First Report of a Severe Outbreak of Aujeszky’s Disease in Cattle in Sicily (Italy)
Abstract
1. Introduction
2. Results
2.1. Description of the Outbreak
2.2. Clinical Examination
2.3. Serological Data
2.4. Post-Mortem Investigations
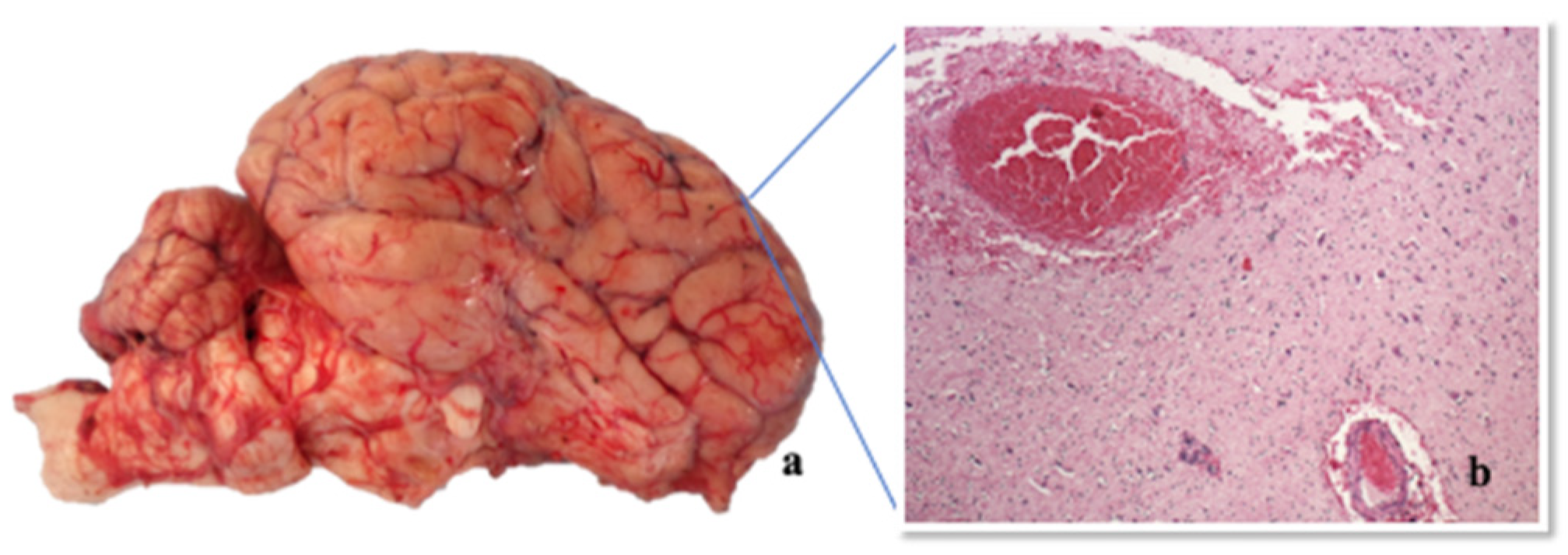
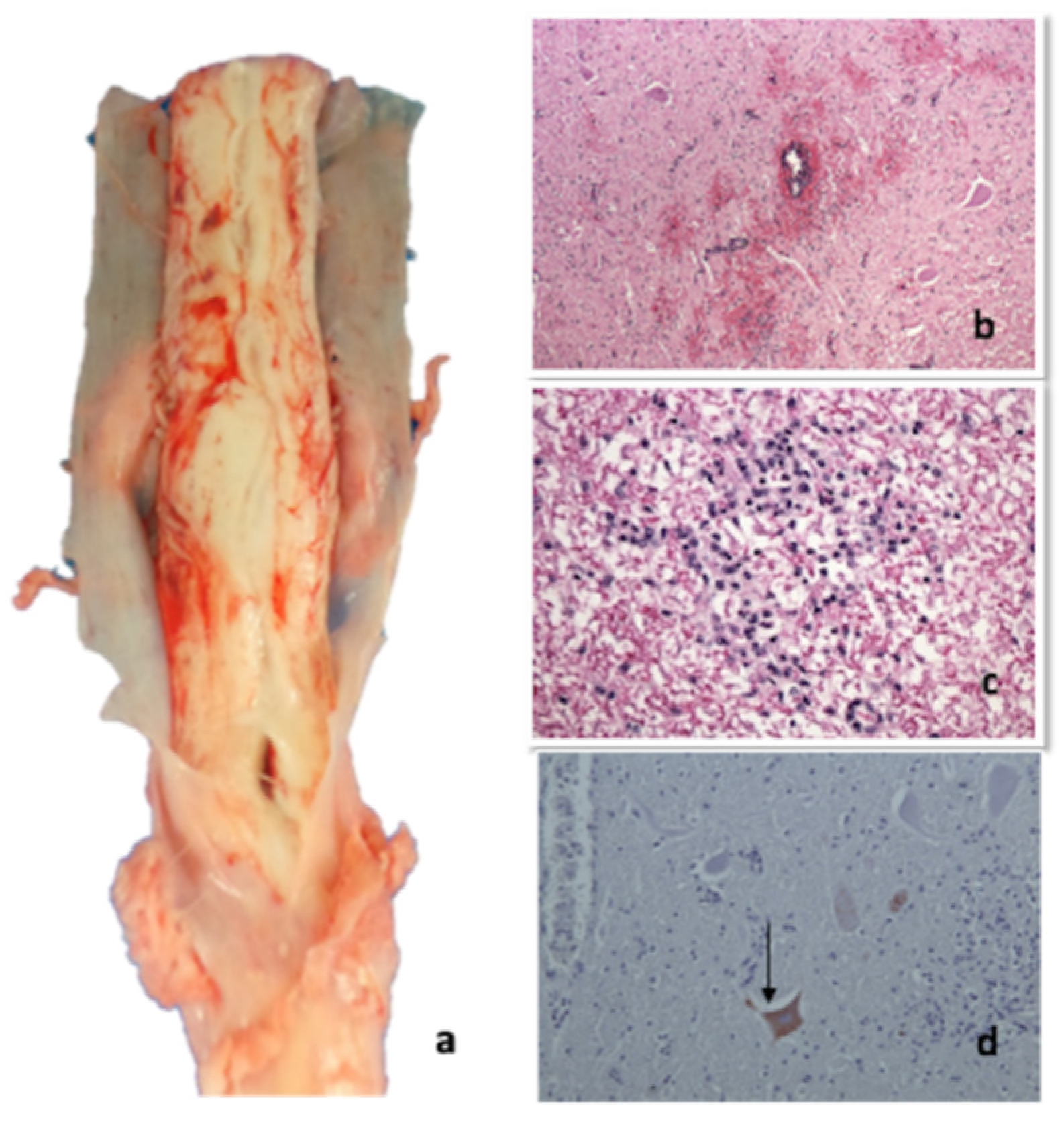
2.4.1. Necropsy
2.4.2. Histopathological Investigations
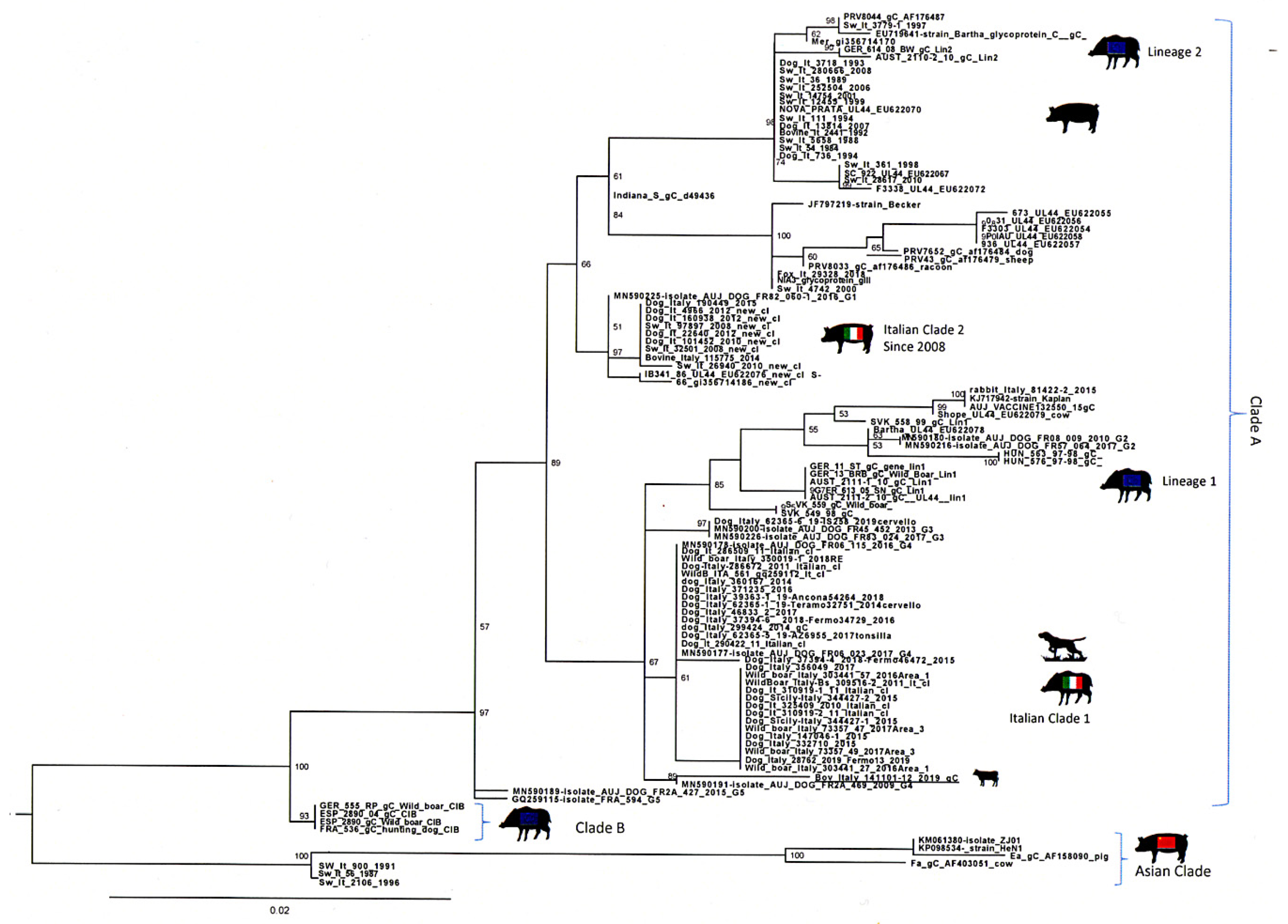
2.5. Microbiological and Molecular Data
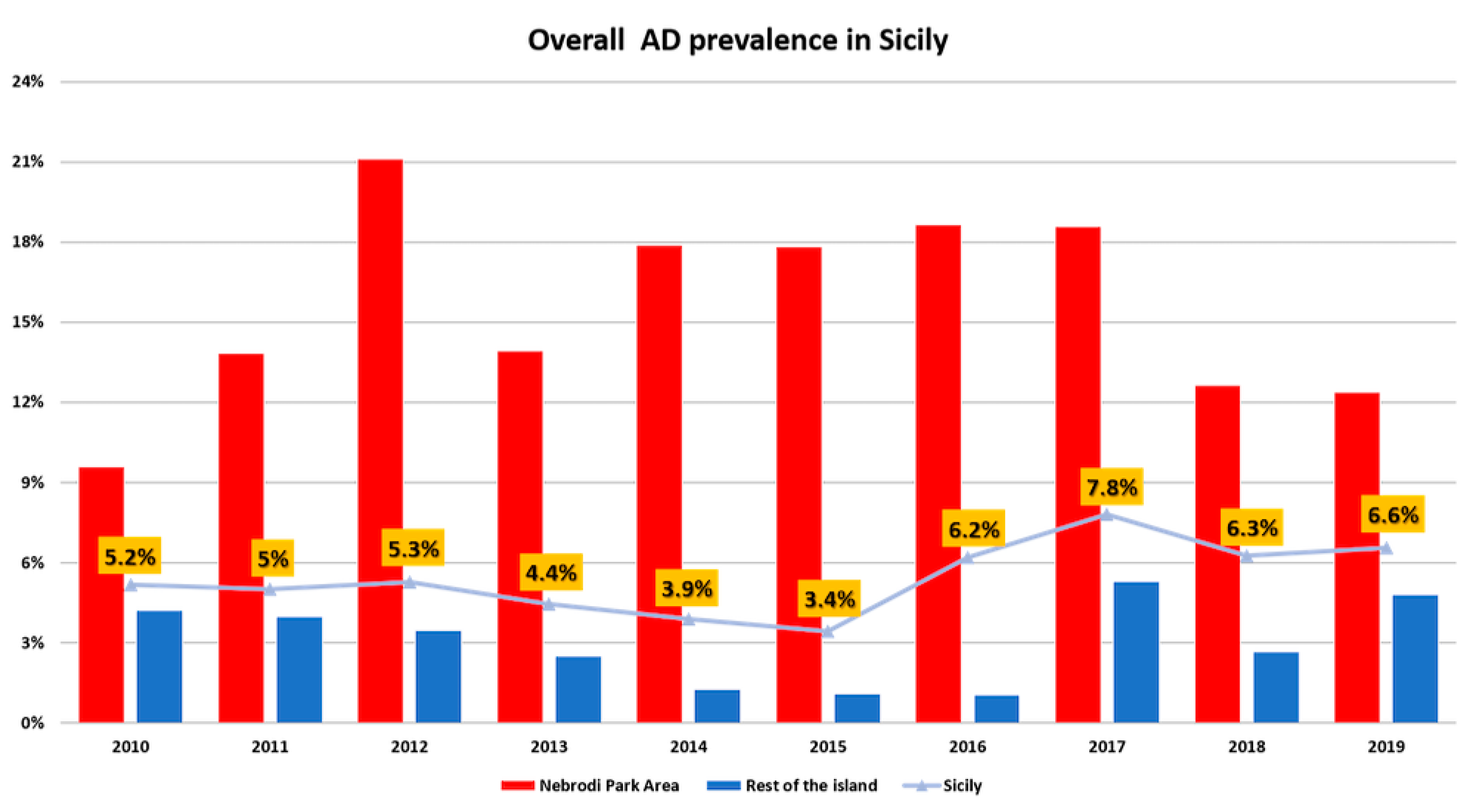
2.6. Retrospective Analysis on the Prevalence of Aujeszky Disease in Pigs in Sicily
3. Discussion
4. Materials and Methods
4.1. Anamnesis, Clinical Examination and Sampling
4.2. Serological Analysis
4.3. Post-Mortem Investigations
4.3.1. Necropsy
4.3.2. Histopathological and Immunohistochemical Investigations
4.4. Molecular and Virologic Investigations
4.5. Retrospective Analysis on the Prevalence of Aujeszky Disease in Pigs in Sicily
4.6. Ethic Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Lari, A.; Lorenzi, D.; Nigrelli, D.; Brocchi, E.; Faccini, S.; Poli, A. Pseudorabies virus in European wild boar from central Italy. J. Wildl. Dis. 2006, 42, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Dondo, A.; Cerutti, F.; Masoero, L.; Rosamilia, A.; Zoppi, S.; D’Errico, V.; Grattarola, C.; Acutis, P.L.; Peletto, S. Aujeszky’s disease in red fox (Vulpes vulpes): Phylogenetic analysis unravels an unexpected epidemiologic link. J. Wildl. Dis. 2014, 50, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Sozzi, E.; Grilli, G.; Gibelli, L.R.; Gelmetti, D.; Lelli, D.; Chiari, M.; Prati, P.; Alborali, G.L.; Boniotti, M.B.; et al. Detection and molecular analysis of Pseudorabies virus strains isolated from dogs and a wild boar in Italy. Vet. Microbiol. 2015, 177, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.K.; Ruiz-Fons, F.; Ryser-Degiorgis, M.P. A picture of trends in Aujeszky’s disease virus exposure in wild boar in the Swiss and European contexts. BMC Vet. Res. 2015, 11, 277. [Google Scholar] [CrossRef]
