Imidocarb Dipropionate Lacks Efficacy against Theileria haneyi and Fails to Consistently Clear Theileria equi in Horses Co-Infected with T. haneyi
Abstract
1. Introduction
2. Results
2.1. T. haneyi Experimental Infection
2.2. Imidocarb Diproprionate Treatment
2.3. Imidocarb Dipropionate Fails to Clear T. haneyi Infection
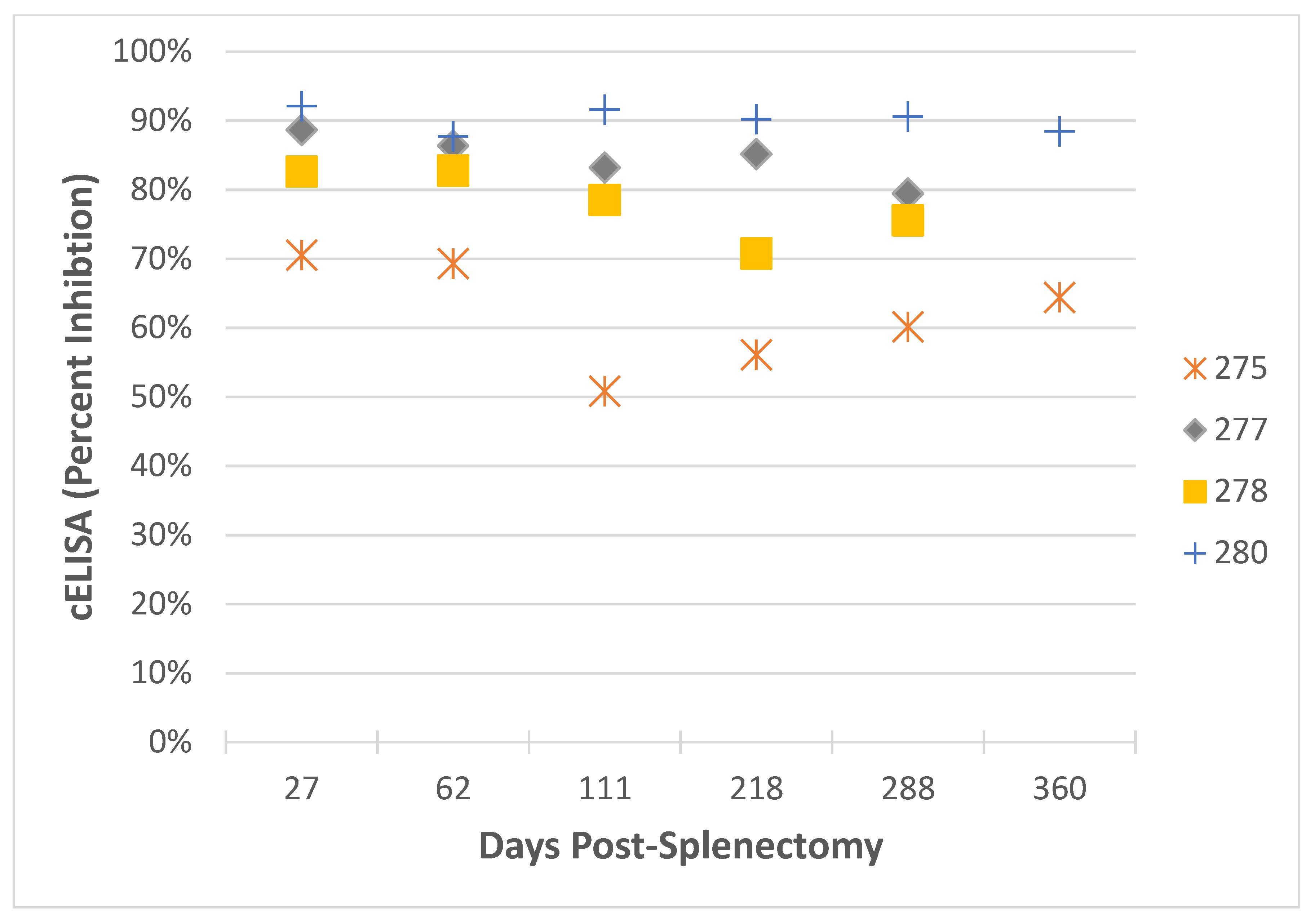
2.4. Imidocarb Dipropionate Fails to Clear T. equi in a Subset of Horses Co-Infected with T. equi and T. haneyi
3. Discussion
4. Materials and Methods
4.1. Horses
4.2. Blood Collection
4.3. Imidocarb Dipropionate Treatment
4.4. T. haneyi Nested PCR (Genomic DNA)
4.5. T. equi Nested PCR (Genomic DNA)
4.6. T. equi cELISA
4.7. Splenectomy of ID-Treated, Co-Infected Horses—T. equi Clearance Confirmation
4.8. Monitoring for T. equi and T. haneyi Recrudescence
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wise, L.N.; Pelzel-McCluskey, A.M.; Mealey, R.H.; Knowles, D.P. Equine piroplasmosis. Vet. Clin. North Am. Equine Pract. 2014, 30, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Ueti, M.W. Vector ecology of equine piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Camino, E.; Pozo, P.; Dorrego, A.; Carvajal, K.A.; Buendia, A.; Gonzalez, S.; de Juan, L.; Dominguez, L.; Cruz-Lopez, F. Importance of equine piroplasmosis antibody presence in Spanish horses prior to export. Ticks Tick Borne Dis. 2020, 11, 101329. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.N.; Kappmeyer, L.S.; Mealey, R.H.; Knowles, D.P. Review of equine piroplasmosis. J. Vet. Intern. Med. 2013, 27, 1334–1346. [Google Scholar] [CrossRef]
- Guimarães, A.M.; Lima, J.D.; Tafuri, W.L.; Ribeiro, M.F.B.; Sciavicco, C.J.S.; Botelho, A.C.C. Clinical and histopathological aspects of splenectomized foals infected by Babesia equi. J. Equine Vet. Sci. 1997, 17, 211–216. [Google Scholar] [CrossRef]
- Kuttler, K.L.; Gipson, C.A.; Goff, W.L.; Johnson, L.W. Experimental Babesia equi infection in mature horses. Am. J. Vet. Res. 1986, 47, 1668–1670. [Google Scholar]
- Singh, B.; Banerjee, D.P.; Gautam, O.P.; Gupta, R.K.P. Clinicopathological changes in splenectomised donkeys infected with Babesia equi. Indian J. Parasitol. 1980, 4, 77–80. [Google Scholar]
- Ueti, M.W.; Mealey, R.H.; Kappmeyer, L.S.; White, S.N.; Kumpula-McWhirter, N.; Pelzel, A.M.; Grause, J.F.; Bunn, T.O.; Schwartz, A.; Traub-Dargatz, J.L.; et al. Re-emergence of the apicomplexan Theileria equi in the united states: Elimination of persistent infection and transmission risk. PLoS ONE 2012, 7, e44713. [Google Scholar] [CrossRef]
