcus suis serotype 2 is strain-dependent and may be beneficial for host survival. Front. Immunol. 2017, 8, 8. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Sarti, A.C.; Falzoni, S.; Di Virgilio, F. The P2X7 receptor-interleukin-1 liaison. Front. Pharmacol. 2017, 8, 35582. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int. Rev. Immunol. 1998, 16, 457–499. [Google Scholar] [CrossRef]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C.; Mancuso, G.; Midiri, A.; Signorino, G.; Domina, M.; Cariccio, V.L.; Venza, M.; Venza, I.; Teti, G.; Beninati, C. Essential role of interleukin-1 signaling in host defenses against group B Streptococcus. mBio 2014, 5, e01428-14. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2017, 281, 8–27. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Müller, C.; Martin, S.; Beyaert, R. Proteolytic processing of interleukin-1 family cytokines: Variations on a common theme. Immunity 2015, 42, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Muruve, D.A.; Tschopp, J. Innate Immunity: Cytoplasmic DNA sensing by the AIM2 inflammasome. Curr. Biol. 2009, 19, R262–R265. [Google Scholar] [CrossRef]
- Zwijnenburg, P.J.; Van Der Poll, T.; Florquin, S.; Roord, J.J.; Van Furth, A.M. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J. Immunol. 2003, 170, 4724–4730. [Google Scholar] [CrossRef]
- Kafka, D.; Ling, E.; Feldman, G.; Benharroch, D.; Voronov, E.; Givon-Lavi, N.; Iwakura, Y.; Dagan, R.; Apte, R.N.; Mizrachi-Nebenzahl, Y. Contribution of IL-1 to resistance to Streptococcus pneumoniae infection. Int. Immunol. 2008, 20, 1139–1146. [Google Scholar] [CrossRef]
- Biondo, C.; Mancuso, G.; Midiri, A.; Signorino, G.; Domina, M.; Lanza Cariccio, V.; Mohammadi, N.; Venza, M.; Venza, I.; Teti, G.; et al. The interleukin-1β/CXCL1/2/neutrophil axis mediates host protection against group B streptococcal infection. Infect. Immun. 2014, 82, 4508–4517. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Nizet, V. Group A Streptococcus encounters with host macrophages. Futur. Microbiol. 2018, 13, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Castiglia, V.; Piersigilli, A.; Ebner, F.; Janos, M.; Goldmann, O.; Dambock, U.; Kroger, A.; Weiss, S.; Knapp, S.; Jamieson, A.M.; et al. Type I interferon signaling prevents IL-1β-driven lethal systemic hyperinflammation during invasive bacterial infection of soft tissue. Cell Host Microbe 2016, 19, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, L.; Lv, W.; Han, L.; Xiang, Y.; Fu, L.; Jin, M.; Zhou, R.; Chen, H.; Zhang, A. An NLRP3 inflammasome-triggered cytokine storm contributes to streptococcal toxic shock-like syndrome (STSLS). PLoS Pathog. 2019, 15, e1007795. [Google Scholar] [CrossRef] [PubMed]
- King, S.J.; Heath, P.J.; Luque, I.; Tarradas, C.; Dowson, C.; Whatmore, A. Distribution and genetic diversity of suilysin in Streptococcus suis isolated from different diseases of pigs and characterization of the genetic basis of suilysin absence. Infect. Immun. 2001, 69, 7572–7582. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Punaro, M.D.L.C.; Segura, M.; Radzioch, D.; Rivest, S.; Gottschalk, M. Comparison of the susceptibilities of C57BL/6 and A/J mouse strains to Streptococcus suis serotype 2 infection. Infect. Immun. 2008, 76, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Letendre, C.; Auger, J.-P.; Lemire, P.; Galbas, T.; Gottschalk, M.; Thibodeau, J.; Segura, M. Streptococcus suis serotype 2 infection impairs interleukin-12 production and the MHC-II-restricted antigen presentation capacity of dendritic cells. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-ĸB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, 1–10. [Google Scholar] [CrossRef]
- Auger, J.-P.; Dolbec, D.; Roy, D.; Segura, M.; Gottschalk, M. Role of the Streptococcus suis serotype 2 capsular polysaccharide in the interactions with dendritic cells is strain-dependent but remains critical for virulence. PLoS ONE 2018, 13, e0200453. [Google Scholar] [CrossRef]