- Verpoest, S.; Cay, A.B.; Favoreel, H.; De Regge, N. Pseudorabies virus isolates from domestic pigs and wild boars show no apparent in vitro differences in replication kinetics and sensitivity to interferon-induced antiviral status. J. Gen. Virol. 2016, 97, 473–479. [Google Scholar] [CrossRef][Green Version]
- Mravak, S.; Bienzle, U.; Feldmeier, H.; Hampl, H.; Habermehl, K.O. Pseudorabies in man. Lancet 1987, 1, 501–502. [Google Scholar] [CrossRef]
- Anusz, Z.; Szweda, W.; PopkoJ, T.E. Is Aujeszky’s disease a zoonosis? Prz. Epidemiol. 1992, 46, 181–186. [Google Scholar]
- Guan, H.; Shen, A.; Lv, X.; Yang, X.; Ren, H.; Zhao, Y.; Zhang, Y.; Gong, Y.; Ni, P.; Wu, H.; et al. Detection of virus in CSF from the cases with meningoencephalitis by next-generation sequencing. J. Neurovirol. 2016, 22, 240–245. [Google Scholar] [CrossRef]
- Ai, J.W.; Weng, S.S.; Cheng, Q.; Cui, P.; Li, Y.J.; Wu, H.L.; Zhu, Y.M.; Xu, B.; Zhang, W.H. Human Endophthalmitis Caused by Pseudorabies Virus Infection, China, 2017. Emerg. Infect. Dis. 2018, 24, 1087–1090. [Google Scholar] [CrossRef]
- Wang, Y.; Nian, H.; Li, Z.; Wang, W.; Wang, X.; Cui, Y. Human encephalitis complicated with bilateral acute retinal necrosis associated with pseudorabies virus infection: A case report. Int. J. Infect. Dis. 2019, 89, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Han, H.; Wang, H.; Cui, Y.; Liu, H.; Ding, S. A Case of Human Viral Encephalitis Caused by Pseudorabies Virus Infection in China. Front. Neurol. 