Assessment of Risk Factors of African Swine Fever in India: Perspectives on Future Outbreaks and Control Strategies
Abstract
1. Introduction
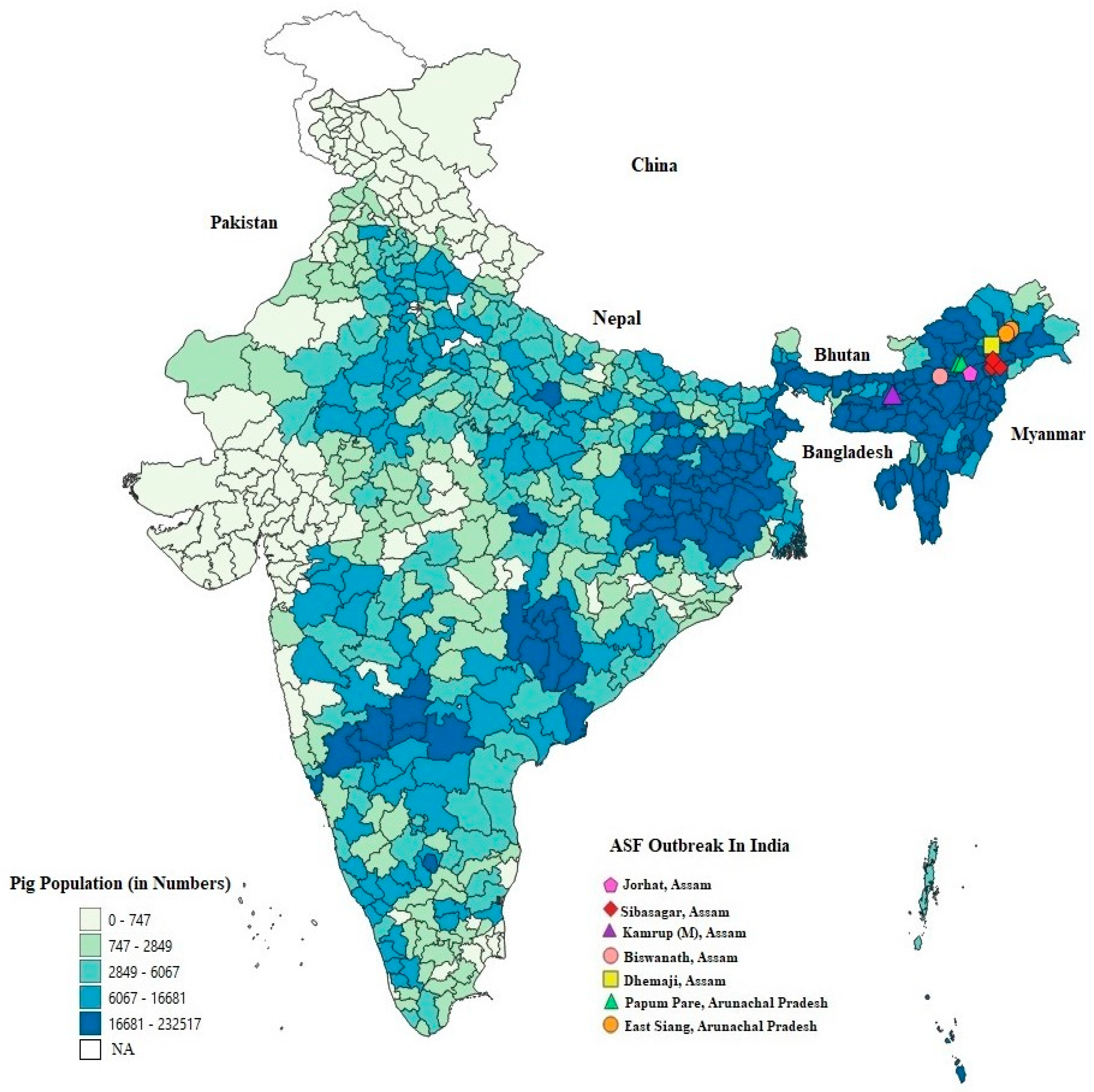
2. First Emergence of ASF in India
3. Etiology and Natural Reservoirs of ASF
4. Epidemiological Cycles of ASF
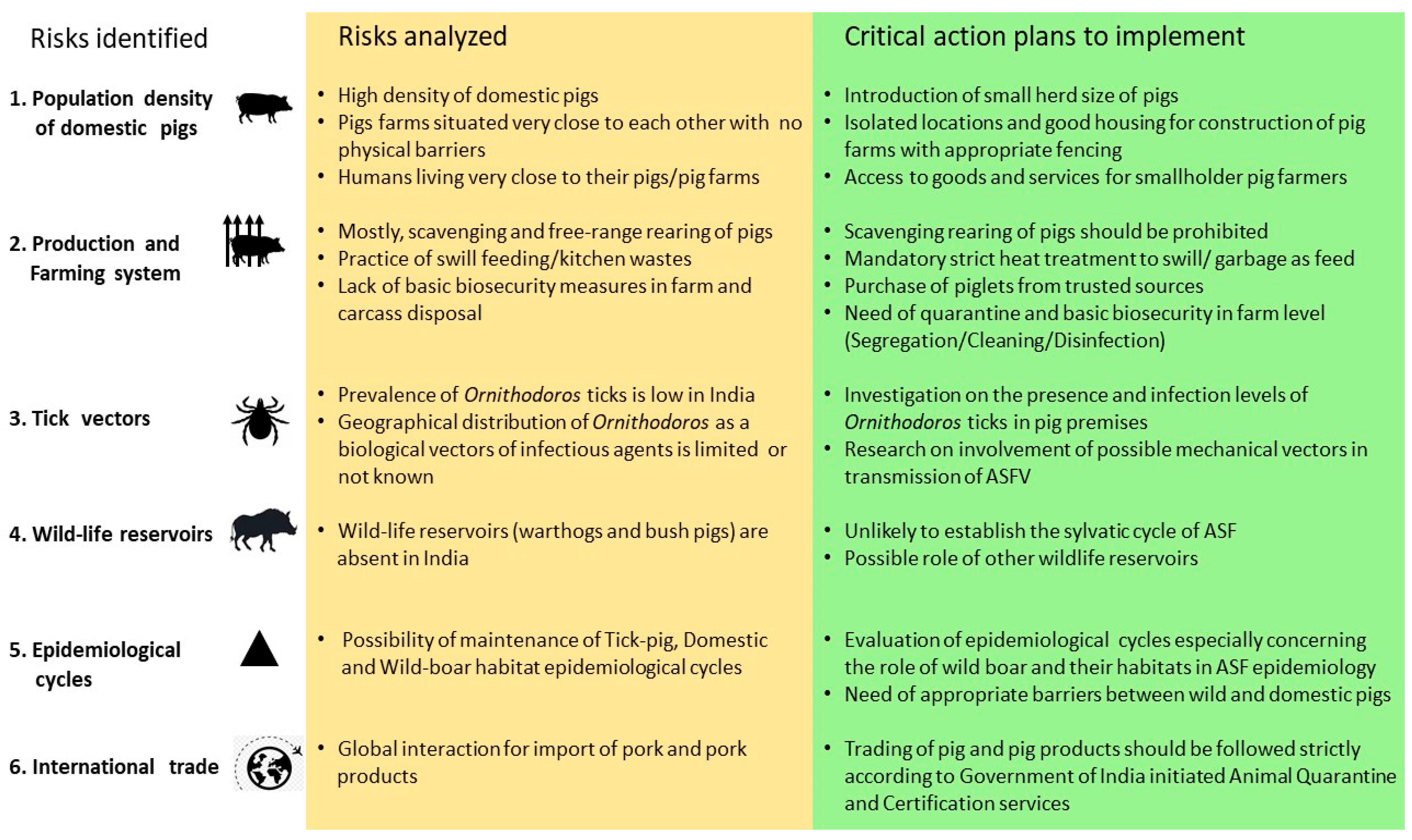
5. Risk Identification and Assessment of ASF
5.1. Population Density of Domestic Pigs
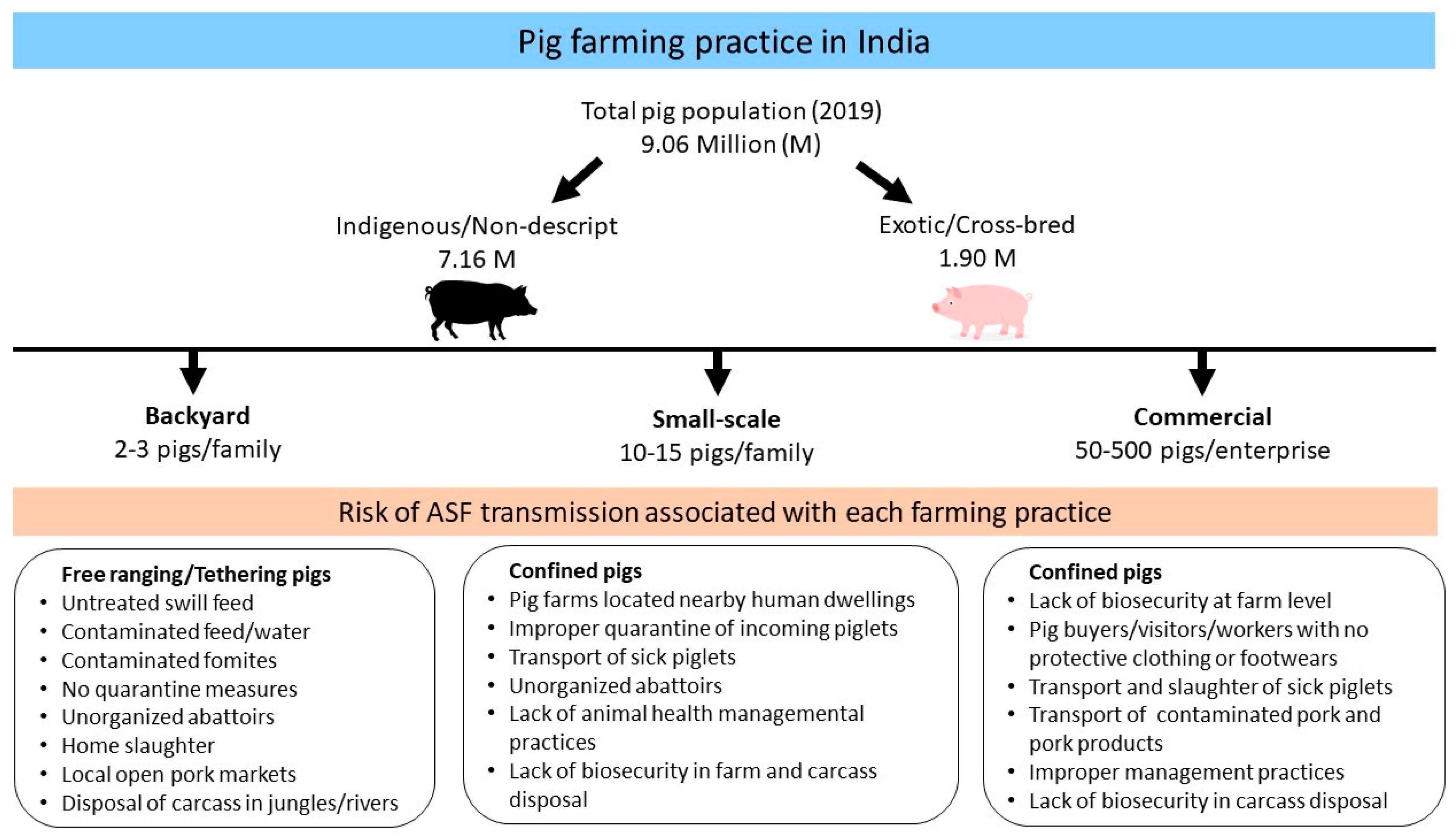
5.2. Farming Practice
5.3. Availability of Tick Vectors
5.4. Availability of Wildlife Reservoirs and Their Habitat
5.5. Correlation between the Epidemiological Cycles of ASF
5.5.1. Sylvatic Cycle
5.5.2. Tick–Pig Cycle
5.5.3. Domestic Cycle
5.5.4. Wild Boar–Habitat Cycle
5.6. International Trade
6. Research Gaps
7. Prevention and Control Strategies
7.1. Establish Control Zones
7.2. Establish Quarantine Facilities
7.3. Prohibition of Scavenging Pig Production Systems
7.4. Enchanced Biosecurity at Backyard and Small-Scale Farms