- Ambawat, H.K.; Malhotra, D.V.; Kumar, S.; Dhar, S. Erythrocyte associated haemato-biochemical changes in Babesia equi infection experimentally produced in donkeys. Vet. Parasitol. 1999, 85, 319–324. [Google Scholar] [CrossRef]
- Cunha, C.W.; McGuire, T.C.; Kappmeyer, L.S.; Hines, S.A.; Lopez, A.M.; Dellagostin, O.A.; Knowles, D.P. Development of specific immunoglobulin Ga (IgGa) and IgGb antibodies correlates with control of parasitemia in Babesia equi infection. Clin. Vaccine Immunol. 2006, 13, 297–300. [Google Scholar] [CrossRef]
- Lewis, M.J.; Wagner, B.; Woof, J.M. The different effector function capabilities of the seven equine IgG subclasses have implications for vaccine strategies. Mol. Immunol. 2008, 45, 818–827. [Google Scholar] [CrossRef]
- Knowles, D.P.; Kappmeyer, L.S.; Stiller, D.; Hennager, S.G.; Perryman, L.E. Antibody to a recombinant merozoite protein epitope identifies horses infected with Babesia equi. J. Clin. Microbiol. 1992, 30, 3122–3126. [Google Scholar] [CrossRef]
- Bishop, R.P.; Kappmeyer, L.S.; Onzere, C.K.; Odongo, D.O.; Githaka, N.; Sears, K.P.; Knowles, D.P.; Fry, L.M. Equid infective Theileria cluster in distinct 18S rRNA gene clades comprising multiple taxa with unusually broad mammalian host ranges. Parasites Vectors 2020, 13, 261. [Google Scholar] [CrossRef]
- Knowles, D.P.; Kappmeyer, L.S.; Haney, D.; Herndon, D.R.; Fry, L.M.; Munro, J.B.; Sears, K.; Ueti, M.W.; Wise, L.N.; Silva, M.; et al. Discovery of a novel species, Theileria haneyi n. sp., infective to equids, highlights exceptional genomic diversity within the genus Theileria: Implications for apicomplexan parasite surveillance. Int. J. Parasitol. 2018, 48, 679–690. [Google Scholar] [CrossRef]
- Sears, K.P.; Kappmeyer, L.S.; Wise, L.N.; Silva, M.; Ueti, M.W.; White, S.; Reif, K.E.; Knowles, D.P. Infection dynamics of Theileria equi and Theileria haneyi, a newly discovered apicomplexan of the horse. Vet. Parasitol. 2019, 271, 68–75. [Google Scholar] [CrossRef]
- Coultous, R.M.; McDonald, M.; Raftery, A.G.; Shiels, B.R.; Sutton, D.G.M.; Weir, W. Analysis of Theileria equi diversity in The Gambia using a novel genotyping method. Transbound. Emerg. Dis. 2019, 67, 1213–1221. [Google Scholar] [CrossRef]