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tao, X.; Fei, M.; Chen, J.; Guo, W.; Li, P.; Wang, J. Human encephalitis caused by pseudorabies virus infection: A case report. J. Neurovirol. 2020, 26, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Xie, C.; Ding, S.; Yang, H.; Guo, S.; Li, J.; Qin, L.; Ban, F.; Wang, D.; et al. A novel human acute encephalitis caused by pseudorabies virus variant strain. Clin. Infect. Dis. 2020, ciaa987. [Google Scholar] [CrossRef]
- Aujeszky, A. Uber eine neue Infektion krankheit bei Haustieren. Zbl. Bakt. Abt. Orig. 1902, 32, 353–357. [Google Scholar]
- Shope, R.E. An experimental study of “mad itch” with especial reference to its relationship to pseudorabies. J. Exp. Med. 1931, 54, 233–248. [Google Scholar] [CrossRef]
- Hagemoser, W.A.; Hill, H.T.; Moss, E.W. Nonfatal pseudorabies in cattle. J. Am. Vet. Med Assoc. 1978, 173, 205–206. [Google Scholar]
- Beasley, V.R.; Crandell, R.A.; Buck, W.B.; Ely, R.W.; Thilsted, J.P. A clinical episode demonstrating variable characteristics of pseudorabies infection in cattle. Vet. Res. Commun. 1980, 4, 125–129. [Google Scholar] [CrossRef]
- Thawley, D.G.; Wright, J.C.; Solorzano, R.F. Epidemiologic monitoring following an episode of pseudorabies involving swine, sheep, and cattle. J. Am. Vet. Med Assoc. 1980, 176, 1001–1003. [Google Scholar]
- Matsuoka, T.; Iijima, Y.; Sakurai, K.; Kurihara, T.; Kounosu, Y.; Tamiya, K.; Oki, M.; Haritani, M.; Imada, T. Outbreak of Aujeszky’s disease in cattle in Japan. Nihon juigakuzasshi. Jpn. J. Vet. Sci. 1987, 49, 507–510. [Google Scholar] [CrossRef]
- Fukusho, A. Aujeszky’s Disease of cattle in the Olsztyn Province in years 1980–1991. JARQ 1998, 16, 131–135. [Google Scholar]
- Szweda, W.; Janowski, H.; Grzechnik, R.; Brzeska, E. Aujeszky’s Disease of cattle in the Olsztyn Province in years 1980–1991. Med. Weter 2006, 13, 947–957. [Google Scholar]