- Schreur, P.J.W.; Rebel, J.M.J.; Smits, M.; Van Putten, J.; Smith, H.E. Lgt processing is an essential step in Streptococcus suis lipoprotein mediated innate immune activation. PLoS ONE 2011, 6, e22299. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tawaratsumida, K.; Kariya, H.; Aoyama, K.; Tamura, T.; Suda, Y. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int. Immunol. 2005, 18, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Tawaratsumida, K.; Kariya, H.; Kiyohara, A.; Suda, Y.; Krikae, F.; Kirikae, T.; Götz, F. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 2006, 177, 3162–3169. [Google Scholar] [CrossRef]
- Stoll, H.; Dengjel, J.; Nerz, C.; Götz, F. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect. Immun. 2005, 73, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Gisch, N.; Kohler, T.; Ulmer, A.J.; Müthing, J.; Pribyl, T.; Fischer, K.; Lindner, B.; Hammerschmidt, S.; Zähringer, U. Structural reevaluation of Streptococcus pneumoniae lipoteichoic acid and new insights into its immunostimulatory potency. J. Biol. Chem. 2013, 288, 15654–15667. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Van De Veerdonk, F.L.; Van Der Meer, J.W.; Dinarello, C.A.; Joosten, L.A. Inflammasome-independent regulation of IL-1-family cytokines. Annu. Rev. Immunol. 2015, 33, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Martinon, F. The AIM2 inflammasome: Sensor of pathogens and cellular perturbations. Immunol. Rev. 2017, 281, 99–114. [Google Scholar] [CrossRef]
- Wang, B.; Yin, Q. AIM2 inflammasome activation and regulation: A structural perspective. J. Struct. Biol. 2017, 200, 279–282. [Google Scholar] [CrossRef]
- Segura, M.; Calzas, C.; Grenier, D.; Gottschalk, M. Initial steps of the pathogenesis of the infection caused by Streptococcus suis: Fighting against nonspecific defenses. FEBS Lett. 2016, 590, 3772–3799. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Rebel, J.M.; Smits, M.A.; van Putten, J.P.; Smith, H.E. Differential activation of the Toll-like receptor 2/6 complex by lipoproteins of Streptococcus suis serotypes 2 and 9. Vet. Microbiol. 2010, 143, 363–370. [Google Scholar] [CrossRef]
- Gisch, N.; Auger, J.-P.; Thomsen, S.; Roy, D.; Xu, J.; Schwudke, D.; Gottschalk, M. Structural analysis and immunostimulatory potency of lipoteichoic acids isolated from three Streptococcus suis serotype 2 strains. J. Biol. Chem. 2018, 293, 12011–12025. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Franchi, L.; Nunez, G. TLR agonists stimulate NLRP3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J. Immunol. 2013, 190, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Englezou, P.C.; Rothwell, S.W.; Ainscough, J.; Brough, D.; Landsiedel, R.; Verkhratsky, A.; Kimber, I.; Kimber, I. P2X7R activation drives distinct IL-1 responses in dendritic cells compared to macrophages. Cytokine 2015, 74, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Tsuchiya, K.; Kawamura, I.; Shen, Y.; Hara, H.; Sakai, S.; Yamamoto, T.; Fernandes-Alnemri, T.; Yang, R.; Hernandez-Cuellar, E.; et al. Critical roles of ASC inflammasomes in caspase-1 activation and host innate resistance to Streptococcus pneumoniae infection. J. Immunol. 2011, 187, 4890–4899. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Higgins, R.; Jacques, M.; Dubreuil, D. Production and characterization of two Streptococcus suis capsular type 2 mutants. Vet. Microbiol. 1992, 30, 59–71. [Google Scholar] [CrossRef]
- Lachance, C.; Gottschalk, M.; Gerber, P.P.; Lemire, P.; Xu, J.; Segura, M. Exacerbated Type II interferon response drives hypervirulence and toxic shock by an emergent epidemic strain of Streptococcus suis. Infect. Immun. 2013, 81, 1928–1939. [Google Scholar] [CrossRef]
- Segura, M.; Gottschalk, M.; Olivier, M. Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect. Immun. 2004, 72, 5322–5330. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.; Allen, A.; May, J.; Bolitho, S.; Lindsay, H.; Maskell, D.J. Mutagenesis of Streptococcus equi and Streptococcus suis by transposon Tn917. Vet. Microbiol. 2003, 93, 197–206. [Google Scholar] [CrossRef]
