Host Factors Affecting Generation of Immunity Against Porcine Epidemic Diarrhea Virus in Pregnant and Lactating Swine and Passive Protection of Neonates
Abstract
1. Introduction
2. PEDV: A Re-Emerging Enteric Coronavirus
3. Host Immune Response to PEDV
3.1. Innate Immunity
3.2. Adaptive Immunity
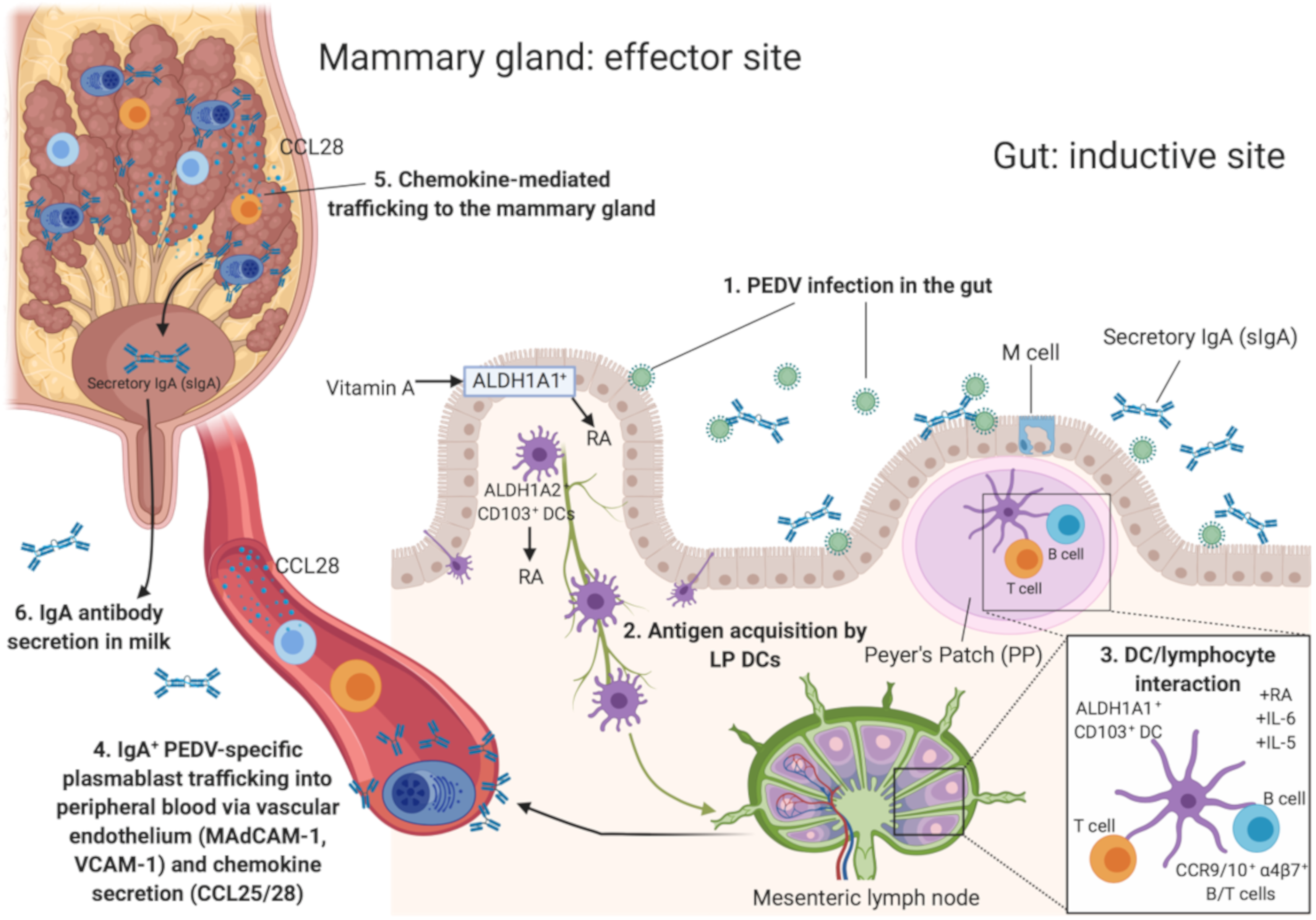
3.3. Generating PEDV Lactogenic Immune Protection
3.4. Inducing Lactogenic Immunity against PEDV—Vaccination Strategies
4. Host Factors Impacting Maternal Immunity in Swine
4.1. Basic Aspects of Lymphocyte Mucosal Trafficking
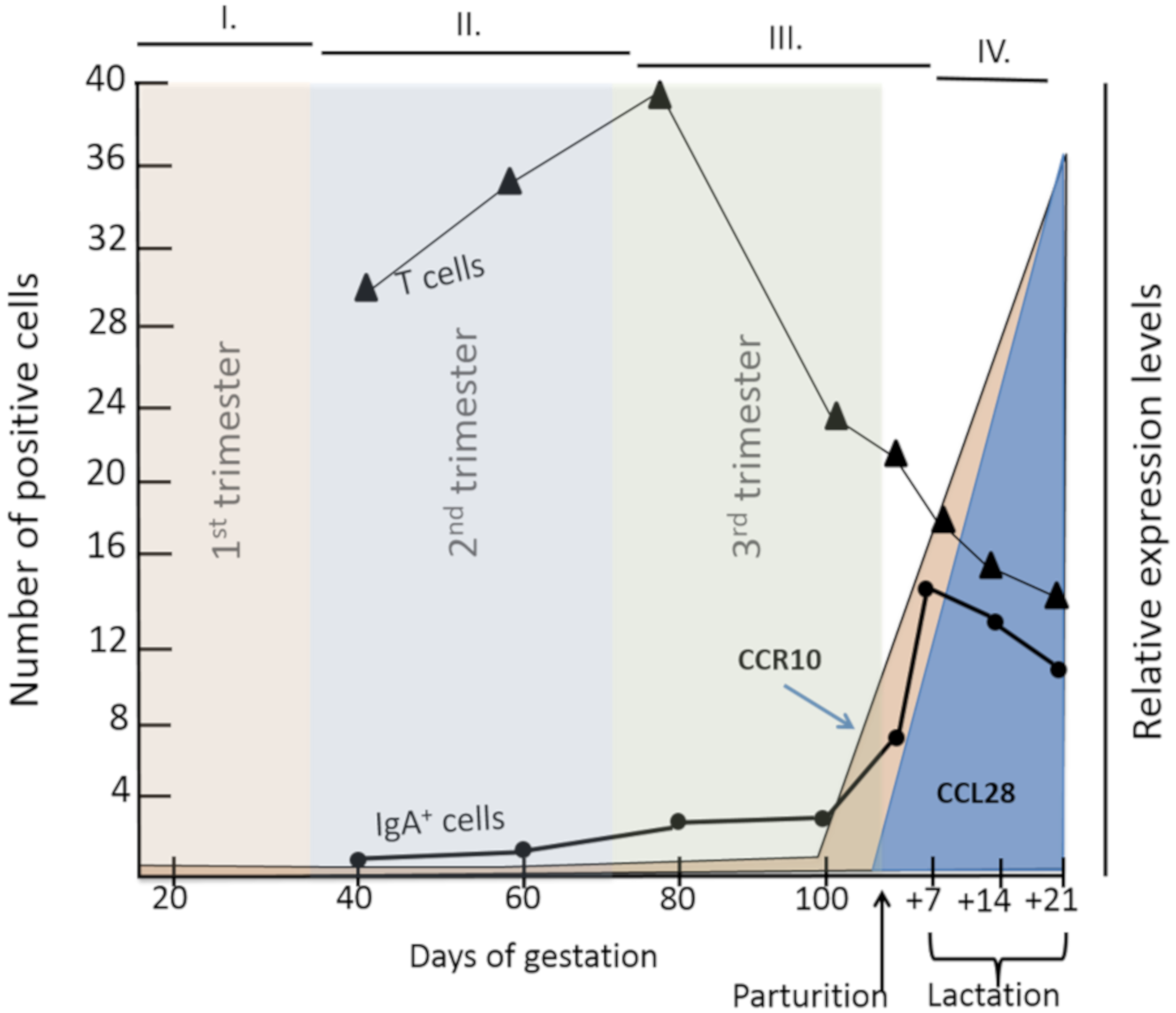
4.2. Role of Pregnancy-Associated Hormones and Gestational Stage in Lymphocyte Mucosal Trafficking
4.3. Other Factors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saif, L.J.; Theil, K.W. Enteric coronaviruses. In Viral Diarrhea of Man and Animals; Saif, L.J., Heckert, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Langel, S.N.; Paim, F.C.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus Res. 2016, 226, 93–107. [Google Scholar] [CrossRef]
- Chattha, K.S.; Roth, J.A.; Saif, L.J. Strategies for design and application of enteric viral vaccines. Annu. Rev. Anim. Biosci. 2015, 3, 375–395. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China,2019. New Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Huang, Y.W.; Dickerman, A.W.; Pineyro, P.; Li, L.; Fang, L.; Kiehne, R.; Opriessnig, T.; Meng, X.J. Origin, evolution and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. MBio 2013, 4, e00737-13. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020. Available online: https://doi.org/10.1016/S0140-6736(20)30251-8 (accessed on 13 February 2020). [CrossRef]
- Anthony, S.J.; Gilardi, K.; Menachery, V.D.; Goldstein, T.; Ssebide, B.; Mbabazi, R.; Navarrete-Macias, I.; Liang, E.; Wells, H.; Hicks, A. Further Evidence for Bats as the Evolutionary Source of Middle East Respiratory Syndrome Coronavirus. mBio 2017, 8, e00373-17. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhao, K.; Shi, Z.-L.; Zhou, P. Bat Coronaviruses in China. Viruses 2019, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Vlasova, A.N.; Kenney, S.P.; Saif, L.J. Emerging and re-emerging coronaviruses in pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef]
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef]
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, A.; Arguello, H.; Martinez-Lobo, F.J.; Costillas, S.; Miranda, R.; PJ, G.d.N.; Rubio, P. Porcine epidemic diarrhoea: New insights into an old disease. Porcine Health Manag. 2015, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J.; Pensaert, M.B.; Sestak, K.; Yeo, S.G.; Jung, K. Coronaviruses. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Takahashi, K.; Okada, K.; Ohshima, K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Nihon Juigaku Zasshi 1983, 45, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Park, B. Porcine epidemic diarrhoea virus: A comprehensive review of molecular epidemiology, diagnosis and vaccines. Virus Genes 2012, 44, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, X.; Wei, S.; Chen, J.; Feng, L. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: A mini-review. J. Vet. Med. Sci. 2016, 78, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takeyama, N.; Katsumata, A.; Tuchiya, K.; Kodama, T.; Kusanagi, K. Mutations in the spike gene of porcine epidemic diarrhea virus associated with growth adaptation in vitro and attenuation of virulence in vivo. Virus Genes 2011, 43, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qiao, S.; Yang, Y.; Su, Y.; Zhao, P.; Zhou, E.; Zhang, G. Phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field strains in central China based on the ORF3 gene and the main neutralization epitopes. Arch. Virol. 2014, 159, 1057–1065. [Google Scholar] [CrossRef]
- Li, R.; Qiao, S.; Yang, Y.; Guo, J.; Xie, S.; Zhou, E.; Zhang, G. Genome sequencing and analysis of a novel recombinant porcine epidemic diarrhea virus strain from Henan, China. Virus Genes 2016, 52, 91–98. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Shi, H.; Qiu, H.; Liu, S.; Chen, X.; Zhang, Z.; Feng, L. Molecular epidemiology of porcine epidemic diarrhea virus in China. Arch. Virol. 2010, 155, 1471–1476. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Huang, L.; Yuan, C.; Wang, J.; Yang, Q. An alternative pathway of enteric PEDV dissemination from nasal cavity to intestinal mucosa in swine. Nat. Commun. 2018, 9, 3811. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Goede, D.P.; Morrison, R.B.; Davies, P.R.; Rovira, A.; Marthaler, D.G.; Torremorell, M. Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Vet. Res. 2014, 45, 73. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Marthaler, D.; Wang, Q.; Culhane, M.R.; Rossow, K.D.; Rovira, A.; Collins, J.; Saif, L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013-February 2014. Emerg. Infect. Dis. 2014, 20, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Marthaler, D.; Jiang, Y.; Otterson, T.; Goyal, S.; Rossow, K.; Collins, J. Complete Genome Sequence of Porcine Epidemic Diarrhea Virus Strain USA/Colorado/2013 from the United States. Genome Announc. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Byrum, B.; Zhang, Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014, 20, 917–919. [Google Scholar] [CrossRef]
- Chen, Q.; Gauger, P.C.; Stafne, M.R.; Thomas, J.T.; Madson, D.M.; Huang, H.; Zheng, Y.; Li, G.; Zhang, J. Pathogenesis comparison between the United States porcine epidemic diarrhoea virus prototype and S-INDEL-variant strains in conventional neonatal piglets. J. General Virol. 2016, 97, 1107–1121. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Lucio de Esesarte, E.; Guo, H.; van den Elzen, P.; Aarts, E.; van den Born, E.; Rottier, P.J.M.; Bosch, B.J. Cell Attachment Domains of the Porcine Epidemic Diarrhea Virus Spike Protein Are Key Targets of Neutralizing Antibodies. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Shi, D.; Shi, H.; Zhang, X.; Yuan, J.; Jiang, S.; Feng, L. Immunogenicity and antigenic relationships among spike proteins of porcine epidemic diarrhea virus subtypes G1 and G2. Arch. Virol. 2016, 161, 537–547. [Google Scholar] [CrossRef]
- Annamalai, T.; Lin, C.M.; Gao, X.; Liu, X.; Lu, Z.; Saif, L.J.; Wang, Q. Cross protective immune responses in nursing piglets infected with a US spike-insertion deletion porcine epidemic diarrhea virus strain and challenged with an original US PEDV strain. Vet. Res. 2017, 48, 61. [Google Scholar] [CrossRef]
- Sato, T.; Oroku, K.; Ohshima, Y.; Furuya, Y.; Sasakawa, C. Efficacy of genogroup 1 based porcine epidemic diarrhea live vaccine against genogroup 2 field strain in Japan. Virol. J. 2018, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ge, X.; Chen, D.; Li, J.; Cai, Y.; Deng, J.; Zhou, L.; Guo, X.; Han, J.; Yang, H. The S Gene Is Necessary but Not Sufficient for the Virulence of Porcine Epidemic Diarrhea Virus Novel Variant Strain BJ2011C. J. Virol. 2018, 92, e00603-18. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, D.C.; Morin, M.; Jolette, J.; Higgins, R.; Marsolais, G.; DiFranco, E. Coronavirus-like particles associated with diarrhea in baby pigs in Quebec. Can. Vet. J. 1980, 21, 100-xxiii. [Google Scholar] [PubMed]
- Wang, J.; Zhao, P.; Guo, L.; Liu, Y.; Du, Y.; Ren, S.; Li, J.; Zhang, Y.; Fan, Y.; Huang, B.; et al. Porcine epidemic diarrhea virus variants with high pathogenicity, China. Emerg. Infect. Dis. 2013, 19, 2048–2049. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Nunoya, T.; Ihara, T.; Kusanagi, K.; Shibuya, K. Dual infection with PCV-2 and porcine epidemic diarrhoea virus in neonatal piglets. Vet. Rec. 2001, 148, 482–484. [Google Scholar] [CrossRef]
- Jung, K.; Ha, Y.; Ha, S.K.; Kim, J.; Choi, C.; Park, H.K.; Kim, S.H.; Chae, C. Identification of porcine circovirus type 2 in retrospective cases of pigs naturally infected with porcine epidemic diarrhoea virus. Vet. J. 2006, 171, 166–168. [Google Scholar] [CrossRef]
- Jung, K.; Kang, B.K.; Lee, C.S.; Song, D.S. Impact of porcine group A rotavirus co-infection on porcine epidemic diarrhea virus pathogenicity in piglets. Res. Vet. Sci. 2008, 84, 502–506. [Google Scholar] [CrossRef]
- Moon, H.W. Epithelial cell migration in the alimentary mucosa of the suckling pig. Proc. Soc. Exp. Biol. Med. 1971, 137, 151–154. [Google Scholar] [CrossRef]
- Annamalai, T.; Saif, L.J.; Lu, Z.; Jung, K. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet. Immunol. Immunopathol. 2015, 168, 193–202. [Google Scholar] [CrossRef]
- Lecce, J.G.; Matrone, G. Porcine neonatal nutrition: The effect of diet on blood serum proteins and performance of the baby pig. J. Nutr. 1960, 70, 13–20. [Google Scholar] [CrossRef]
- Stetson, D.B.; Medzhitov, R. Type I Interferons in Host Defense. Immunity 2006, 25, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Antiviral Actions of Interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Delhase, M. The IκB kinase (IKK) and NF-κB: Key elements of proinflammatory signalling. Semin. Immunol. 2000, 12, 85–98. [Google Scholar] [CrossRef]