7.5. Control Interstate Movement
7.6. Ban/Control Illegal Import of Contaminated Pork and Pork Products
7.7. Improve ASF Surveillance
7.8. Quick Elimination of Infected Animals and Proper Disposal
7.9. Disinfection of Infected Premises
7.10. Investigations on Prevalence of Soft Ticks
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Penrith, M.L.; Vosloo, W. Review of African swine fever: Transmission, spread and control. J. S. Afr. Vet. Assoc. 2009, 80, 58–62. [Google Scholar] [CrossRef] [PubMed]
- OIE. OIE-Listed Diseases, Infections and Infestations in Force in 2020. 2020. Available online: https://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2020/ (accessed on 13 August 2020).
- Montgomery, R.E. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African swine fever status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef]
- Matsuyama, T.; Takano, T.; Nishiki, I.; Fujiwara, A.; Kiryu, I.; Inada, M.; Sakai, T.; Terashima, S.; Matsuura, Y.; Isowa, K.; et al. A novel Asfarvirus-like virus identified as a potential cause of mass mortality of abalone. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vizcaino, J.M. Early detection and contingency plans for African swine fever. In Compendium of Technical Items Presented to the OIE World Assembly of Delegates and to OIE Regional Commissions; World Organization for Animal Health: Paris, France, 2010; pp. 129–168. [Google Scholar]
- Walczak, M.; Żmudzki, J.; Mazur-Panasiuk, N.; Juszkiewicz, M.; Woźniakowski, G. Analysis of the Clinical Course of Experimental Infection with Highly Pathogenic African Swine Fever Strain, Isolated from an Outbreak in Poland. Aspects Related to the Disease Suspicion at the Farm Level. Pathogens 2020, 9, 237. [Google Scholar] [CrossRef]
- OIE. African Swine Fever, India. 2020. Available online: https://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=34283 (accessed on 27 October 2020).