- Heß, N.; Waldow, F.; Kohler, T.P.; Rohde, M.; Kreikemeyer, B.; Mejia, A.G.; Hain, T.; Schwudke, D.; Vollmer, W.; Hammerschmidt, S.; et al. Lipoteichoic acid deficiency permits normal growth but impairs virulence of Streptococcus pneumoniae. Nat. Commun. 2017, 8, 2093. [Google Scholar] [CrossRef]
- Franchi, L.; Amer, A.; Body-Malapel, M.; Kanneganti, T.D.; Ozoren, N.; Jagirdar, R.; Inohara, N.; Vandenabeele, P.; Bertin, J.; Coyle, A.; et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin-1β in salmonella-infected macrophages. Nat. Immunol. 2006, 7, 576–582. [Google Scholar] [CrossRef]
- Franchi, L.; Kamada, N.; Nakamura, Y.; Burberry, A.; Kuffa, P.; Suzuki, S.; Shaw, M.H.; Kim, Y.G.; Nunez, G. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat. Immunol. 2012, 13, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Su, Z.; Piccirillo, C.; Stevenson, M.M. Impairment of dendritic cell function by excretory-secretory products: A potential mechanism for nematode-induced immunosuppression. Eur. J. Immunol. 2007, 37, 1887–1904. [Google Scholar] [CrossRef] [PubMed]
- Gratz, N.; Hartweger, H.; Matt, U.; Kratochvill, F.; Janos, M.; Sigel, S.; Drobits, B.; Li, X.-D.; Knapp, S.; Kovarik, P. Type I interferon production induced by Streptococcus pyogenes-derived nucleic acids is required for host protection. PLoS Pathog. 2011, 7, e1001345. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Gambuzza, M.; Midiri, A.; Biondo, C.; Papasergi, S.; Akira, S.; Teti, G.; Beninati, C. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat. Immunol. 2009, 10, 587–594. [Google Scholar] [CrossRef]
- Segura, M.; Stankova, J.; Gottschalk, M. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect. Immun. 1999, 67, 4646–4654. [Google Scholar] [CrossRef]

| Strain | General Characteristics | Reference |
|---|---|---|
| 89-1591 | Virulent North American ST25 strain isolated from a case of pig sepsis in Canada | [56] |
| 89-1591ΔcpsF | Non-encapsulated isogenic mutant derived from 89-1591; in frame deletion of cpsF gene | [40] |
| 89-1591Δlgt | Isogenic mutant strain derived from 89-1591; in frame deletion of lgt gene | [51] |
| P1/7 | Classical highly virulent ST1 strain isolated from a pig with meningitis in the United Kingdom, used for comparison | [59] |
| Primer Name | Sequence (5′ – 3′) |
|---|---|
| Atp5b | F: ACC AGC CCA CCC TAG CCA CC R: TGC AGG GGC AGG GTC AGT CA |
| Gapdh | F: CCC GTA GAC AAA ATG GTG AAG R: GAC TGT GCC GTT GAA TTT G |
| Il1b | F: AGG TCA AAG GTT TGG AAG CA R: TGA AGC TAT GGC AAC TG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavagna, A.; Auger, J.-P.; Giradin, S.E.; Gisch, N.; Segura, M.; Gottschalk, M. Recognition of Lipoproteins by Toll-like Receptor 2 and DNA by the AIM2 Inflammasome Is Responsible for Production of Interleukin-1β by Virulent Suilysin-Negative Streptococcus suis Serotype 2. Pathogens 2020, 9, 147. https://doi.org/10.3390/pathogens9020147
Lavagna A, Auger J-P, Giradin SE, Gisch N, Segura M, Gottschalk M. Recognition of Lipoproteins by Toll-like Receptor 2 and DNA by the AIM2 Inflammasome Is Responsible for Production of Interleukin-1β by Virulent Suilysin-Negative Streptococcus suis Serotype 2. Pathogens. 2020; 9(2):147. https://doi.org/10.3390/pathogens9020147
Chicago/Turabian StyleLavagna, Agustina, Jean-Philippe Auger, Stephen E. Giradin, Nicolas Gisch, Mariela Segura, and Marcelo Gottschalk. 2020. "Recognition of Lipoproteins by Toll-like Receptor 2 and DNA by the AIM2 Inflammasome Is Responsible for Production of Interleukin-1β by Virulent Suilysin-Negative Streptococcus suis Serotype 2" Pathogens 9, no. 2: 147. https://doi.org/10.3390/pathogens9020147
APA StyleLavagna, A., Auger, J.-P., Giradin, S. E., Gisch, N., Segura, M., & Gottschalk, M. (2020). Recognition of Lipoproteins by Toll-like Receptor 2 and DNA by the AIM2 Inflammasome Is Responsible for Production of Interleukin-1β by Virulent Suilysin-Negative Streptococcus suis Serotype 2. Pathogens, 9(2), 147. https://doi.org/10.3390/pathogens9020147