- Sharma, S.; TenOever, B.R.; Grandvaux, N.; Zhou, G.P.; Lin, R.; Hiscott, J. Triggering the interferon antiviral response through an IKK-related pathway. Science 2003, 300, 1148–1151. [Google Scholar] [CrossRef]
- Li, L.; Fu, F.; Xue, M.; Chen, W.; Liu, J.; Shi, H.; Chen, J.; Bu, Z.; Feng, L.; Liu, P. IFN-lambda preferably inhibits PEDV infection of porcine intestinal epithelial cells compared with IFN-alpha. Antivir. Res. 2017, 140, 76–82. [Google Scholar] [CrossRef]
- Lee, S.; Baldridge, M.T. Interferon-Lambda: A Potent Regulator of Intestinal Viral Infections. Front. Immunol. 2017, 8, 749. [Google Scholar] [CrossRef]
- Lazear, H.M.; Nice, T.J.; Diamond, M.S. Interferon-λ: Immune functions at barrier surfaces and beyond. Immunity 2015, 43, 15–28. [Google Scholar] [CrossRef]
- Kindler, E.; Thiel, V. To sense or not to sense viral RNA--essentials of coronavirus innate immune evasion. Curr. Opin. Microbiol. 2014, 20, 69–75. [Google Scholar] [CrossRef]
- Zhang, Q.; Ke, H.; Blikslager, A.; Fujita, T.; Yoo, D. Type III Interferon Restriction by Porcine Epidemic Diarrhea Virus and the Role of Viral Protein nsp1 in IRF1 Signaling. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, K.; Yoo, D. Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology 2016, 489, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; van Geelen, A.; Buckley, A.C.; O’Brien, A.; Pillatzki, A.; Lager, K.M.; Faaberg, K.S.; Baker, S.C. Coronavirus Endoribonuclease Activity in Porcine Epidemic Diarrhea Virus Suppresses Type I and Type III Interferon Responses. J. Virol. 2019, 93, e02000-18. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ke, H.; Kim, J.; Yoo, D.; Su, Y.; Boley, P.; Chepngeno, J.; Vlasova, A.N.; Saif, L.J.; Wang, Q. Engineering a Live Attenuated Porcine Epidemic Diarrhea Virus Vaccine Candidate via Inactivation of the Viral 2′-O-Methyltransferase and the Endocytosis Signal of the Spike Protein. J. Virol. 2019, 93, e00406-19. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Zhang, Q.; Dong, J.; Liang, Y.; Huang, Y.; Liu, H.J.; Tong, D. Porcine epidemic diarrhea virus E protein causes endoplasmic reticulum stress and up-regulates interleukin-8 expression. Virol. J. 2013, 10, 26. [Google Scholar] [CrossRef]
- Ding, Z.; Fang, L.; Jing, H.; Zeng, S.; Wang, D.; Liu, L.; Zhang, H.; Luo, R.; Chen, H.; Xiao, S. Porcine Epidemic Diarrhea Virus Nucleocapsid Protein Antagonizes Beta Interferon Production by Sequestering the Interaction between IRF3 and TBK1. J. Virol. 2014, 88, 8936–8945. [Google Scholar] [CrossRef]
- Wang, D.; Fang, L.; Shi, Y.; Zhang, H.; Gao, L.; Peng, G.; Chen, H.; Li, K.; Xiao, S. Porcine Epidemic Diarrhea Virus 3C-Like Protease Regulates Its Interferon Antagonism by Cleaving NEMO. J. Virol. 2016, 90, 2090–2101. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, J.; Tu, J.; Zhang, B.; Chen, X.; Shi, H.; Baker, S.C.; Feng, L.; Chen, Z. The papain-like protease of porcine epidemic diarrhea virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase. J. General Virol. 2013, 94, 1554–1567. [Google Scholar] [CrossRef]
- Yang, L.; Xu, J.; Guo, L.; Guo, T.; Zhang, L.; Feng, L.; Chen, H.; Wang, Y. Porcine Epidemic Diarrhea Virus-Induced Epidermal Growth Factor Receptor Activation Impairs the Antiviral Activity of Type I Interferon. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Guo, L.; Luo, X.; Li, R.; Xu, Y.; Zhang, J.; Ge, J.; Bu, Z.; Feng, L.; Wang, Y. Porcine Epidemic Diarrhea Virus Infection Inhibits Interferon Signaling by Targeted Degradation of STAT1. J. Virol. 2016, 90, 8281–8292. [Google Scholar] [CrossRef]
- Onomoto, K.; Yoneyama, M.; Fung, G.; Kato, H.; Fujita, T. Antiviral innate immunity and stress granule responses. Trends Immunol. 2014, 35, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Zhong, S.; Diel, D.G.; Hou, Y.; Wang, Q.; Nelson, E.; Wang, X. GTPase-activating protein-binding protein 1 (G3BP1) plays an antiviral role against porcine epidemic diarrhea virus. Vet. Microbiol. 2019, 236, 108392. [Google Scholar] [CrossRef] [PubMed]
