Cystic Echinococcosis: Clinical, Immunological, and Biomolecular Evaluation of Patients from Sardinia (Italy)
Abstract
1. Introduction
2. Results
2.1. Radiological Examination
2.2. Serology Analysis
2.3. Surgery
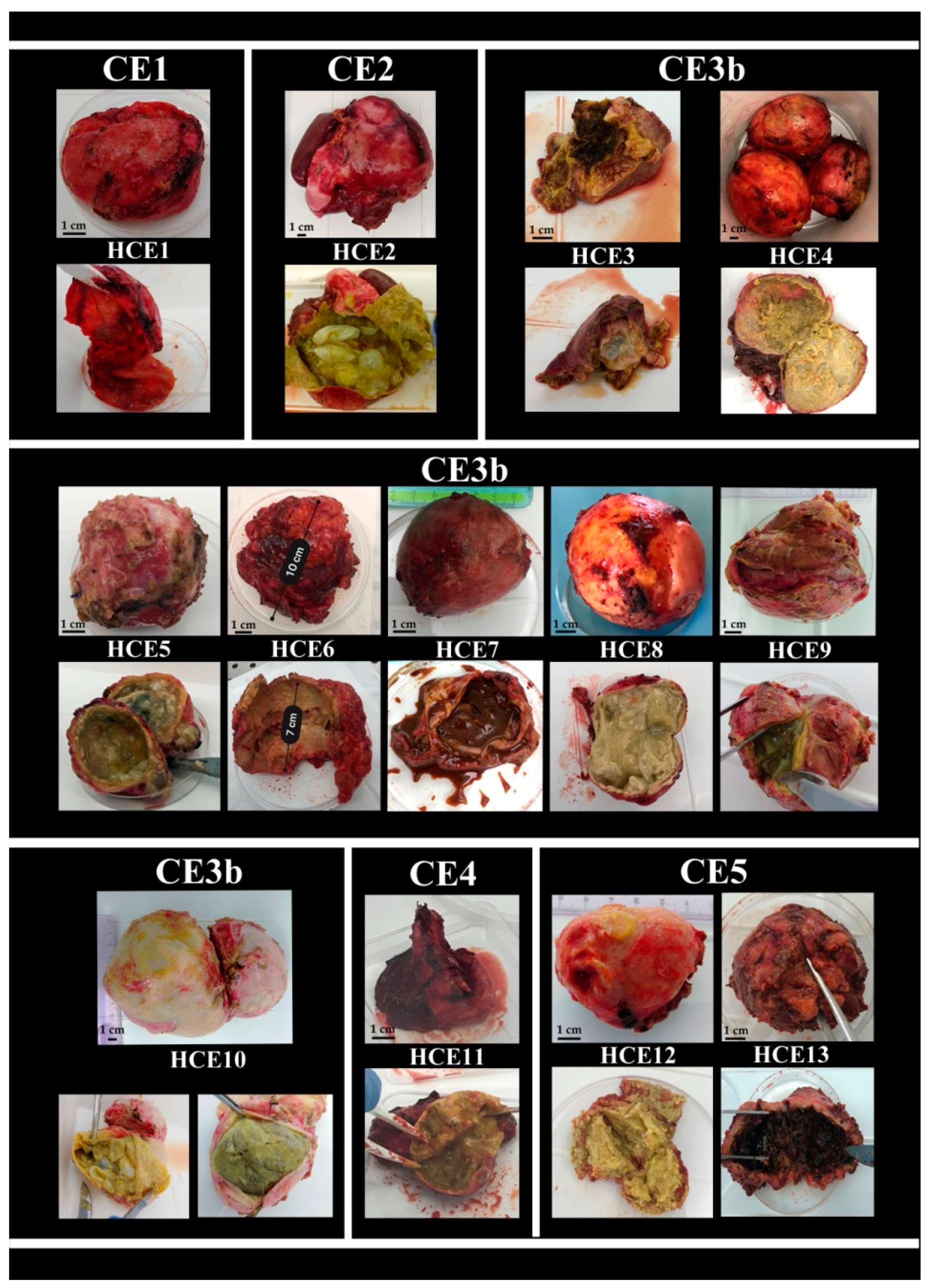
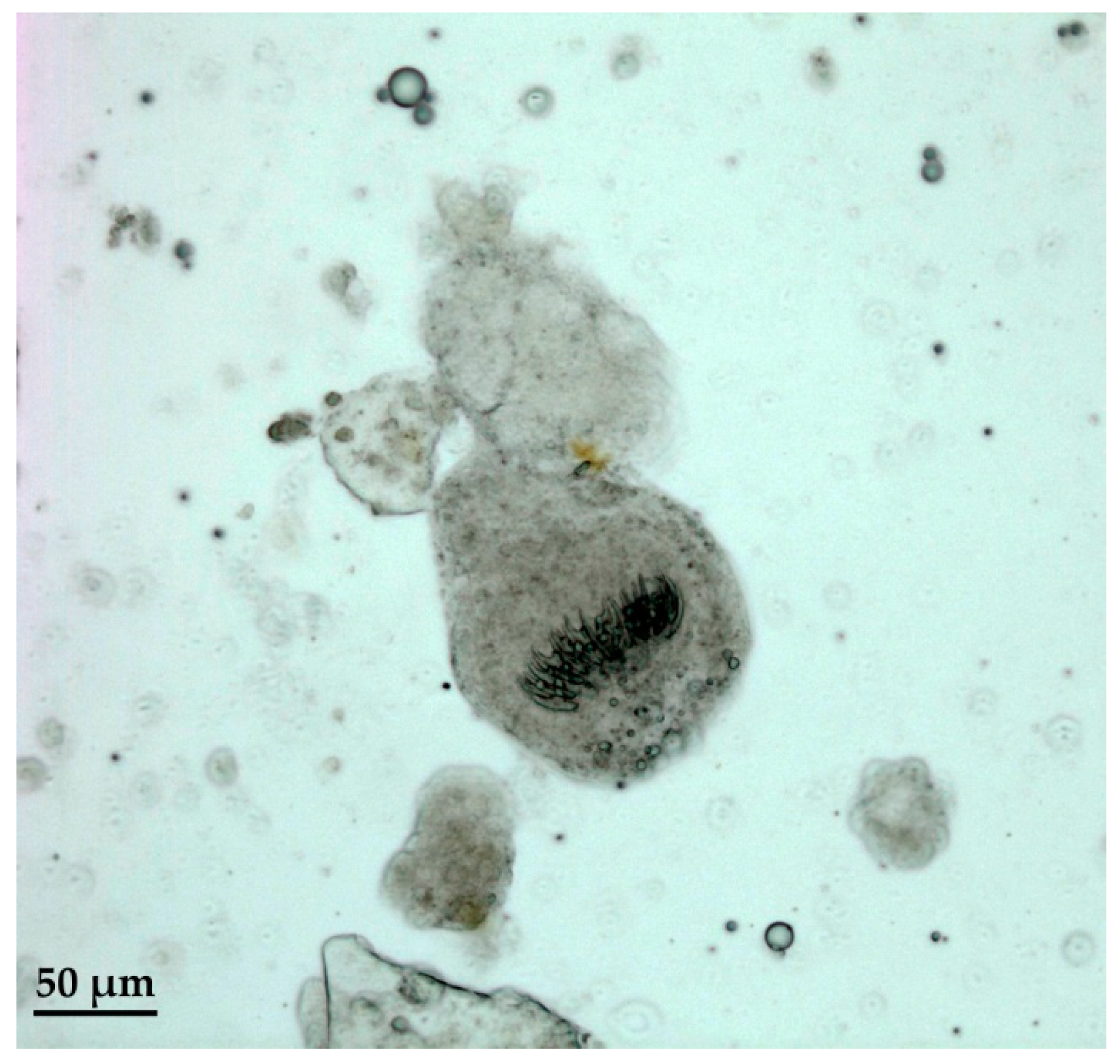
2.4. Hydatid Cyst Examination
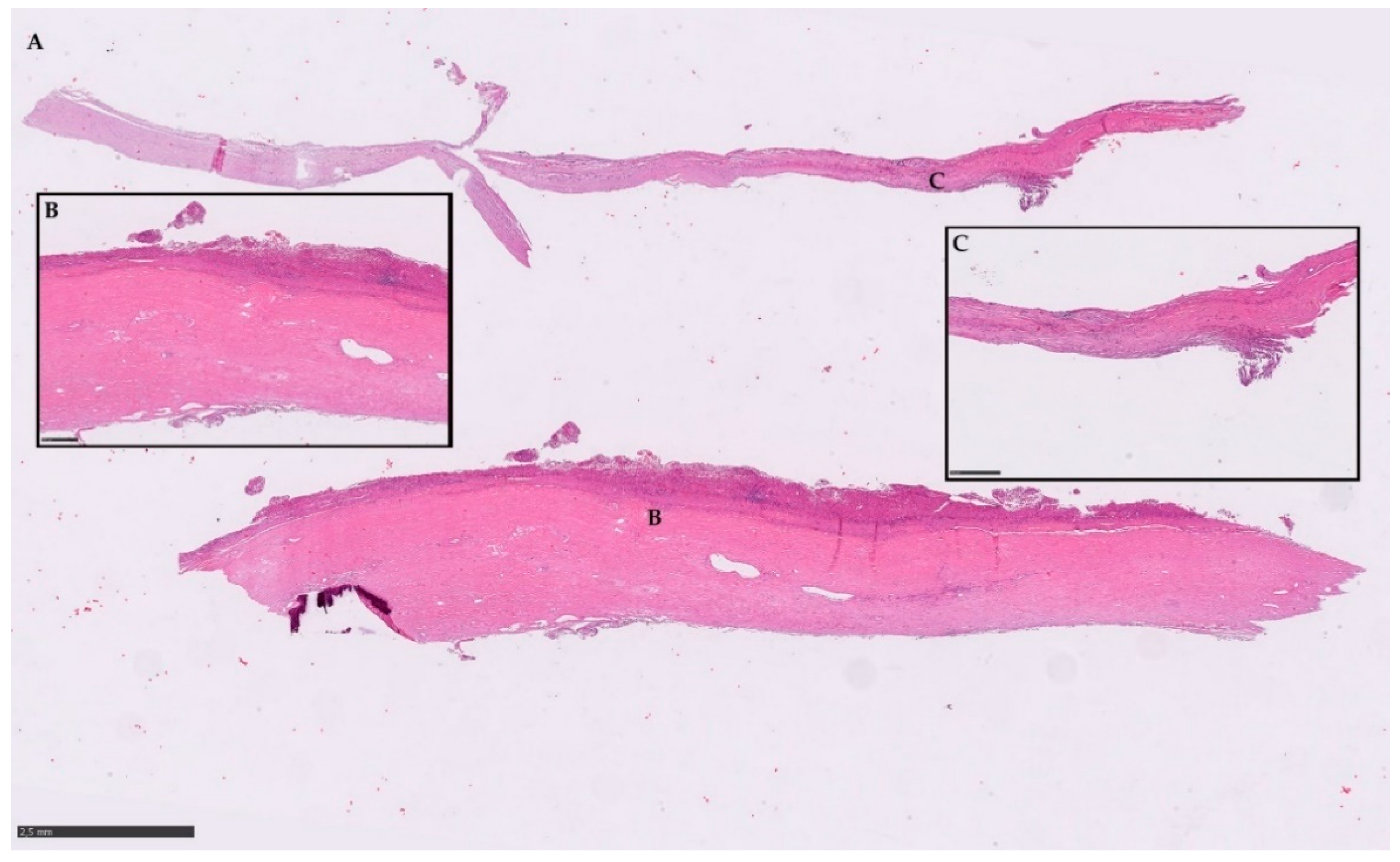
2.5. Histopathological Examination
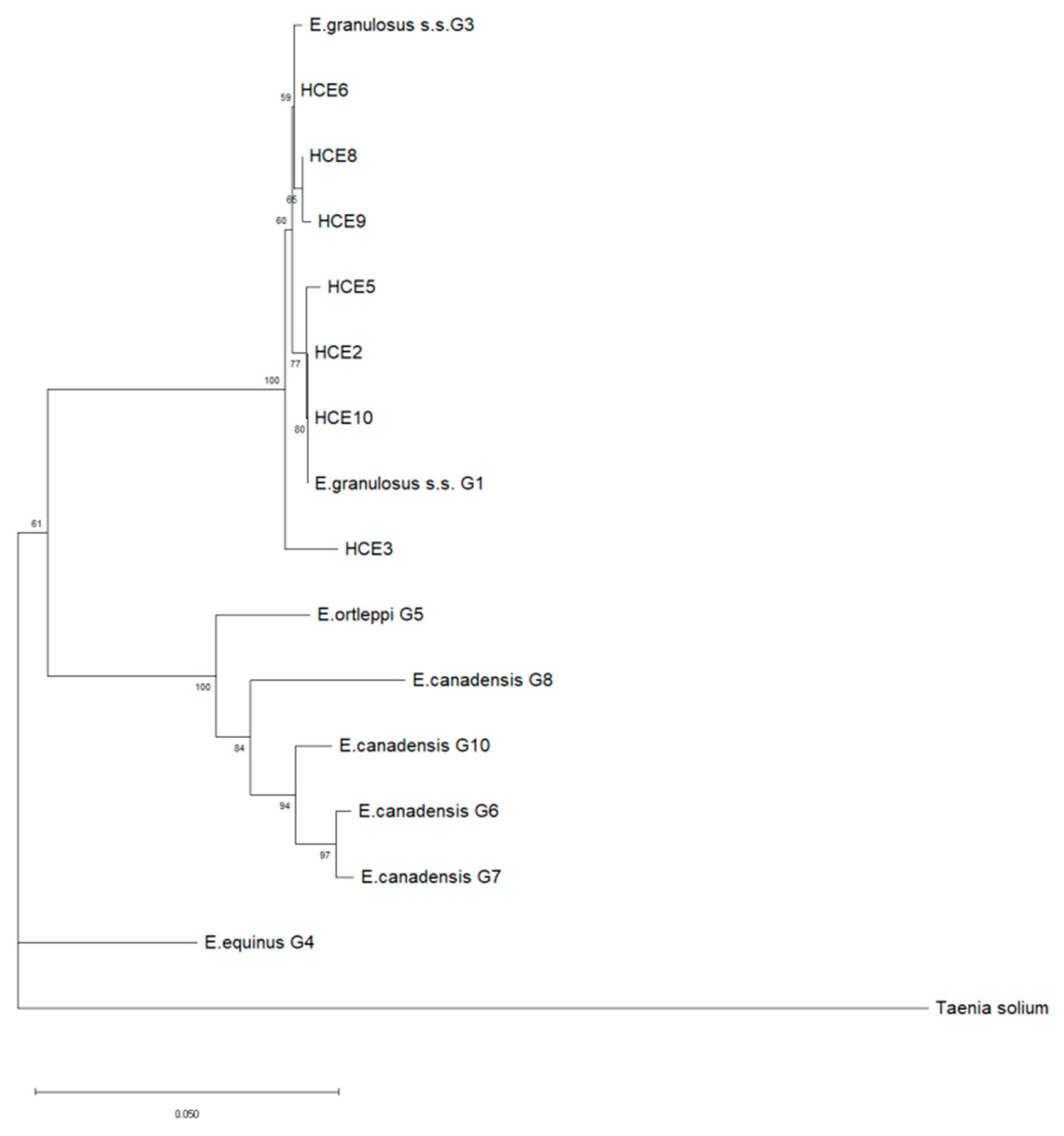
2.6. DNA Amplification and Sequencing
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Patients Involved in the Study
4.3. Ethical Statement
4.4. Radiological Examination
4.5. Serology Analysis
4.6. Surgery
4.7. Hydatid Cyst Examination
4.8. Histopathological Examination
4.9. DNA Extraction from Parasite Tissue
4.10. DNA Amplification
- The PCR for E.g.s.s. [59] method was able to determine the species and the genotype of E. granulosus s.s. G1 or G3 in only one step, avoiding the sequencing step. This assay was performed by using the primers pairs that amplified the Cal gene of 1001 bp (F5′: CAATTTACGGTAAAGCAT-3′-R5′: CCTCATCTCCACTCTCT-3′) (Table 6). Primers were first prepared in a solution of 25 pmol/µL and 1.6 µM (1 pmol/µL final concentration), then 1 μL was used for the amplification of DNA along with 1 µL of DNA (3 ng/µL final concentration); in addition, 4 µL of Milli-Q water RNAse-free, 12.5 µL (1× final concentration) of 2x QuantiTect Probe PCR Master Mix (Qiagen), and 5.5 µL of Milli-Q water RNAse-free were added. The protocol consisted of an initial denaturation step at 95 °C for 15 min, followed by 35 cycles of 94 °C for 1 min, 56 °C for 30 s, and 72 °C for 1 min, and a final extension step at 72 °C of 5 min. After the end of the reaction, amplicons were stored at 4 °C before electrophoresis.
- PCR COX1 [60] was employed to obtain an amplicon for the sequencing analysis of 880 bp, useful to differentiate between G1 and G3. The amplification of DNA was performed by using primers specific for the gene sequence of the enzyme cytochrome oxidase subunit I (COX1) (F 5′-TTTTTTGGCCATCCTGAGGTTTAT-3′ e R 5′-TAACGACATAACATAATGAAAATG-3′) (Table 6). Primers were first prepared in a solution of 25 pmol/µL and 1.6 µM (1 pmol/µL final concentration); then, 1 μL was used for the amplification of DNA, along with 1 µL of DNA (3 ng/µL final concentration), in addition to 4 µL of Milli-Q water RNAse-free, and 12.5 µL (1X final concentration) of 2x QuantiTect Probe PCR Master Mix (Qiagen). Finally, 5.5 µL of Milli-Q water RNAse-free were added. The protocol for the amplification was performed as follows: 1 cycle of 15 min at 95 °C, 40 cycles of 1 min at 94 °C, then 30 s at 58 °C, and 1 min at 72 °C, and 1 cycle of 5 min at 72 °C. After the end of the reaction, amplicons were stored at 4 °C before electrophoresis.