- Barman, N.N.; Bora, D.P.; Khatoon, E.; Mandal, S.; Rakshit, A.; Rajbongshi, G.; Depner, K.; Chakraborty, A.; Kumar, S. Classical swine fever in wild hog: Report of its prevalence in northeast India. Transbound. Emerg. Dis. 2016, 63, 540–547. [Google Scholar] [CrossRef]
- Rajkhowa, T.K.; Jagan Mohanarao, G.; Gogoi, A.; Hauhnar, L.; Isaac, L. Porcine reproductive and respiratory syndrome virus (PRRSV) from the first outbreak of India shows close relationship with the highly pathogenic variant of China. Vet. Q. 2015, 35, 186–193. [Google Scholar] [CrossRef]
- Mukherjee, P.; Karam, A.; Barkalita, L.; Borah, P.; Chakraborty, A.K.; Das, S.; Puro, K.; Sanjukta, R.; Ghatak, S.; Shakuntala, I.; et al. Porcine circovirus 2 in the North Eastern region of India: Disease prevalence and genetic variation among the isolates from areas of intensive pig rearing. Acta Trop. 2018, 182, 166–172. [Google Scholar] [CrossRef]
- Galindo, I.; Alonso, C. African swine fever virus: A review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.D.S.; Penrith, M.-L.; Crucière, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; Thomson, G.R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.A.; Tcherepanov, V.; Upton, C.; Dixon, L.K. Comparison of the genome sequences of non-pathogenic and pathogenic African swine fever virus isolates. J. Gen. Virol. 2008, 89, 397–408. [Google Scholar] [CrossRef] [PubMed]
- OIE. African Swine Fever. 2019. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/AFRICAN_SWINE_FEVER.pdf (accessed on 27 October 2020).
- Mulumba-Mfumu, L.K.; Saegerman, C.; Dixon, L.K.; Madimba, K.C.; Kazadi, E.; Mukalakata, N.T.; Oura, C.A.L.; Chenais, E.; Masembe, C.; Ståhl, K.; et al. African swine fever: Update on Eastern, Central and Southern Africa. Transbound. Emerg. Dis. 2019, 66, 1462–1480. [Google Scholar] [CrossRef]
- Jia, N.; Ou, Y.; Pejsak, Z.; Zhang, Y.; Zhang, J. Roles of African swine fever virus structural proteins in viral infection. J. Vet. Res. 2017, 61, 135–143. [Google Scholar] [CrossRef]
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaino, J.M.; Pfeiffer, D.U. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef]
- Kimberling, C.V.; Teegarden, R.M. African Swine Fever. Available online: https://mountainscholar.org/bitstream/handle/10217/182374/AEXT_ucsu206228015.pdf?sequence=1&isAllowed=y (accessed on 27 August 2020).
- Penrith, M.L. African swine fever. Onderstepoort J. Vet. Res. 2009, 76, 91–95. [Google Scholar] [CrossRef]
- Burrage, T.G. African swine fever virus infection in Ornithodoros ticks. Virus Res. 2013, 173, 131–139. [Google Scholar] [CrossRef]
- Frant, M.; Woźniakowski, G.; Pejsak, Z. African swine fever (ASF) and ticks. No risk of tick-mediated ASF spread in Poland and Baltic states. J. Vet. Res. 2017, 61, 375–380. [Google Scholar] [CrossRef]
- Costard, S.; Wieland, B.; De Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef]
- CSFPH. African Swine Fever. The Centre for Food Security and Public Health. 2019. Available online: http://www.cfsph.iastate.edu/Factsheets/pdfs/african_swine_fever.pdf (accessed on 27 October 2020).