- Temeeyasen, G.; Sinha, A.; Gimenez-Lirola, L.G.; Zhang, J.Q.; Piñeyro, P.E. Differential gene modulation of pattern-recognition receptor TLR and RIG-I-like and downstream mediators on intestinal mucosa of pigs infected with PEDV non S-INDEL and PEDV S-INDEL strains. Virology 2018, 517, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.C.; Lanier, L.L. NK cells in host responses to viral infections. Curr. Opin. Immunol. 2017, 44, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhao, S.; Qin, T.; Yin, Y.; Yang, Q. Effects of porcine epidemic diarrhea virus on porcine monocyte-derived dendritic cells and intestinal dendritic cells. Vet. Microbiol. 2015, 179, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Parra-Sánchez, H.; Bustamante-Córdova, L.; Reséndiz, M.; Mata-Haro, V.; Pinelli-Saavedra, A.; Hernández, J. Analysis of Swine Conventional Dendritic Cells, DEC205+CD172a+/−CADM1+, from Blood and Spleen in Response to PRRSV and PEDV. Viruses 2019, 11, 1001. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Cao, D.; Tian, D.; Cao, Q.M.; Overend, C.; Yugo, D.M.; Matzinger, S.R.; Rogers, A.J.; Heffron, C.L.; Catanzaro, N.; et al. Efficient priming of CD4 T cells by Langerin-expressing dendritic cells targeted with porcine epidemic diarrhea virus spike protein domains in pigs. Virus Res. 2017, 227, 212–219. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, X.; Jiang, Y.; Tang, L.; Xu, Y.; Qiao, X.; Min, L.; Wen, C.; Ma, G.; Li, Y. Oral Immunization against PEDV with Recombinant Lactobacillus casei Expressing Dendritic Cell-Targeting Peptide Fusing COE Protein of PEDV in Piglets. Viruses 2018, 10, 106. [Google Scholar] [CrossRef]
- Wang, X.; Ohnstad, M.; Nelsen, A.; Nelson, E. Porcine epidemic diarrhea virus does not replicate in porcine monocyte-derived dendritic cells but activates the transcription of type I interferon and chemokine. Vet. Microbiol. 2017, 208, 77–81. [Google Scholar] [CrossRef]
- Liu, X.; Lin, C.-M.; Annamalai, T.; Gao, X.; Lu, Z.; Esseili, M.A.; Jung, K.; El-Tholoth, M.; Saif, L.J.; Wang, Q. Determination of the infectious titer and virulence of an original US porcine epidemic diarrhea virus PC22A strain. Vet. Res. 2015, 46, 109. [Google Scholar] [CrossRef]
- Thomas, J.T.; Chen, Q.; Gauger, P.C.; Giménez-Lirola, L.G.; Sinha, A.; Harmon, K.M.; Madson, D.M.; Burrough, E.R.; Magstadt, D.R.; Salzbrenner, H.M.; et al. Effect of Porcine Epidemic Diarrhea Virus Infectious Doses on Infection Outcomes in Naïve Conventional Neonatal and Weaned Pigs. PLoS ONE 2015, 10, e0139266. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Wang, Q.; Scheuer, K.A.; Lu, Z.; Zhang, Y.; Saif, L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014, 20, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Madson, D.M.; Arruda, P.H.; Magstadt, D.R.; Burrough, E.R.; Hoang, H.; Sun, D.; Bower, L.P.; Bhandari, M.; Gauger, P.C.; Stevenson, G.W.; et al. Characterization of Porcine Epidemic Diarrhea Virus Isolate US/Iowa/18984/2013 Infection in 1-Day-Old Cesarean-Derived Colostrum-Deprived Piglets. Vet. Pathol. 2016, 53, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Niederwerder, M.C.; Nietfeld, J.C.; Bai, J.; Peddireddi, L.; Breazeale, B.; Anderson, J.; Kerrigan, M.A.; An, B.; Oberst, R.D.; Crawford, K.; et al. Tissue localization, shedding, virus carriage, antibody response and aerosol transmission of Porcine epidemic diarrhea virus following inoculation of 4-week-old feeder pigs. J. Vet. Diagn. Investig. 2016, 28, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Paim, F.C.; Alhamo, M.A.; Buckley, A.; Van Geelen, A.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Stage of Gestation at Porcine Epidemic Diarrhea Virus Infection of Pregnant Swine Impacts Maternal Immunity and Lactogenic Immune Protection of Neonatal Suckling Piglets. Front. Immunol. 2019, 10, 727. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front. Immunol. 2014, 5, 253. [Google Scholar] [CrossRef]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Mor, G. Inflammation and Implantation. Am. J. Reprod. Immunol. 2010, 63, 17–21. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef]
- Kay, A.W.; Bayless, N.L.; Fukuyama, J.; Aziz, N.; Dekker, C.L.; Mackey, S.; Swan, G.E.; Davis, M.M.; Blish, C.A. Pregnancy Does Not Attenuate the Antibody or Plasmablast Response to Inactivated Influenza Vaccine. J. Infect. Dis. 2015, 212, 861–870. [Google Scholar] [CrossRef]
- Le Gars, M.; Kay, A.W.; Bayless, N.L.; Aziz, N.; Dekker, C.L.; Swan, G.E.; Davis, M.M.; Blish, C.A. Increased Proinflammatory Responses of Monocytes and Plasmacytoid Dendritic Cells to Influenza A Virus Infection During Pregnancy. J. Infect. Dis. 2016, 214, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Barriga, C.; Rodriguez, A.B.; Ortega, E. Increased phagocytic activity of polymorphonuclear leukocytes during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 57, 43–46. [Google Scholar] [CrossRef]
- Sacks, G.P.; Studena, K.; Sargent, I.L.; Redman, C.W.G. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am. J. Obstet. Gynecol. 1998, 179, 80–86. [Google Scholar] [CrossRef]