- Bhoora, R.V.; Collins, N.E.; Schnittger, L.; Troskie, C.; Marumo, R.; Labuschagne, K.; Smith, R.M.; Dalton, D.L.; Mbizeni, S. Molecular genotyping and epidemiology of equine piroplasmids in South Africa. Ticks Tick Borne Dis. 2019, 11, 101358. [Google Scholar] [CrossRef]
- Mshelia, P.W.; Kappmeyer, L.; Johnson, W.C.; Kudi, C.A.; Oluyinka, O.O.; Balogun, E.O.; Richard, E.E.; Onoja, E.; Sears, K.P.; Ueti, M.W. Molecular detection of Theileria species and Babesia caballi from horses in Nigeria. Parasitol. Res. 2020, 119, 2955–2963. [Google Scholar] [CrossRef]
- Frerichs, W.M.; Allen, P.C.; Holbrook, A.A. Equine piroplasmosis (Babesia equi): Therapeutic trials of imidocarb dihydrochloride in horses and donkeys. Vet. Rec. 1973, 93, 73–75. [Google Scholar] [CrossRef]
- Grause, J.F.; Ueti, M.W.; Nelson, J.T.; Knowles, D.P.; Kappmeyer, L.S.; Bunn, T.O. Efficacy of imidocarb dipropionate in eliminating Theileria equi from experimentally infected horses. Vet. J. 2013, 196, 541–546. [Google Scholar] [CrossRef]
- Meyer, C.; Guthrie, A.J.; Stevens, K.B. Clinical and clinicopathological changes in 6 healthy ponies following intramuscular administration of multiple doses of imidocarb dipropionate. J. S. Afr. Vet. Assoc. 2005, 76, 26–32. [Google Scholar] [CrossRef]
- Adams, L.G. Clinicopathological aspects of imidocarb dipropionate toxicity in horses. Res. Vet. Sci. 1981, 31, 54–61. [Google Scholar] [CrossRef]
- Kutscha, J.; Sutton, D.G.; Preston, T.; Guthrie, A.J. Equine piroplasmosis treatment protocols: Specific effect on orocaecal transit time as measured by the lactose 13c-ureide breath test. Equine Vet. J. 2012, 44, 62–67. [Google Scholar] [CrossRef]
- Wise, L.N.; Kappmeyer, L.S.; Silva, M.G.; White, S.N.; Grause, J.F.; Knowles, D.P. Verification of post-chemotherapeutic clearance of Theileria equi through concordance of nested PCR and immunoblot. Ticks Tick Borne Dis. 2018, 9, 135–140. [Google Scholar] [CrossRef]
- Hines, S.A.; Ramsay, J.D.; Kappmeyer, L.S.; Lau, A.O.; Ojo, K.K.; Van Voorhis, W.C.; Knowles, D.P.; Mealey, R.H. Theileria equi isolates vary in susceptibility to imidocarb dipropionate but demonstrate uniform in vitro susceptibility to a bumped kinase inhibitor. Parasites Vectors 2015, 8, 33. [Google Scholar] [CrossRef][Green Version]
- Pasolini, M.P.; Pagano, T.B.; Costagliola, A.; Biase, D.; Lamagna, B.; Auletta, L.; Fatone, G.; Greco, M.; Coluccia, P.; Vincenzo, V.; et al. Inflammatory myopathy in horses with chronic piroplasmosis. Vet. Pathol. 2018, 55, 133–143. [Google Scholar] [CrossRef]