- Cheng, Z.; Kong, Z.; Liu, P.; Fu, Z.; Zhang, J.; Liu, M.; Shang, Y. Natural infection of a variant pseudorabies virus leads to bovine death in China. Transbound. Emerg. Dis. 2020, 67, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Toma, B.; Gilet, J. Etude D’un Foyer de MaladieD’Aujeszky Chez les Bovins Avec cas de Guérison Spontanée. Rec. Med. Vet. 1978, 154, 425–429. [Google Scholar]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. MMBR 2005, 69, 462–500. [Google Scholar] [CrossRef]
- Callan, R.J.; Van Metre, D.C. Viral diseases of the ruminant nervous system. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 327-vii. [Google Scholar] [CrossRef]
- Wittmann, G. Aujeszky’s disease. Rev. Sci. Tech. Off. Int. Epiz. 1986, 5, 959–977. [Google Scholar] [CrossRef]
- Kong, H.; Zhang, K.; Liu, Y.; Shang, Y.; Wu, B.; Liu, X. Attenuated live vaccine (Bartha-K16) caused Pseudorabies (Aujeszky’s Disease) in sheep. Vet. Res. Commun. 2013, 37, 329–332. [Google Scholar] [CrossRef]
- Yildirim, S.; Özkan, O.; Yener, Z.; Çetin, M.; Kozat, S. Immunohistochemical diagnosis of Pseudorabies (Aujeszky’s Disease) in a Cow in Van, Turkey. Kafkas. Univ. Vet. Fak. Derg. 2016, 23, 173–176. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Part 3, Section 3.1, Chapter 3.1.2; OIE: Paris, France, 2019. [Google Scholar]
- Deblanc, C.; Oger, A.; Simon, G.; Le Potier, M.F. Genetic Diversity among Pseudorabies Viruses Isolated from Dogs in France from 2006 to 2018. Pathogens 2019, 8, 266. [Google Scholar] [CrossRef]