- Kraus, T.A.; Engel, S.M.; Sperling, R.S.; Kellerman, L.; Lo, Y.; Wallenstein, S.; Escribese, M.M.; Garrido, J.L.; Singh, T.; Loubeau, M.; et al. Characterizing the pregnancy immune phenotype: Results of the viral immunity and pregnancy (VIP) study. J. Clin. Immunol. 2012, 32, 300–311. [Google Scholar] [CrossRef]
- Lycke, N.Y.; Bemark, M. The regulation of gut mucosal IgA B-cell responses: Recent developments. Mucosal. Immunol. 2017, 10, 1361–1374. [Google Scholar] [CrossRef]
- Ohno, H. Intestinal M cells. J. Biochem. 2016, 159, 151–160. [Google Scholar] [CrossRef]
- Fleeton, M.N.; Contractor, N.; Leon, F.; Wetzel, J.D.; Dermody, T.S.; Kelsall, B.L. Peyer’s patch dendritic cells process viral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J. Exp. Med. 2004, 200, 235–245. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Chen, Y.; Liu, Z.; Zheng, J.; Wang, T.; Luo, H.; Liu, Y.; Shan, Y.; Fang, W.; et al. Effect of route of inoculation on innate and adaptive immune responses to porcine epidemic diarrhea virus infection in suckling pigs. Vet. Microbiol. 2019, 228, 83–92. [Google Scholar] [CrossRef]
- Zhang, N.; Bevan, M.J. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef]
- Subramaniam, S.; Yugo, D.M.; Heffron, C.L.; Rogers, A.J.; Sooryanarain, H.; LeRoith, T.; Overend, C.; Cao, D.; Meng, X.J. Vaccination of sows with a dendritic cell-targeted porcine epidemic diarrhea virus S1 protein-based candidate vaccine reduced viral shedding but exacerbated gross pathological lesions in suckling neonatal piglets. J. General Virol. 2018, 99, 230–239. [Google Scholar] [CrossRef]
- Gimenez-Lirola, L.G.; Zhang, J.; Carrillo-Avila, J.A.; Chen, Q.; Magtoto, R.; Poonsuk, K.; Baum, D.H.; Pineyro, P.; Zimmerman, J. Reactivity of Porcine Epidemic Diarrhea Virus Structural Proteins to Antibodies against Porcine Enteric Coronaviruses: Diagnostic Implications. J. Clin. Microbiol. 2017, 55, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- De Arriba, M.L.; Carvajal, A.; Pozo, J.; Rubio, P. Mucosal and systemic isotype-specific antibody responses and protection in conventional pigs exposed to virulent or attenuated porcine epidemic diarrhoea virus. Vet. Immunol. Immunopathol. 2002, 85, 85–97. [Google Scholar] [CrossRef]
- De Arriba, M.L.; Carvajal, A.; Pozo, J.; Rubio, P. Isotype-specific antibody-secreting cells in systemic and mucosal associated lymphoid tissues and antibody responses in serum of conventional pigs inoculated with PEDV. Vet. Immunol. Immunopathol. 2002, 84, 1–16. [Google Scholar] [CrossRef]
- Bjustrom-Kraft, J.; Woodard, K.; Giménez-Lirola, L.; Rotolo, M.; Wang, C.; Sun, Y.; Lasley, P.; Zhang, J.; Baum, D.; Gauger, P.; et al. Porcine epidemic diarrhea virus (PEDV) detection and antibody response in commercial growing pigs. BMC Vet. Res. 2016, 12, 99. [Google Scholar] [CrossRef]
- Gerber, P.F.; Opriessnig, T. Detection of immunoglobulin (Ig) A antibodies against porcine epidemic diarrhea virus (PEDV) in fecal and serum samples. MethodsX 2015, 2, 368–373. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Kao, C.-F.; Chang, C.-Y.; Jeng, C.-R.; Tsai, P.-S.; Pang, V.F.; Chiou, H.-Y.; Peng, J.-Y.; Cheng, I.-C.; Chang, H.-W. Evaluation and Comparison of the Pathogenicity and Host Immune Responses Induced by a G2b Taiwan Porcine Epidemic Diarrhea Virus (Strain Pintung 52) and Its Highly Cell-Culture Passaged Strain in Conventional 5-Week-Old Pigs. Viruses 2017, 9, 121. [Google Scholar] [CrossRef]
- VanCott, J.L.; Brim, T.A.; Simkins, R.A.; Saif, L.J. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J. Immunol. 1993, 150, 3990–4000. [Google Scholar]
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses and possible vaccination strategies. Front. Immunol. 2014, 5, 446. [Google Scholar] [CrossRef]
- Segre, D.; Kaeberle, M.L. The immunologic behavior of baby pigs. I. Production of antibodies in three-week-old pigs. J. Immunol. 1962, 89, 782–789. [Google Scholar]
- Saif, L.J. Enteric viral infections of pigs and strategies for induction of mucosal immunity. Adv. Vet. Med. 1999, 41, 429–446. [Google Scholar]
- Bohl, E.H.; Frederick, T.; Saif, L.J. Passive immunity in transmissible gastroenteritis of swine: Intramuscular injection of pregnant swine with a modified live-virus vaccine. Am. J. Vet. Res. 1975, 36, 267–271. [Google Scholar]
- Bohl, E.H.; Gupta, R.K.; Olquin, M.V.; Saif, L.J. Antibody responses in serum, colostrum and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect. Immun. 1972, 6, 289–301. [Google Scholar] [CrossRef]