- Chenais, E.; Ståhl, K.; Guberti, V.; Depner, K. Identification of wild boar–habitat epidemiologic cycle in African swine fever epizootic. Emerg. Infect. Dis. 2018, 24, 810. [Google Scholar] [CrossRef] [PubMed]
- Plowright, W.; Thomson, G.R.; Neser, J.A. African swine fever. In Infectious Diseases of Livestock, with Special Reference to Southern Africa, 1st ed.; Coetzer, J.A.W., Thomson, G.R., Tutsin, R.C., Eds.; Oxford University Press: Cape Town, South Africa, 1994; Volume 1, pp. 567–599. [Google Scholar]
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. Handbook on African Swine Fever in Wild Boar and Biosecurity during Hunting; World Organization for Animal Health: Paris, France, 2018. [Google Scholar]
- Wilkinson, P.J. The persistence of African swine fever in Africa and the Mediterranean. Prev. Vet. Med. 1984, 2, 71–82. [Google Scholar] [CrossRef]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Ståhl, K. Epidemiological considerations on African swine fever in Europe 2014–2018. Porc. Health Manag. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- NRC Pig. National Research Station in Pig. Vision 2030. 2011. Available online: http://www.nrcp.in/pdf/NRCP_vision_2030_protected.pdf (accessed on 1 September 2020).
- Talukdar, P.; Talukdar, D.; Sarma, K.; Saikia, K. Prospects and Potentiality of Improving Pig Farming in North Eastern Hill Region of India: An Overview. Int. J. Livest. Res. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Kumar, R.; Prakash, N.; Naskar, S. Livestock management practices by the small holders of north eastern region. Indian J. Anim. Sci. 2004, 74, 882–886. [Google Scholar]
- Jeyakumar, S.; Sunder, J.; Kundu, A.; Balakrishnan, P.; Kundu, M.S.; Srivastava, R.C. Nicobari pig: An indigenous pig germplasm of the Nicobar group of Islands, India. Anim. Genet. Resour./Resourc. Génét. Anim. 2014, 55, 77–86. [Google Scholar] [CrossRef]
- Chauhan, A.; Patel, B.H.M.; Maurya, R.; Kumar, S.; Shukla, S.; Kumar, S. Pig production system as a source of livelihood in Indian scenario: An overview. Int. J. Sci. Environ. Technol. 2016, 5, 2089–2096. [Google Scholar]
- Sahu, S.; Sarangi, A.; Gulati, H.K.; Verma, A. Pig farming in Haryana: A review. Int. J. Environ. Sci. Technol. 2018, 7, 624–632. [Google Scholar]
- Livestock Census. 20th Livestock Census. All India Report. 2019. Available online: http://dahd.nic.in/division/provisional-key-results-20th-livestock-census (accessed on 1 September 2020).
- dahd.nic.in. Department of Animal Husbandry, Dairying. Government of India. Ministry of Fisheries, Animal Husbandry and Dairying. Available online: http://dahd.nic.in/sites/default/filess/NAP%20on%20Pig%20.pdf (accessed on 3 September 2020).
- Nunn, C.L.; Jordán, F.; McCabe, C.M.; Verdolin, J.L.; Fewell, J.H. Infectious disease and group size: More than just a numbers game. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140111. [Google Scholar] [CrossRef]
- Kumar, A.; Staal, S.J.; Elumalai, K.; Singh, D.K. Livestock sector in north-eastern region of India: An appraisal of performance. Agric. Econ. Res. Rev. 2007, 20, 255–272. [Google Scholar]
- Patra, M.K.; Begum, S.; Deka, B.C. Problems and prospects of traditional pig farming for tribal livelihood in Nagaland. Agric. Econ. Res. Rev. 2014, 14, 6–11. [Google Scholar]
- Singh, N.M.; Singh, S.B.; Singh, S.K. Small Scale Pig Farming in Manipur, India: A Studyon Socio-Personal and Socio-Economical Status of the SmallScale Tribal Farming Community. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1158–1168. [Google Scholar] [CrossRef]
- Kumaresan, A.; Bujarbaruah, K.M.; Pathak, K.A.; Das, A.; Bardoloi, R.K. Integrated resource driven pig production systems in a mountainous area of Northeast India: Production practices and pig performance. Trop. Anim. Health Prod. 2009, 41, 1187. [Google Scholar] [CrossRef] [PubMed]
- Shyam, J.; Tripathi, H.E.; Balaraju, B.L. Backyard Pig Rearing Practices among Tribals of Assam. Adv. Life Sci. 2016, 5, 2278–3849. [Google Scholar]
- Muthuramalingam, T.; Gnanaraj, P.; Sivakumar, T.; Murallidharan, R.; Murugan, M. Influence of Heat-treated swill feed on the performance of large white yorkshire pigs. Indian J. Vet. Anim. Sci. 2011, 7, 312–314. [Google Scholar]