4.11. DNA Sequencing and Phylogenetic Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, R.C.A. Chapter Two—Biology and Systematics of Echinococcus. In Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 95, pp. 65–109. [Google Scholar] [CrossRef]
- Agudelo Higuita, N.I.; Brunetti, E.; McCloskey, C. Cystic echinococcosis. J. Clin. Microbiol. 2016, 54, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Gemmell, M.A.; Meslin, F.-X.; Pawłowski, Z.S. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; World Organization for Animal Health: Paris, France, 2001; p. 265. [Google Scholar]
- Alvarez Rojas, C.A.; Romig, T.; Lightowlers, M.W. Echinococcus granulosus sensu lato genotypes infecting humans—Review of current knowledge. Int. J. Parasitol. 2014, 44, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Marcinkute, A.; Šarkunas, M.; Moks, E.; Saarma, U.; Jokelainen, P.; Bagrade, G.; Laivacuma, S.; Strupas, K.; Sokolovas, V.; Deplazes, P. Echinococcus infections in the Baltic region. Vet. Parasitol. 2015, 213, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Chapter Six—Global Distribution of Alveolar and Cystic Echinococcosis. In Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 95, pp. 315–493. [Google Scholar] [CrossRef]
- Casulli, A. Recognising the substantial burden of neglected pandemics cystic and alveolar echinococcosis. Lancet Glob. Health 2020, 8, e470–e471. [Google Scholar] [CrossRef]