- Saif, L.J.; Bohl, E.H.; Gupta, R.K. Isolation of porcine immunoglobulins and determination of the immunoglobulin classes of transmissible gastroenteritis viral antibodies. Infect. Immun. 1972, 6, 600–609. [Google Scholar] [CrossRef]
- Goede, D.; Murtaugh, M.P.; Nerem, J.; Yeske, P.; Rossow, K.; Morrison, R. Previous infection of sows with a “mild” strain of porcine epidemic diarrhea virus confers protection against infection with a “severe” strain. Vet. Microbiol. 2015, 176, 161–164. [Google Scholar] [CrossRef]
- Poonsuk, K.; Zhang, J.; Chen, Q.; Gonzalez, W.; da Silva Carrion, L.C.; Sun, Y.; Ji, J.; Wang, C.; Main, R.; Zimmerman, J.; et al. Quantifying the effect of lactogenic antibody on porcine epidemic diarrhea virus infection in neonatal piglets. Vet. Microbiol. 2016, 197, 83–92. [Google Scholar] [CrossRef]
- Poonsuk, K.; Gimenez-Lirola, L.G.; Zhang, J.; Arruda, P.; Chen, Q.; Correa da Silva Carrion, L.; Magtoto, R.; Pineyro, P.; Sarmento, L.; Wang, C.; et al. Does Circulating Antibody Play a Role in the Protection of Piglets against Porcine Epidemic Diarrhea Virus? PLoS ONE 2016, 11, e0153041. [Google Scholar] [CrossRef]
- Leidenberger, S.; Schroder, C.; Zani, L.; Auste, A.; Pinette, M.; Ambagala, A.; Nikolin, V.; de Smit, H.; Beer, M.; Blome, S. Virulence of current German PEDV strains in suckling pigs and investigation of protective effects of maternally derived antibodies. Sci. Rep. 2017, 7, 10825. [Google Scholar] [CrossRef]
- Bertasio, C.; Giacomini, E.; Lazzaro, M.; Perulli, S.; Papetti, A.; Lavazza, A.; Lelli, D.; Alborali, G.; Boniotti, M.B. Porcine Epidemic Diarrhea Virus Shedding and Antibody Response in Swine Farms: A Longitudinal Study. Front. Microbiol. 2016, 7, 2009. [Google Scholar] [CrossRef]
- Lin, C.M.; Annamalai, T.; Liu, X.; Gao, X.; Lu, Z.; El-Tholoth, M.; Hu, H.; Saif, L.J.; Wang, Q. Experimental infection of a US spike-insertion deletion porcine epidemic diarrhea virus in conventional nursing piglets and cross-protection to the original US PEDV infection. Vet. Res. 2015, 46, 134. [Google Scholar] [CrossRef]
- Poonsuk, K.; Zimmerman, J. Historical and contemporary aspects of maternal immunity in swine. Anim. Health Res. Rev. 2018, 19, 31–45. [Google Scholar] [CrossRef]
- Joshi, L.R.; Okda, F.A.; Singrey, A.; Maggioli, M.F.; Faccin, T.C.; Fernandes, M.H.V.; Hain, K.S.; Dee, S.; Bauermann, F.V.; Nelson, E.A.; et al. Passive immunity to porcine epidemic diarrhea virus following immunization of pregnant gilts with a recombinant orf virus vector expressing the spike protein. Arch. Virol. 2018, 163, 2327–2335. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Q. Emerging Highly Virulent Porcine Epidemic Diarrhea Virus: Molecular Mechanisms of Attenuation and Rational Design of Live Attenuated Vaccines. Int. J. Mol. Sci. 2019, 20, E5478. [Google Scholar] [CrossRef]
- Kweon, C.H.; Kwon, B.J.; Lee, J.G.; Kwon, G.O.; Kang, Y.B. Derivation of attenuated porcine epidemic diarrhea virus (PEDV) as vaccine candidate. Vaccine 1999, 17, 2546–2553. [Google Scholar] [CrossRef]
- Lin, C.-M.; Ghimire, S.; Hou, Y.; Boley, P.; Langel, S.N.; Vlasova, A.N.; Saif, L.J.; Wang, Q. Pathogenicity and immunogenicity of attenuated porcine epidemic diarrhea virus PC22A strain in conventional weaned pigs. BMC Vet. Res. 2019, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Iglesias, A.; Sanchez, C.M.; Penzes, Z.; Sola, I.; Enjuanes, L.; Zuñiga, S. Recombinant Chimeric Transmissible Gastroenteritis Virus (TGEV)—Porcine Epidemic Diarrhea Virus (PEDV) Virus Provides Protection against Virulent PEDV. Viruses 2019, 11, 682. [Google Scholar] [CrossRef]
- Gillespie, T.; Song, Q.; Inskeep, M.; Stone, S.; Murtaugh, M.P. Effect of Booster Vaccination with Inactivated Porcine Epidemic Diarrhea Virus on Neutralizing Antibody Response in Mammary Secretions. Viral. Immunol. 2018, 31, 62–68. [Google Scholar] [CrossRef]
- Jang, G.; Won, H.; Lee, D.-U.; Noh, Y.-H.; Lee, S.-C.; Choi, H.-W.; Yoon, I.-J.; Lee, Y.J.; Sang Yoo, H.; Lee, C. Assessment of the safety and efficacy of an attenuated live vaccine based on highly virulent genotype 2b porcine epidemic diarrhea virus in nursing piglets. Vet. Microbiol. 2019, 231, 120–128. [Google Scholar] [CrossRef]
- Wen, Z.; Xu, Z.; Zhou, Q.; Li, W.; Wu, Y.; Du, Y.; Chen, L.; Xue, C.; Cao, Y. A heterologous ‘prime-boost’ anti-PEDV immunization for pregnant sows protects neonatal piglets through lactogenic immunity against PEDV. Lett. Appl. Microbiol. 2019, 69, 258–263. [Google Scholar] [CrossRef]