- Fonseca, A.A., Jr.; Camargos, M.F.; de Oliveira, A.M.; Ciacci-Zanella, J.R.; Patrício, M.A.; Braga, A.C.; Cunha, E.S.; D’Ambros, R.; Heinemann, M.B.; Leite, R.C.; et al. Molecular epidemiology of Brazilian pseudorabies viral isolates. Vet. Microbiol. 2010, 141, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Laval, K.; Enquist, L.W. The Neuropathic Itch Caused by Pseudorabies Virus. Pathogens 2020, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Hopp, W.; Witte, K.H.; Prager, D. ZurPathogenese und Klinik der AujeszkyschenKrankheit des RindesnachexperimentellerInfektionüber den Atmungs-, Verdauungs- und Geschlechtsapparatsowieüber die Haut [Pathogenesis and clinical aspects of Aujeszky’s disease in cattle following an experimental infection through the respiratory, digestive and genital organs and through the skin]. Zent. Vet. Reihe B J. Vet. Med. Ser. B 1985, 32, 287–305. [Google Scholar] [CrossRef]
- Bitsch, V. A study of outbreaks of Aujeszky’s disease in cattle. I. Virological and epidemiological findings. Acta Vet. Scand. 1975, 16, 420–433. [Google Scholar]
- Power, E.P.; O’Connor, M.; Donnelly, W.J.; Dolan, C.E. Aujeszky’s disease in a cow. Vet. Rec. 1990, 126, 13–15. [Google Scholar]
- Cantile, C.; Youssef, S. Nervous system. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Academic Press: New York, NY, USA, 2016; Volume 1, pp. 251–406. [Google Scholar]
- Cano-Manuel, F.J.; López-Olvera, J.; Fandos, P.; Soriguer, R.C.; Pérez, J.M.; Granados, J.E. Long-term monitoring of 10 selected pathogens in wild boar (Sus scrofa) in Sierra Nevada National Park, southern Spain. Vet. Microbiol. 2014, 174, 148–154. [Google Scholar] [CrossRef]
- Grieco, V.; Gelmetti, D.; Finazzi, G.; Brocchi, E.; Finazzi, M. Immunohistologic diagnosis of pseudorabies (Aujeszky’s disease) using monoclonal antibodies. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 1997, 9, 326–328. [Google Scholar] [CrossRef]