- Brundu, D.; Piseddu, T.; Stegel, G.; Masu, G.; Ledda, S.; Masala, G. Retrospective study of human cystic echinococcosis in Italy based on the analysis of hospital discharge records between 2001 and 2012. Acta Trop. 2014, 140, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Piseddu, T.; Brundu, D.; Stegel, G.; Loi, F.; Rolesu, S.; Masu, G.; Ledda, S.; Masala, G. The disease burden of human cystic echinococcosis based on HDRs from 2001 to 2014 in Italy. PLoS Negl. Trop. Dis. 2017, 11, e0005771. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.S.; Hegglin, D.; Lightowlers, M.W.; Torgerson, P.R.; Wang, Q. Echinococcosis: Control and prevention. Adv. Parasitol. 2017, 96, 55–158. [Google Scholar] [PubMed]
- Siko, S.; Deplazes, P.; Ceica, C.; Tivadar, C.S.; Mogolin, I.; Popescu, S.; Cozma, V. Echinococcus multilocularis in southeastern Europe Romania. Parasitology 2011, 10, 103–107. [Google Scholar]
- Groeneveld, F.; Enstra, A.; Eding, H.; Toro, M.A.; Scherf, B.; Pilling, D. Genetic diversity in farm animals a review. Anim. Genet. 2010, 41, S6–S16. [Google Scholar] [CrossRef]
- World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases. In Third WHO Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO). Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites. Microbiological Risk Assessment Series MRA no 23 Rome FAO/WHO. 2014. Available online: http://www.fao.org/publications/card/en/c/ee07c6ae-b86c-4d5f-915c-94c93ded7d9e/ (accessed on 7 September 2012).