- Butler, C.M.; Werners, A.H.; de Haseth, O.B.; van Maanen, C. Strain variations might determine the effectiveness of multiple high doses of imidocarb dipropionate treatment in T. equi carrier horses. In Proceedings of the 6th Congress of the European College of Equine Internal Medicine, Le Touquet, France, 7–9 February 2013; pp. 696–710. [Google Scholar]
- De Waal, D.T.; Van Heerden, J.; Van den Berg, S.S.; Stegmann, G.F.; Potgieter, F.T. Isolation of pure Babesia equi and Babesia caballi organisms in splenectomized horses from endemic areas in South Africa. Onderstepoort J. Vet. Res. 1988, 55, 33–35. [Google Scholar]
- Bushman, M.; Morton, L.; Duah, N.; Quashie, N.; Abuaku, B.; Koram, K.A.; Dimbu, P.R.; Plucinski, M.; Gutman, J.; Lyaruu, P.; et al. Within-host competition and drug resistance in the human malaria parasite plasmodium falciparum. Proc. Biol. Sci. 2016, 283, 20153038. [Google Scholar] [CrossRef]
- Hansen, J.; Day, T. Coinfection and the evolution of drug resistance. J. Evol. Biol. 2014, 27, 2595–2604. [Google Scholar] [CrossRef]
- Karvonen, A.; Jokela, J.; Laine, A.L. Importance of sequence and timing in parasite coinfections. Trends Parasitol. 2019, 35, 109–118. [Google Scholar] [CrossRef]
- Gimenez, F.; Hines, S.A.; Evanoff, R.; Ojo, K.K.; Van Voorhis, W.C.; Maly, D.J.; Vidadala, R.S.R.; Mealey, R.H. In vitro growth inhibition of Theileria equi by bumped kinase inhibitors. Vet. Parasitol. 2018, 251, 90–94. [Google Scholar] [CrossRef]
- Silva, M.G.; Knowles, D.P.; Antunes, S.; Domingos, A.; Esteves, M.A.; Suarez, C.E. Inhibition of the in vitro growth of Babesia bigemina, Babesia caballi and Theileria equi parasites by trifluralin analogues. Ticks Tick Borne Dis. 2017, 8, 593–597. [Google Scholar] [CrossRef]
- Silva, M.G.; Villarino, N.F.; Knowles, D.P.; Suarez, C.E. Assessment of Draxxin® (tulathromycin) as an inhibitor of in vitro growth of Babesia bovis, Babesia bigemina and Theileria equi. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 265–270. [Google Scholar] [CrossRef]
- Bowden, G.D.; Reis, P.M.; Rogers, M.B.; Bone Relat, R.M.; Brayton, K.A.; Wilson, S.K.; Di Genova, B.M.; Knoll, L.J.; Nepveux, V.F.; Tai, A.K.; et al. A conserved coccidian gene is involved in Toxoplasma sensitivity to the anti-apicomplexan compound, tartrolon E. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 1–7. [Google Scholar] [CrossRef]