- Ramesh, V.; Kumar, V.; Sivakumar, K.; Singh, D.; Thiruvenkadan, A.K. Effect of swill feeding on the reproductive performance of large white yorkshire pigs. Indian J. Field Vet. 2012, 8, 42–45. [Google Scholar]
- Mohakud, S.S.; Hazarika, R.A.; Sonowal, S.; Bora, D.P.; Talukdar, A.; Tamuly, S.; Lindahl, J.F. The extent and structure of pig rearing system in urban and peri-urban areas of Guwahati. Infect. Ecol. Epidemiol. 2020, 10, 1711576. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef]
- Manzano-Román, R.; Díaz-Martín, V.; de la Fuente, J.; Pérez-Sánchez, R. Soft ticks as pathogen vectors: Distribution, surveillance and control. Parasitology 2012, 7, 125–162. [Google Scholar]
- Hess, W.R.; Endris, R.G.; Lousa, A.; Caiado, J.M. Clearance of African swine fever virus from infected tick (Acari) colonies. J. Med. Entomol. 1989, 26, 314–317. [Google Scholar] [CrossRef]
- Plowright, W.; Perry, C.T.; Peirce, M.A. Transovarial infection with African swine fever virus in the argasid tick, Ornithodoros moubata porcinus, Walton. Res. Vet. Sci. 1970, 11, 582–584. [Google Scholar] [CrossRef]
- Boinas, F.S.; Wilson, A.J.; Hutchings, G.H.; Martins, C.; Dixon, L.J. The persistence of African swine fever virus in field-infected Ornithodoros erraticus during the ASF endemic period in Portugal. PLoS ONE 2011, 6, e20383. [Google Scholar] [CrossRef] [PubMed]
- Shyma, K.P.; Singh, V.; Gupta, J.P. Susceptibility Level of Ornithodoros spp (Acari: Argasidae) to Some Commercial Synthetic Pyrethroids in North Gujarat. Int. J. Livest. Res. 2019, 9, 62–67. [Google Scholar] [CrossRef]
- Kalra, S.L.; Rao, K.N.A. Observations on the epidemiology of relapsing fever in Kashmir. Indian J. Med. Res. 1951, 39, 313–321. [Google Scholar]
- Aher Atul, R.; Shah, H.; Rastogi, V.; Tukaram, P.K.; Choudhury, R.C. A case report of relapsing fever. Indian J. Pathol. Microbiol. 2008, 51, 292–293. [Google Scholar] [CrossRef]
- Veena, S.; Seema, V.; Babu, R. Borreliosis: Recurrent fever due to spirochetes. Ann. Trop. Med. Public Health 2013, 6, 482. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, V.; Shyma, K.P.; Parsani, H.R. Comparative efficacy of commercial preparation of deltamethrin and cypermethrin against Ornithodoros spp. of North Gujarat. J. Parasit. Dis. 2017, 41, 1139–1142. [Google Scholar] [CrossRef]
- Kumar, K.; Balakrishnan, N.; Sharma, A.K. Studies on the vertical distribution of ticks of domestic animals and their public health importance in Nilgiri Hills and adjoining areas of Tamil Nadu State (India). Int. J. Zool. 2014, 2014, 359812. [Google Scholar] [CrossRef]
- Netherton, C.L.; Connell, S.; Benfield, C.T.; Dixon, L.K. The genetics of life and death: Virus-host interactions underpinning resistance to African swine fever, a viral hemorrhagic disease. Front. Genet. 2019, 10, 402. [Google Scholar] [CrossRef]
- Calisher, C.; Fenner, F. Macroecology and Microecology of Viruses of Terrestrial Mammals. In Viral Ecology; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- De Jong, Y.A.; Cumming, D.; d’Huart, J.; Butynski, T. Phacochoerus africanus (errata version published in 2017). The IUCN Red List of Threatened Species 2016: E.T41768A109669842. 2016. Available online: https://www.iucnredlist.org/species/41768/109669842 (accessed on 14 September 2020).
- De Jong, Y.A.; Butynski, T.M.; d’Huart, J.P. Phacochoerus aethiopicus. The IUCN Red List of Threatened Species 2016: E.T41767A99376685. 2016. Available online: https://www.iucnredlist.org/species/41767/99376685 (accessed on 17 September 2020).
- Ravaomanana, J.; Jori, F.; Vial, L.; Pérez-Sánchez, R.; Blanco, E.; Michaud, V.; Roger, F. Assessment of interactions between African swine fever virus, bushpigs (Potamochoerus larvatus), Ornithodoros ticks and domestic pigs in north-western Madagascar. Transbound. Emerg. Dis. 2011, 58, 247–254. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on African swine fever. EFSA J. 2014, 12, 3628. [Google Scholar]
- FAO. ASF Virology. 2020. Available online: http://www.fao.org/ag/againfo/programmes/en/empres/ASF/Virology.html (accessed on 19 September 2020).