- Mableson, H.E.; Okello, A.; Picozzi, K.; Welburn, S.C. Neglected zoonotic diseases-the long and winding road to advocacy. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef]
- Romig, T.; Ebi, D.; Wassermann, M. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet. Parasitol. 2015, 213, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Yanagida, T.; Konyaev, S.; Lavikainen, A.; Odnokurtsev, V.A.; Zaikov, V.A.; Ito, A. Mitochondrial phylogeny of the genus Echinococcus Cestoda: Taeniidae with emphasis on relationships among Echinococcus canadensis genotypes. Parasitology 2013, 140, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Thompson, R.C.; Bucklar, H.; Bilger, B.; Deplazes, P. Efficacy evaluation of epsiprantel Cestex against Echinococcus mutilocularis in dogs and cats. Berl. Munch. Tierarztl. Wochenschr. 2001, 1143–1144, 121–126. [Google Scholar]
- Thompson, R.C. The taxonomy, phylogeny and transmission of Echinococcus. Exp. Parasitol. 2008, 1194, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.; Irshadullah, M.; Mobedi, I. Rostellar hook morphology of larval Echinococcus granulosus isolates from the Indian buffalo and Iranian sheep, cattle and camel. J. Helminthol. 2011, 853, 239–245. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 542, 165–173. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; McManus, D.P. Molecular genetic characterization of the cervid strain ‘northern form’ of Echinococcus granulosus. Parasitology 1994, 109, 215–221. [Google Scholar] [CrossRef]
- Lavikainen, A.; Lehtinen, M.J.; Meri, T.; Hirvelä-Koski, V.; Meri, S. Molecular genetic characterization of the Fennoscandian cervid strain, a new genotypic group G10 of Echinococcus granulosus. Parasitology 2003, 127, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; McManus, D.P.; Schantz, P.M.; Craig, P.S.; Ito, A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology 2007, 134, 713–722. [Google Scholar] [CrossRef]
- Huttner, M.; Nakao, M.; Wassermann, T.; Siefert, L.; Boomker, J.D.; Dinkel, A.; Sako, Y.; Mackenstedt, U.; Romig, T.; Ito, A. Genetic characterization and phylogenetic position of Echinococcus felidis Cestoda: Taeniidae from the African lion. Int. J. Parasitol. 2008, 387, 861–868. [Google Scholar] [CrossRef]
- Saarma, U.; Jõgisalu, I.; Moks, E.; Varcasia, A.; Lavikainen, A.; Oksanen, A.; Simsek, S.; Andresiuk, V.; Denegri, G.; González, L.M.; et al. A novel phylogeny for the genus Echinococcus, based on nuclear data, challenges relationships based on mitochondrial evidence. Parasitology 2009, 1363, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.; Nakao, M.; Yanagida, T.; Okamoto, M.; Saarma, U.; Lavikainen, A.; Ito, A. Phylogenetic relationships within Echinococcus and Taenia tapeworms Cestoda: Taeniidae: An inference from nuclear protein-coding genes. Mol. Phylogenet. Evol. 2011, 613, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Moks, E.; Jõgisalu, I.; Saarma, U.; Talvik, H.; Järvis, T.; Valdmann, H. Helminthologic survey of the wolf Canis lupus in Estonia, with an emphasis on Echinococcus granulosus. J. Wildl. Dis. 2006, 422, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Moks, E.; Jõgisalu, I.; Valdmann, H.; Saarma, U. First report of Echinococcus granulosus G8 in Eurasia and a reappraisal of the phylogenetic relationships of ‘genotypes’G5-G10. Parasitology 2008, 1355, 647–654. [Google Scholar] [CrossRef]
- Deplazes, P.; van Knapen, F.; Schweiger, A.; Overgaauw, P.A. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet. Parasitol 2011, 1821, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Laurimae, L.; Davison, J.; Süld, K.; Plumer, L.; Oja, R.; Moks, E.; Keis, M.; Hindrikson, M.; Kinkar, L.; Laurimäe, T.; et al. First report of highly pathogenic Echinococcus granulosus genotype G1 in dogs in a European urban environment. Parasit. Vectors 2015, 268, 182. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, P.; Masu, G.; Dei Giudici, S.; Pintus, D.; Peruzzu, A.; Piseddu, T.; Santucciu, C.; Cossu, A.; Demurtas, N.; Masala, G. Cystic echinococcosis in a domestic cat Felis catus in Italy. Parasite 2018, 25, 25. [Google Scholar] [CrossRef]