- Bohl, E.H.; Saif, L.J. Passive immunity in transmissible gastroenteritis of swine: Immunoglobulin characteristics of antibodies in milk after inoculating virus by different routes. Infect. Immun. 1975, 11, 23–32. [Google Scholar] [CrossRef]
- Fink, K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front. Immunol. 2012, 3, 78. [Google Scholar] [CrossRef]
- Salmon, H.; Berri, M.; Gerdts, V.; Meurens, F. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 2009, 33, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Butcher, E.C.; Picker, L.J. Lymphocyte homing and homeostasis. Science 1996, 272, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Nguyen, L.P.; Hadeiba, H.; Butcher, E.C. Leukocyte Trafficking to the Small Intestine and Colon. Gastroenterology 2016, 150, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, E.J.; Butcher, E.C. Plasma-cell homing. Nat. Rev. Immunol. 2003, 3, 822–829. [Google Scholar] [CrossRef]
- Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Alon, R.; Feigelson, S.W. Chemokine-triggered leukocyte arrest: Force-regulated bi-directional integrin activation in quantal adhesive contacts. Curr. Opin. Cell Biol. 2012, 24, 670–676. [Google Scholar] [CrossRef]
- Umemoto, E.; Hayasaka, H.; Bai, Z.; Cai, L.; Yonekura, S.; Peng, X.; Takeda, A.; Tohya, K.; Miyasaka, M. Novel regulators of lymphocyte trafficking across high endothelial venules. Crit. Rev. Immunol. 2011, 31, 147–169. [Google Scholar] [CrossRef]
- Briskin, M.; Winsor-Hines, D.; Shyjan, A.; Cochran, N.; Bloom, S.; Wilson, J.; McEvoy, L.M.; Butcher, E.C.; Kassam, N.; Mackay, C.R.; et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 1997, 151, 97–110. [Google Scholar]
- Marui, N.; Offermann, M.K.; Swerlick, R.; Kunsch, C.; Rosen, C.A.; Ahmad, M.; Alexander, R.W.; Medford, R.M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J. Clin. Investig. 1993, 92, 1866–1874. [Google Scholar] [CrossRef]
- Stenstad, H.; Ericsson, A.; Johansson-Lindbom, B.; Svensson, M.; Marsal, J.; Mack, M.; Picarella, D.; Soler, D.; Marquez, G.; Briskin, M.; et al. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood 2006, 107, 3447–3454. [Google Scholar] [CrossRef]
- Wurbel, M.A.; Malissen, M.; Guy-Grand, D.; Malissen, B.; Campbell, J.J. Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria. J. Immunol. 2007, 178, 7598–7606. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Soto, H.; Oldham, E.R.; Buchanan, M.E.; Homey, B.; Catron, D.; Jenkins, N.; Copeland, N.G.; Gilbert, D.J.; Nguyen, N.; et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J. Biol. Chem. 2000, 275, 22313–22323. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Butcher, E.C. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J. Exp. Med. 2004, 200, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Weisz-Carrington, P.; Roux, M.E.; McWilliams, M.; Phillips-Quagliata, J.M.; Lamm, M.E. Hormonal induction of the secretory immune system in the mammary gland. Proc. Natl. Acad. Sci. USA 1978, 75, 2928–2932. [Google Scholar] [CrossRef]
- Kunkel, E.J.; Kim, C.H.; Lazarus, N.H.; Vierra, M.A.; Soler, D.; Bowman, E.P.; Butcher, E.C. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J. Clin. Investig. 2003, 111, 1001–1010. [Google Scholar] [CrossRef]
- Hu, S.; Yang, K.; Yang, J.; Li, M.; Xiong, N. Critical roles of chemokine receptor CCR10 in regulating memory IgA responses in intestines. Proc. Natl. Acad. Sci. USA 2011, 108, E1035–E1044. [Google Scholar] [CrossRef]
- Berri, M.; Meurens, F.; Lefevre, F.; Chevaleyre, C.; Zanello, G.; Gerdts, V.; Salmon, H. Molecular cloning and functional characterization of porcine CCL28: Possible involvement in homing of IgA antibody secreting cells into the mammary gland. Mol. Immunol. 2008, 45, 271–277. [Google Scholar] [CrossRef]
- Robertson, H.A.; King, G.J. Plasma concentrations of progesterone, oestrone, oestradiol-17beta and of oestrone sulphate in the pig at implantation, during pregnancy and at parturition. J. Reprod. Fertil. 1974, 40, 133–141. [Google Scholar] [CrossRef]
- Kraeling, R.R.; Barb, C.R.; Rampacek, G.B. Prolactin and luteinizing hormone secretion in the pregnant pig. J. Anim. Sci. 1992, 70, 3521–3527. [Google Scholar] [CrossRef]
- Foisnet, A.; Farmer, C.; David, C.; Quesnel, H. Farrowing induction induces transient alterations in prolactin concentrations and colostrum composition in primiparous sows. J. Anim. Sci. 2011, 89, 3048–3059. [Google Scholar] [CrossRef]
- Meurens, F.; Berri, M.; Whale, J.; Dybvig, T.; Strom, S.; Thompson, D.; Brownlie, R.; Townsend, H.G.; Salmon, H.; Gerdts, V. Expression of TECK/CCL25 and MEC/CCL28 chemokines and their respective receptors CCR9 and CCR10 in porcine mucosal tissues. Vet. Immunol. Immunopathol. 2006, 113, 313–327. [Google Scholar] [CrossRef]