- He, H. Feral Swine Diseases Prevention and Control in China. International Workshop on Feral Swine Disease and Risk Management. 2014. Available online: http://www.cvmbs.colostate.edu/aphi/feralswine/PDFs/HongxuanHe%20Feral%20swine%20disease%20control%20in%20China.pdf (accessed on 25 September 2020).
- Naish, D. Fabulous Crested Indian Wild Pigs. 2015. Available online: https://blogs.scientificamerican.com/tetrapod-zoology/fabulous-crested-indian-wild-pigs/#:~:text=cristatus%20of%20India%2C%20Sri%20Lanka,it%20a%20sometimes%20prominent%20beard (accessed on 25 September 2020).
- Probst, C.; Globig, A.; Knoll, B.; Conraths, F.J.; Depner, K. Behaviour of free ranging wild boar towards their dead fellows: Potential implications for the transmission of African swine fever. R. Soc. Open Sci. 2017, 4, 170054. [Google Scholar] [CrossRef] [PubMed]
- Allwin, B.; Swaminathan, R.; Mohanraj, A.; Suhas, G.N.; Vedaminckam, S.; Gopal, S.; Kumar, M. The Wild Pig (Sus scrofa) Behavior—A Retrospective Study. JoVST 2016, 7, 333. [Google Scholar]
- Chauhan, N.P.S.; Barwal, K.S.; Kumar, D. Human-wild pig conflict in selected states in India and mitigation strategies. Acta Silv. Lignaria Hung. 2009, 5, 189–197. [Google Scholar]
- Meijaard, E.; Narayan, G.; Deka, P. Porcula salvania. The IUCN Red List of Threatened Species 2019: E.T21172A44139115. 2019. Available online: https://www.iucnredlist.org/species/21172/44139115 (accessed on 27 September 2020).
- Barman, N.N.; Bora, D.P.; Tiwari, A.K.; Kataria, R.S.; Desai, G.S.; Deka, P.J. Classical swine fever in the pygmy hog. Rev. Sci. Tech. 2012, 31, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Labonté, R.; Mohindra, K.S.; Lencucha, R. Framing international trade and chronic disease. Glob. Health 2011, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Evans, I. African Swine Fever Poses a Serious Threat to Global Food Security Experts say. Elsevier Connect. 2019. Available online: https://www.elsevier.com/connect/african-swine-fever-poses-aserious-threat-to-global-food-security-experts-say (accessed on 29 September 2020).
- Huang, Y. The Biggest Animal Disease Outbreak in China. 2020. Available online: https://www.cfr.org/blog/biggest-animal-disease-outbreak-china (accessed on 29 September 2020).
- USDA FAS. Global Agricultural Information Network. 2016. Available online: https://www.fas.usda.gov/data/india-pork-2016 (accessed on 29 September 2020).
- Álvarez, J.; Bicout, D.; Boklund, A.; Bøtner, A.; Depner, K.; More, S.J.; Roberts, H.; Stahl, K.; Thulke, H.; Viltrop, A.; et al. Research gap analysis on African swine fever. EFSA J. 2019, 17, e05811. [Google Scholar]
- Gaudreault, N.N.; Richt, J.A. Subunit vaccine approaches for African swine fever virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef]
- Rock, D.L. Challenges for African swine fever vaccine development “… perhaps the end of the beginning”. Vet. Microbiol. 2017, 206, 52–58. [Google Scholar] [CrossRef]
- O’Donnell, V.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Carlson, J.; Sanford, B.J.; Alfano, M.; Kramer, E.J.; Lu, Z.; Arzt, J.; et al. African swine fever virus Georgia 2007 with a deletion of virulence-associated gene 9GL (B119L), when administered at low doses, leads to virus attenuation in swine and induces an effective protection against homologous challenge. J. Virol. 2015, 89, 8556–8566. [Google Scholar] [CrossRef]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, P.L.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71ΔCD2: A new recombinant live attenuated African swine fever virus with cross-protective capabilities. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.L.; Goatley, L.C.; Jabbar, T.; Sanchez-Cordon, P.J.; Netherton, C.L.; Chapman, D.A.; Dixon, L.K. Deletion of the African swine fever virus gene DP148R does not reduce virus replication in culture but reduces virus virulence in pigs and induces high levels of protection against challenge. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- FAO. Food and Agricultural Organization. Manual on Livestock Disease Surveillance and Information Systems. Animal Health Manual No. 8. 1999. Available online: http://www.fao.org/3/Y0510E/Y0510E06.htm (accessed on 1 October 2020).