- Bhutani, N.; Kajal, P. Hepatic echinococcosis: A review. Ann. Med. Surg. 2018, 36, 99–105. [Google Scholar] [CrossRef]
- Chiboub, H.; Boutayeb, F.; Wahbi, S.; El Yacoubi, M.; Ouazzani, N.; Hermas, M. Echinococcosis of the pelvic bone: Four cases. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2001, 874, 397–401. [Google Scholar]
- Zhang, W.; Wen, H.; Li, J.; Lin, R.; McManus, D.P. Immunology and immunodiagnosis of cystic echinococcosis: An update. Clin. Dev. Immunol. 2012, 2012, 101895. [Google Scholar] [CrossRef]
- Díaz, A.; Casaravilla, C.; Irigoín, F. Understanding the laminated layer of larval Echinococcus I: Structure. Trends Parasitol. 2011, 27, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Casaravilla, C.; Allen, J.E. Understanding the laminated layer of larval Echinococcus II: Immunology. Trends Parasitol. 2011, 27, 264–273. [Google Scholar] [CrossRef]
- Kern, P.; Menezes da Silva, A.; Akhan, O.; Müllhaupt, B.; Vizcaychipi, K.A.; Budke, C.; Vuitton, D.A. Chapter Four—The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. In Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 96, pp. 259–369. [Google Scholar] [CrossRef]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, E.; Tamarozzi, F.; Macpherson, C.; Filice, C.; Piontek, M.S.; Kabaalioglu, A.; Dong, Y.; Atkinson, N.; Richter, J.; Schreiber-Dietrich, D.; et al. Ultrasound and Cystic Echinococcosis. Ultrasound Int. Open 2018, 43, E70–E78. [Google Scholar] [CrossRef] [PubMed]
- Hosch, W.; Junghanss, T.; Stojkovic, M.; Brunetti, E.; Heye, T.; Kauffmann, G.W.; Hull, W.E. Metabolic viability assessment of cystic echinococcosis using highfield 1H MRS of cyst contents. NMR Biomed. 2008, 21, 734–754. [Google Scholar] [CrossRef] [PubMed]
- Siles-Lucas, M.; Casulli, A.; Conraths, F.J.; Müller, N. Chapter Three—Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. In Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 96, pp. 159–257. [Google Scholar] [CrossRef]
- Zhang, W.; McManus, D.P. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol. Med. Microbiol. 2006, 471, 24–41. [Google Scholar] [CrossRef]
- Carmena, D.; Benito, A.; Eraso, E. Antigens for the immunodiagnosis of Echinococcus granulosus infection: An update. Acta Trop. 2006, 981, 74–86. [Google Scholar] [CrossRef]
- Barnes, T.S.; Deplazes, P.; Gottstein, B.; Jenkins, D.J.; Mathis, A.; Siles-Lucas, M.; Torgerson, P.R.; Ziadinov, I.; Heath, D.D. Challenges for diagnosis and control of cystic hydatid disease. Acta Trop. 2012, 1231, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sarkari, B.; Rezaei, Z. Immunodiagnosis of human hydatids disease: Where do we stand? World J. Methodol. 2015, 54, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Roman, R.; Sanchez-Ovejero, C.; Hernandez-Gonzales, A.; Casulli, A.; Siles-Lucas, M. Serological diagnosis and follow-up of human cystic echinococcosis: A new hope for the future? Biomed. Res. Int. 2015, 2015, 428205. [Google Scholar] [CrossRef] [PubMed]
- Lissandrin, R.; Tamarozzi, F.; Piccoli, L.; Tinelli, C.; De Silvestri, A.; Mariconti, M.; Meroni, V.; Genco, F.; Brunetti, E. Factors influencing the serological response in hepatic Echinococcus granulosus infection. Am. J. Trop. Med. Hyg. 2016, 94, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Patkowski, W.; Krasnodębski, M.; Grąt, V.; Masior, Ł.; Krawczyk, M. Surgical treatment of hepatic Echinococcus granulosus. Gastroenterol. Rev. 2017, 12, 199–202. [Google Scholar] [CrossRef]