- Bourges, D.; Meurens, F.; Berri, M.; Chevaleyre, C.; Zanello, G.; Levast, B.; Melo, S.; Gerdts, V.; Salmon, H. New insights into the dual recruitment of IgA+ B cells in the developing mammary gland. Mol Immunol. 2008, 45, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, K.M.; Reed, G.W.; Mitchel, E.F.; Simonsen, L.; Griffin, M.R. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am. J. Epidemiol. 1998, 148, 1094–1102. [Google Scholar] [CrossRef]
- Louie, J.K.; Acosta, M.; Jamieson, D.J.; Honein, M.A.; California Pandemic Working, G. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. New Engl. J. Med. 2010, 362, 27–35. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Okoko, B.J.; Enwere, G.; Ota, M.O. The epidemiology and consequences of maternal malaria: A review of immunological basis. Acta Trop. 2003, 87, 193–205. [Google Scholar] [CrossRef]
- Price, M.E.; Fisher-Hoch, S.P.; Craven, R.B.; McCormick, J.B. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. BMJ 1988, 297, 584–587. [Google Scholar] [CrossRef]
- Brown, C.C.; Noelle, R.J. Seeing through the dark: New insights into the immune regulatory functions of vitamin A. Eur. J. Immunol. 2015, 45, 1287–1295. [Google Scholar] [CrossRef]
- Villamor, E.; Fawzi, W.W. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 2005, 18, 446–464. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Chattha, K.S.; Kandasamy, S.; Siegismund, C.S.; Saif, L.J. Prenatally acquired vitamin A deficiency alters innate immune responses to human rotavirus in a gnotobiotic pig model. J. Immunol. 2013, 190, 4742–4753. [Google Scholar] [CrossRef]
- Sommer, A. Vitamin A deficiency and childhood mortality. Lancet 1992, 340, 488–489. [Google Scholar] [CrossRef]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; von Andrian, U.H. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin. Immunol. 2009, 21, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Paim, F.C.; Alhamo, M.A.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Oral vitamin A supplementation of porcine epidemic diarrhea virus infected gilts enhances IgA and lactogenic immune protection of nursing piglets. Vet. Res. 2019, 50, 101. [Google Scholar] [CrossRef] [PubMed]
- Teymoori-Rad, M.; Shokri, F.; Salimi, V.; Marashi, S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019, 29, e2032. [Google Scholar] [CrossRef]
- Yang, J.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; Mao, X.; He, J.; Luo, Y.; Luo, J.; Huang, Z.; et al. Dietary 25-Hydroxyvitamin D(3) Supplementation Alleviates Porcine Epidemic Diarrhea Virus Infection by Improving Intestinal Structure and Immune Response in Weaned Pigs. Animals 2019, 9, 627. [Google Scholar] [CrossRef]
- Chepngeno, J.; Diaz, A.; Paim, F.C.; Saif, L.J.; Vlasova, A.N. Rotavirus C: Prevalence in suckling piglets and development of virus-like particles to assess the influence of maternal immunity on the disease development. Vet. Res. 2019, 50, 84. [Google Scholar] [CrossRef]
- Ozawa, M.; Matsuu, A.; Yonezawa, K.; Igarashi, M.; Okuya, K.; Kawabata, T.; Ito, K.; Tsukiyama-Kohara, K.; Taneno, A.; Deguchi, E. Efficient Isolation of Swine Influenza Viruses by Age-Targeted Specimen Collection. J. Clin. Microbiol. 2015, 53, 1331–1338. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langel, S.N.; Wang, Q.; Vlasova, A.N.; Saif, L.J. Host Factors Affecting Generation of Immunity Against Porcine Epidemic Diarrhea Virus in Pregnant and Lactating Swine and Passive Protection of Neonates. Pathogens 2020, 9, 130. https://doi.org/10.3390/pathogens9020130
Langel SN, Wang Q, Vlasova AN, Saif LJ. Host Factors Affecting Generation of Immunity Against Porcine Epidemic Diarrhea Virus in Pregnant and Lactating Swine and Passive Protection of Neonates. Pathogens. 2020; 9(2):130. https://doi.org/10.3390/pathogens9020130
Chicago/Turabian StyleLangel, Stephanie N., Qiuhong Wang, Anastasia N. Vlasova, and Linda J. Saif. 2020. "Host Factors Affecting Generation of Immunity Against Porcine Epidemic Diarrhea Virus in Pregnant and Lactating Swine and Passive Protection of Neonates" Pathogens 9, no. 2: 130. https://doi.org/10.3390/pathogens9020130
APA StyleLangel, S. N., Wang, Q., Vlasova, A. N., & Saif, L. J. (2020). Host Factors Affecting Generation of Immunity Against Porcine Epidemic Diarrhea Virus in Pregnant and Lactating Swine and Passive Protection of Neonates. Pathogens, 9(2), 130. https://doi.org/10.3390/pathogens9020130