- Yoon, H.A.; Eo, S.K.; Aleyas, A.G.; Park, S.O.; Lee, J.H.; Chae, J.S.; Cho, J.G.; Song, H.J. Molecular survey of latent pseudorabies virus infection in nervous tissues of slaughtered pigs by nested and real-time PCR. J. Microbiol. 2005, 43, 430–436. [Google Scholar]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]





| Bovine | Nasal Swabs | Blood | Sera | Necroscopy | IHC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Id. | Group | Clinical | SHV-1 RT-PRC | SHV-1 RT-PRC | gB AB ELISA | IDEXX gB AB ELISA | IDEXX gE AB ELISA | VNT SHV-1 | ||
| 1 | 1 | S | N | N | 256 | P | N | N | X | P |
| 2 | 1 | S | N | N | 64 | D | N | N | X | N |
| 3 | 1 | S | N | N | >256 | P | N | N | X | N |
| 4 | 1 | S | N | N | 64 | D | N | N | N | |
| 5 | 1 | N | N | 64 | D | N | N | |||
| 6 | 1 | N | N | 256 | D | N | N | |||
| 7 | 2 | S | N | N | >256 | P | N | N | X | N |
| 8 | 2 | S | N | N | >256 | NA | N | N | ||
| 9 | 2 | S | N | N | 64 | P | N | 64/128 | N | |
| 10 | 2 | S | N | N | 16 | D | N | N | N | |
| 11 | 2 | S | N | N | 256 | P | N | N | N | |
| 12 | 2 | N | N | >256 | P | N | N | N | ||
| 13 | 2 | N | N | 64 | N | N | N | N | ||
| 14 | 2 | N | N | 256 | P | N | N | N | ||
| 15 | 2 | N | N | 64 | N | N | N | N | ||
| 16 | 2 | N | N | 16 | D | N | N | N | ||
| 17 | 2 | N | N | >256 | P | N | N | N | ||
| 18 | 2 | N | N | >256 | P | N | N | N | ||
| 19 | 2 | N | N | >256 | D | N | N | N | ||
| 20 | 2 | N | N | 16 | N | N | N | N | ||
| 21 | 2 | N | N | >256 | P | N | N | |||
| 22 | 2 | N | N | 256 | P | N | N | |||
| 23 | 2 | N | N | 256 | NA | N | N | |||
| 24 | 2 | N | N | >256 | P | N | N | |||
| 25 | 2 | N | N | 16 | N | N | N | |||
| 26 | 2 | N | N | >256 | P | N | N | |||
| 27 | 2 | N | N | 64 | P | N | N | |||
| 28 | 2 | N | N | >256 | P | N | N | |||
| 29 | 2 | N | N | 64 | N | N | N | |||
| 30 | 2 | N | N | >256 | P | N | N | |||
| 31 | 2 | N | N | >256 | P | N | 16/32 | |||