- O’Connor, R.M.; Nepveux, V.F.; Abenoja, J.; Bowden, G.; Reis, P.; Beaushaw, J.; Bone Relat, R.M.; Driskell, I.; Gimenez, F.; Riggs, M.W.; et al. A symbiotic bacterium of shipworms produces a compound with broad spectrum anti-apicomplexan activity. PLoS Pathog. 2020, 16, e1008600. [Google Scholar] [CrossRef]
- Bertone, J.G.H.; Sislak, M.; Agnic, H.; Heidmiller, M.; Stanley, S.; Wickler, S. Pharmacokinetics, Pharmacodynamics, and Safety of N-Butylscopolammonium Bromide Administered Intramuscularly Versus Intravenously. In Proceedings of the American Association of Equine Practitioners, Baltimore, MD, USA, 4–8 December 2010; pp. 279–280. [Google Scholar]
- Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Kappmeyer, L.S.; Knowles, D.P. Persistently infected horses are reservoirs for intrastadial tick-borne transmission of the apicomplexan parasite Babesia equi. Infect. Immun. 2008, 76, 3525–3529. [Google Scholar] [CrossRef]
- Roberts, M.C.; Groenendyk, S. Splenectomy in the horse. Aust. Vet. J. 1978, 54, 196–197. [Google Scholar] [CrossRef]

| 1st Course of Treatment | 2nd Course of Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Horse Number | Group | CK | AST | GGT | Injection Site Swelling | CK | AST | GGT | Injection Site Swelling |
| 270 | 1 | ↑ | ↑ | N | ↑ | ↑ | N | ||
| 364 | 1 | ↑ | ↑ | N | ↑ | ↑ | ↑ | N | |
| 776 | 1 | ↑ | ↑ | ↑ | Y | N | |||
| 777 | 1 | ↑ | ↑ | Y | N | ||||
| 784 | 1 | ↑ | ↑ | Y | N | ||||
| 273 | 2 | ↑ | Y | ↑ | Y | ||||
| 280 | 2 | ↑ | N | ↑ | N | ||||
| 283 | 2 | ↑ | ↑ | N | ↑ | N | |||
| 275 | 3 | ↑ | ↑ | ↑ | Y | ↑ | ↑ | Y | |
| 277 | 3 | ↑ | ↑ | ↑ | Y | ↑ | ↑ | ↑ | Y |
| 278 | 3 | ↑ | ↑ | ↑ | N | ↑ | ↑ | ↑ | Y |
| T. haneyi nPCR | |||||||
| Horse No. | Group | Pre-Treatment 1 | Post-Treatment 1 a | Pre-Treatment 2 | Post-Treatment 2 a | Pre-Surgery | Post-Surgery |
| 270 | 1 | + | + | + | + | N/A | N/A |
| 364 | 1 | + | + | + | + | N/A | N/A |
| 776 | 1 | + | + | + | + | N/A | N/A |
| 777 | 1 | + | + | + | + | N/A | N/A |
| 784 | 1 | + | + | + | + | N/A | N/A |
| 273 | 2 | + | + | + | + | + | + |
| 280 | 2 | + | + | + | + | + | + |
| 283 | 2 | + | + | + | + | + | + |
| 275 | 3 | + | + | + | + | + | + |
| 277 | 3 | + | + | + | + | + | + |
| 278 | 3 | + | + | + | + | + | + |
| T. equi nPCR | |||||||