- Yahya, A.I.; Shwereif, H.E.; Ekheil, M.A.; Ahmed, S.; Algader, T.K.A.; Gyaed, F.O.; Aldarat, A.S. The role of emergency surgery in hydatid liver disease. World J. Emerg. Surg. 2014, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Dinc, T.; Kayilioglu, S.I.; Akturk, O.M.; Coskun, F. Surgical management of liver hydatid cyst related non-traumatic emergencies: Single center experience. Iran. J. Parasitol. 2016, 11, 574–579. [Google Scholar] [PubMed]
- Porcu, A.; Fancellu, A.; Cherchi, G.; Nigri, G. The role of emergency surgery in hydatid liver disease. In The Surgical Management of Parasitic Diseases; Tsoulfas, G., Hoballah, J., Velmahos, G., Ho, Y.H., Eds.; Springer Nature: Cham, Switzerland, 2020; Volume 13, pp. 199–207. [Google Scholar]
- Panteleev, V.; Nartaylakov, M.; Mustafin, A.; Abdeyev, R.; Salimgareyev, I.; Samorodov, A.; Musharapov, D. Surgical treatment of liver echinococcosis and alveococcosis. Infez. Med. 2019, 27, 422–428. [Google Scholar] [PubMed]
- Lissandrin, R.; Tamarozzi, F.; Mariconti, M.; Manciulli, T.; Brunetti, E.; Vola, A. Watch and Wait Approach for Inactive Echinococcal Cyst of the Liver: An Update. Am. J. Trop Med. Hyg. 2018, 99, 375–379. [Google Scholar] [CrossRef]
- Pagnozzi, D.; Addis, M.F.; Biosa, G.; Roggio, A.M.; Tedde, V.; Mariconti, M.; Tamarozzi, F.; Meroni, V.; Masu, G.; Masala, G.; et al. Diagnostic Accuracy of Antigen 5-Based ELISAs for Human Cystic Echinococcosis. PLoS Negl. Trop. Dis. 2016, 10, e0004585. [Google Scholar] [CrossRef] [PubMed]
- Pagnozzi, D.; Tamarozzi, F.; Roggio, A.M.; Tedde, V.; Addis, M.F.; Pisanu, S.; Masu, G.; Santucciu, C.; Vola, A.; Casulli, A.; et al. Structural and Immunodiagnostic Characterization of Synthetic Antigen B Subunits From Echinococcus granulosus and Their Evaluation as Target Antigens for Cyst Viability Assessment. Clin. Infect. Dis. 2018, 66, 1342–1351. [Google Scholar] [CrossRef]
- Krige, J.E.J.; Beckingham, I.J. Liver abscesses and hydatid disease. BMJ 2001, 322, 537–540. [Google Scholar] [CrossRef]
- Hernandez-Gonzalez, A.; Santivanez, S.; Garcia, H.H.; Rodriguez, S.; Munoz, S.; Ramos, G.; Orduna, A. Improved serodiagnosis of cystic echinococcosis using the new recombinant 2B2t antigen. PLoS Negl. Trop. Dis. 2012, 6, e1714. [Google Scholar] [CrossRef]
- Santucciu, C.; Masu, G.; Mura, A.; Peruzzu, A.; Piseddu, T.; Bonelli, P.; Masala, G. Validation of a one-step PCR assay for the molecular identification of Echinococcus granulosus sensu stricto G1-G3 genotype. Mol. Biol. Rep. 2019, 462, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Sako, Y.; Yokoama, N.; Fukunaga, M.; Ito, A. Mitochondrial genetic code in cestodes. Mol. Biochem. Parasitol. 2000, 111, 415–424. [Google Scholar] [CrossRef]
| Patients Cyst Id | Cyst Stadium | Serological Findings | ||
|---|---|---|---|---|
| ELISA (DRG) (OD) 1 | IB (LDBIO) 2 | |||
| HCE1 | CE1 | negative | / | negative |
| HCE2 | CE2 | positive | (2.6) | positive |
| HCE3 | CE3b | positive | (3.6) | positive |
| HCE4 | CE3b 3 | positive | (0.7) | positive |
| HCE5 | CE3b | positive | (1.2) | positive |
| HCE6 | CE3b | positive | (2.3) | positive |
| HCE7 | CE3b | negative | / | negative |
| HCE8 | CE3b | negative | / | positive |
| HCE9 | CE3b | positive | (2.2) | positive |
| HCE10 | CE3b (CE5) 4 | positive | (2.3) | positive |
| HCE11 | CE4 | negative | / | negative |
| HCE12 | CE5 (CE3b) 4 | positive | (2.5) | positive |
| HCE13 | CE5 | negative | / | negative |
| Patients Cyst Id | Cyst Stadium | Protoscoleces |
|---|---|---|
| HCE1 | CE1 | Not available |
| HCE2 | CE2 | Non-viable |
| HCE3 | CE3b | Non-viable |
| HCE4 | CE3b | Non-viable |
| HCE5 | CE3b | Non-viable |
| HCE6 | CE3b | Non-viable |
| HCE7 | CE3b | Negative |
| HCE8 | CE3b | Non-viable |
| HCE9 | CE3b | Non-viable |
| HCE10 | CE3b | Non-viable |
| HCE11 | CE4 | Negative |
| HCE12 | CE5 | Negative |
| HCE13 | CE5 | Negative |
| Cyst Id | Cyst Stadium | PCR for Gene CAL of 1001 bp | PCR for Gene COX1 of 880 bp | Genotype | Sequence |
|---|---|---|---|---|---|
| HCE1 | CE1 | negative | negative | / | / |
| HCE2 | CE2 | positive | positive | G1 | MK780827 |