| 32 | 2 | N | N | 64 | P | N | N | |||
| 33 | 2 | N | N | >256 | NT+ | N | N | |||
| 34 | 2 | N | N | N | NT | N | N | |||
| 35 | 2 | N | N | N | NT | N | N | |||
| 36 | 2 | NA * | N | N | NT | N | N | |||
| 37 | 2 | N | N | 16 | NT | N | N | |||
| 38 | 2 | N | N | NA | NT | N | N | |||
| 39 | 2 | NA | N | 16 | NT | N | N | |||
| Animal Category | n° Positive /n° of Examined ELISA gE | n° Positive /n° of Examined ELISA gB |
|---|---|---|
| Fattening pigs | 32/32 | 32/32 |
| Breeding pigs | 12/12 | 12/12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciarello, F.P.; Capucchio, M.T.; Ippolito, D.; Colombino, E.; Gibelli, L.R.M.; Fiasconaro, M.; Moreno Martin, A.M.; Di Marco Lo Presti, V. First Report of a Severe Outbreak of Aujeszky’s Disease in Cattle in Sicily (Italy). Pathogens 2020, 9, 954. https://doi.org/10.3390/pathogens9110954
Ciarello FP, Capucchio MT, Ippolito D, Colombino E, Gibelli LRM, Fiasconaro M, Moreno Martin AM, Di Marco Lo Presti V. First Report of a Severe Outbreak of Aujeszky’s Disease in Cattle in Sicily (Italy). Pathogens. 2020; 9(11):954. https://doi.org/10.3390/pathogens9110954
Chicago/Turabian StyleCiarello, Flavia Pruiti, Maria Teresa Capucchio, Dorotea Ippolito, Elena Colombino, Lucia Rita Maria Gibelli, Michele Fiasconaro, Ana Maria Moreno Martin, and Vincenzo Di Marco Lo Presti. 2020. "First Report of a Severe Outbreak of Aujeszky’s Disease in Cattle in Sicily (Italy)" Pathogens 9, no. 11: 954. https://doi.org/10.3390/pathogens9110954
APA StyleCiarello, F. P., Capucchio, M. T., Ippolito, D., Colombino, E., Gibelli, L. R. M., Fiasconaro, M., Moreno Martin, A. M., & Di Marco Lo Presti, V. (2020). First Report of a Severe Outbreak of Aujeszky’s Disease in Cattle in Sicily (Italy). Pathogens, 9(11), 954. https://doi.org/10.3390/pathogens9110954