| Horse No. | Group | Pre-Treatment 1 | Post-Treatment 1 a | Pre-Treatment 2 | Post-Treatment 2 a | Pre-Surgery | Post-Surgery |
| 273 | 2 | + | + | − | − | +/− | + |
| 280 | 2 | + | − | − | − | − | − |
| 283 | 2 | + | + | + | + | +/− | + |
| 275 | 3 | + | − | − | − | − | − |
| 277 | 3 | + | − | − | − | − | − |
| 278 | 3 | + | − | − | − | − | − |
| Horse Number | Group | Time to Peak PPE (Days) | Peak PPE (%) | HCT Nadir (%) | Outcome | T. equi nPCR |
|---|---|---|---|---|---|---|
| 280 | 2 | 17 | 7.69 | 17.8 | Survived | Negative |
| 273 | 2 | 9 | 25.1 | 19.5 | Euthanized | Positive |
| 283 | 2 | 10 | 40.46 | 28.9 | Euthanized | Positive |
| 275 | 3 | 14 | 7.5 | 23.4 | Survived | Negative |
| 277 | 3 | 17 | 10.58 | 12.6 | Survived | Negative |
| 278 | 3 | 19 | 14.4 | 10 | Survived | Negative |
| Horse ID | Group Number | Inoculation |
|---|---|---|
| 270 | 1 | T. haneyi |
| 364 | T. haneyi | |
| 776 | T. haneyi | |
| 777 | T. haneyi | |
| 784 | T. haneyi | |
| 273 | 2 | T. haneyi/T. equi |
| 280 | T. haneyi/T. equi | |
| 283 | T. haneyi/T. equi | |
| 275 | 3 | T. equi/T. haneyi |
| 277 | T. equi/T. haneyi | |
| 278 | T. equi/T. haneyi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sears, K.; Knowles, D.; Dinkel, K.; Mshelia, P.W.; Onzere, C.; Silva, M.; Fry, L. Imidocarb Dipropionate Lacks Efficacy against Theileria haneyi and Fails to Consistently Clear Theileria equi in Horses Co-Infected with T. haneyi. Pathogens 2020, 9, 1035. https://doi.org/10.3390/pathogens9121035
Sears K, Knowles D, Dinkel K, Mshelia PW, Onzere C, Silva M, Fry L. Imidocarb Dipropionate Lacks Efficacy against Theileria haneyi and Fails to Consistently Clear Theileria equi in Horses Co-Infected with T. haneyi. Pathogens. 2020; 9(12):1035. https://doi.org/10.3390/pathogens9121035
Chicago/Turabian StyleSears, Kelly, Donald Knowles, Kelcey Dinkel, Philip W. Mshelia, Cynthia Onzere, Marta Silva, and Lindsay Fry. 2020. "Imidocarb Dipropionate Lacks Efficacy against Theileria haneyi and Fails to Consistently Clear Theileria equi in Horses Co-Infected with T. haneyi" Pathogens 9, no. 12: 1035. https://doi.org/10.3390/pathogens9121035
APA StyleSears, K., Knowles, D., Dinkel, K., Mshelia, P. W., Onzere, C., Silva, M., & Fry, L. (2020). Imidocarb Dipropionate Lacks Efficacy against Theileria haneyi and Fails to Consistently Clear Theileria equi in Horses Co-Infected with T. haneyi. Pathogens, 9(12), 1035. https://doi.org/10.3390/pathogens9121035