- FAO; Food and Agriculture Organization of the United Nations; World Organisation for Animal Health; World Bank. Good Practices for Biosecurity in the Pig Sector—Issues and Options in Developing and Transition Countries; FAO Animal Production and Health; Paper No. 169; FAO: Roma, Italy, 2010. [Google Scholar]
- Adkin, A.; Coburn, H.; England, T.; Hall, S.; Hartnett, E.; Marooney, C.; Wooldridge, M.; Watson, E.; Cooper, J.; Cox, T.; et al. Risk Assessment for the Illegal Import of Contaminated Meat and Meat Products into Great Britain and the Subsequent Exposure of GB Livestock (IIRA): Foot and Mouth Disease (FMD), Classical Swine Fever (CSF), African Swine Fever (ASF), Swine Vesicular Disease (SVD); Veterinary Laboratories Agency: New Haw, UK, 2004.
- Gervasi, V.; Marcon, A.; Bellini, S.; Guberti, V. Evaluation of the Efficiency of Active and Passive Surveillance in the Detection of African Swine Fever in Wild Boar. Vet. Sci. 2020, 7, 5. [Google Scholar] [CrossRef]
- Goswami, P.; Borkataki, S. Pen Side Diagnosis of Infectious disease—A Current Status. IJARR 2017, 2, 44–55. [Google Scholar]
- Cappai, S.; Sanna, G.; Loi, F.; Coccollone, A.; Marrocu, E.; Oggiano, A.; Brundu, D.; Rolesu, S.; Bandino, E. African swine fever detection on field with antigen rapid kit test. J. Anim. Sci. Res. 2018, 2. [Google Scholar] [CrossRef]
- OIE. Chapter 7.6. Killing of Animals for Disease Control Purposes. Terresterial Animal Health Code. 2019. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_aw_killing.pdf (accessed on 2 October 2020).
- Davies, K.; Goatley, L.C.; Guinat, C.; Netherton, C.L.; Gubbins, S.; Dixon, L.K.; Reis, A.L. Survival of African swine fever virus in excretions from pigs experimentally infected with the Georgia 2007/1 isolate. Transbound. Emerg. Dis. 2017, 64, 425–431. [Google Scholar] [CrossRef]
- Plowright, W.; Parker, J. The stability of African swine fever virus with particular reference to heat and pH inactivation. Arch. Gesamte Virusforsch. 1967, 21, 383–402. [Google Scholar] [CrossRef]
- Mebus, C.; Arias, M.; Pineda, J.M.; Tapiador, J.; House, C.; Sanchez-Vizcaino, J.M. Survival of several porcine viruses in different Spanish dry-cured meat products. Food Chem. 1997, 59, 555–559. [Google Scholar] [CrossRef]
- DADF. National Action Plan for Control, Containment and Eradication of African Swine Fever; Ministry of Fisheries, Animal Husbandry and Dairying Department of Animal Husbandry and Dairying Government of India: New Delhi, India, 2020. Available online: http://dadf.gov.in/sites/default/filess/ASF_NAP_Booklet.pdf (accessed on 7 October 2020).
- Estrada-Peña, A.; Ayllón, N.; De La Fuente, J. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bora, M.; Bora, D.P.; Manu, M.; Barman, N.N.; Dutta, L.J.; Kumar, P.P.; Poovathikkal, S.; Suresh, K.P.; Nimmanapalli, R. Assessment of Risk Factors of African Swine Fever in India: Perspectives on Future Outbreaks and Control Strategies. Pathogens 2020, 9, 1044. https://doi.org/10.3390/pathogens9121044
Bora M, Bora DP, Manu M, Barman NN, Dutta LJ, Kumar PP, Poovathikkal S, Suresh KP, Nimmanapalli R. Assessment of Risk Factors of African Swine Fever in India: Perspectives on Future Outbreaks and Control Strategies. Pathogens. 2020; 9(12):1044. https://doi.org/10.3390/pathogens9121044
Chicago/Turabian StyleBora, Mousumi, Durlav Prasad Bora, Mohan Manu, Nagendra Nath Barman, Lakshya Jyoti Dutta, Pesingi Pavan Kumar, Suvaneeth Poovathikkal, Kuralayanapalya Puttahonnappa Suresh, and Ramadevi Nimmanapalli. 2020. "Assessment of Risk Factors of African Swine Fever in India: Perspectives on Future Outbreaks and Control Strategies" Pathogens 9, no. 12: 1044. https://doi.org/10.3390/pathogens9121044
APA StyleBora, M., Bora, D. P., Manu, M., Barman, N. N., Dutta, L. J., Kumar, P. P., Poovathikkal, S., Suresh, K. P., & Nimmanapalli, R. (2020). Assessment of Risk Factors of African Swine Fever in India: Perspectives on Future Outbreaks and Control Strategies. Pathogens, 9(12), 1044. https://doi.org/10.3390/pathogens9121044