| HCE3 | CE3b | positive | positive | G3 | MK780842 |
| HCE4 | CE3b | positive | positive | / * | / * |
| HCE5 | CE3b | positive | positive | G1 | MK780830 |
| HCE6 | CE3b | positive | positive | G3 | MK780839 |
| HCE7 | CE3b | negative | negative | / | / |
| HCE8 | CE3b | positive | positive | G3 | MK780843 |
| HCE9 | CE3b | positive | positive | G3 | MT991983 |
| HCE10 | CE3b | positive | positive | G1 | MK780827 |
| HCE11 | CE4 | negative | negative | / | / |
| HCE12 | CE5 | negative | negative | / | / |
| HCE13 | CE5 | negative | negative | / | / |
| Patients Cyst Id | Gender | Age | Nationality |
|---|---|---|---|
| HCE1 | Female | 68 | Italian |
| HCE2 | Male | 53 | Italian |
| HCE3 | Male | 41 | Chinese |
| HCE4 | Male | 75 | Italian |
| HCE5 | Female | 75 | Italian |
| HCE6 | Male | 43 | Italian |
| HCE7 | Female | 67 | Italian |
| HCE8 | Female | 60 | Italian |
| HCE9 | Male | 64 | Italian |
| HCE10 | Female | 42 | Romanian |
| HCE11 | Male | 80 | Italian |
| HCE12 | Female | 18 | Moroccan |
| HCE13 | Female | 67 | Italian |
| Patients Cyst Id | Reason for Surgical Intervention |
|---|---|
| HCE1 | Large viable cyst (6 × 7cm) in contact with hepatic veins |
| HCE2 | Giant cyst (17 × 15 cm) with daughter cysts |
| HCE3 | Recurrent large hydatid cyst (8 × 7 cm) complicated by abscessualization and septic shock |
| HCE4 | Multiple large hydatid cysts (from 3 to 7 cm) with obstructive jaundice |
| HCE5 | Large cyst (6 × 7 cm) causing recurrent cholangitis |
| HCE6 | Large cyst (7 × 10 cm) ruptured into the biliary tree with jaundice and severe cholangitis |
| HCE7 | Large viable cyst (7 × 6 cm) infiltrating the diaphragm and the chest wall |
| HCE8 | Cyst (7 × 8 cm) in contact with the inferior vena cava causing recurrent cholangitis |
| HCE9 | Large cyst (11 × 11 cm) abutting the diaphragm with biliary-bronchial fistula |
| HCE10 | Multiple large cysts (from 7 to 10 cm) with recurrent cholangitis |
| HCE11 | Large cyst (6 × 7 cm) with signs of abscessualization |
| HCE12 | Multiple large cyst (6 × 7 cm), in contact with the portal vein, with daughter cysts and caseous |
| HCE13 | Cyst (7 × 8 cm) fistulated into the biliary tree with recurrent cholangitis |
| Method | Gene Amplified | Primers | Length |
|---|---|---|---|
| PCR for E.g.s.s. | Calreticulin (CAL) l | F5′: CAA TTT ACG GTA AAG CAT-3′- R5′: CCT CAT CTC CAC TCTCT-3′ | 1001 bp |
| PCR COX1 | Cytochrome oxidase subunit (COX) I | F 5′-TTGAATGCTTTGAGTGCTTG-3′ R 5′-GAACCTAACGACATAACATAATGA-3′ | 880 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santucciu, C.; Bonelli, P.; Peruzzu, A.; Fancellu, A.; Marras, V.; Carta, A.; Mastrandrea, S.; Bagella, G.; Piseddu, T.; Profili, S.; et al. Cystic Echinococcosis: Clinical, Immunological, and Biomolecular Evaluation of Patients from Sardinia (Italy). Pathogens 2020, 9, 907. https://doi.org/10.3390/pathogens9110907
Santucciu C, Bonelli P, Peruzzu A, Fancellu A, Marras V, Carta A, Mastrandrea S, Bagella G, Piseddu T, Profili S, et al. Cystic Echinococcosis: Clinical, Immunological, and Biomolecular Evaluation of Patients from Sardinia (Italy). Pathogens. 2020; 9(11):907. https://doi.org/10.3390/pathogens9110907
Chicago/Turabian StyleSantucciu, Cinzia, Piero Bonelli, Angela Peruzzu, Alessandro Fancellu, Vincenzo Marras, Antonello Carta, Scilla Mastrandrea, Giorgio Bagella, Toni Piseddu, Stefano Profili, and et al. 2020. "Cystic Echinococcosis: Clinical, Immunological, and Biomolecular Evaluation of Patients from Sardinia (Italy)" Pathogens 9, no. 11: 907. https://doi.org/10.3390/pathogens9110907
APA StyleSantucciu, C., Bonelli, P., Peruzzu, A., Fancellu, A., Marras, V., Carta, A., Mastrandrea, S., Bagella, G., Piseddu, T., Profili, S., Porcu, A., & Masala, G. (2020). Cystic Echinococcosis: Clinical, Immunological, and Biomolecular Evaluation of Patients from Sardinia (Italy). Pathogens, 9(11), 907. https://doi.org/10.3390/pathogens9110907







