Surveillance of Human Rotavirus in Wuhan, China (2011–2019): Predominance of G9P[8] and Emergence of G12
Abstract
1. Introduction
2. Results
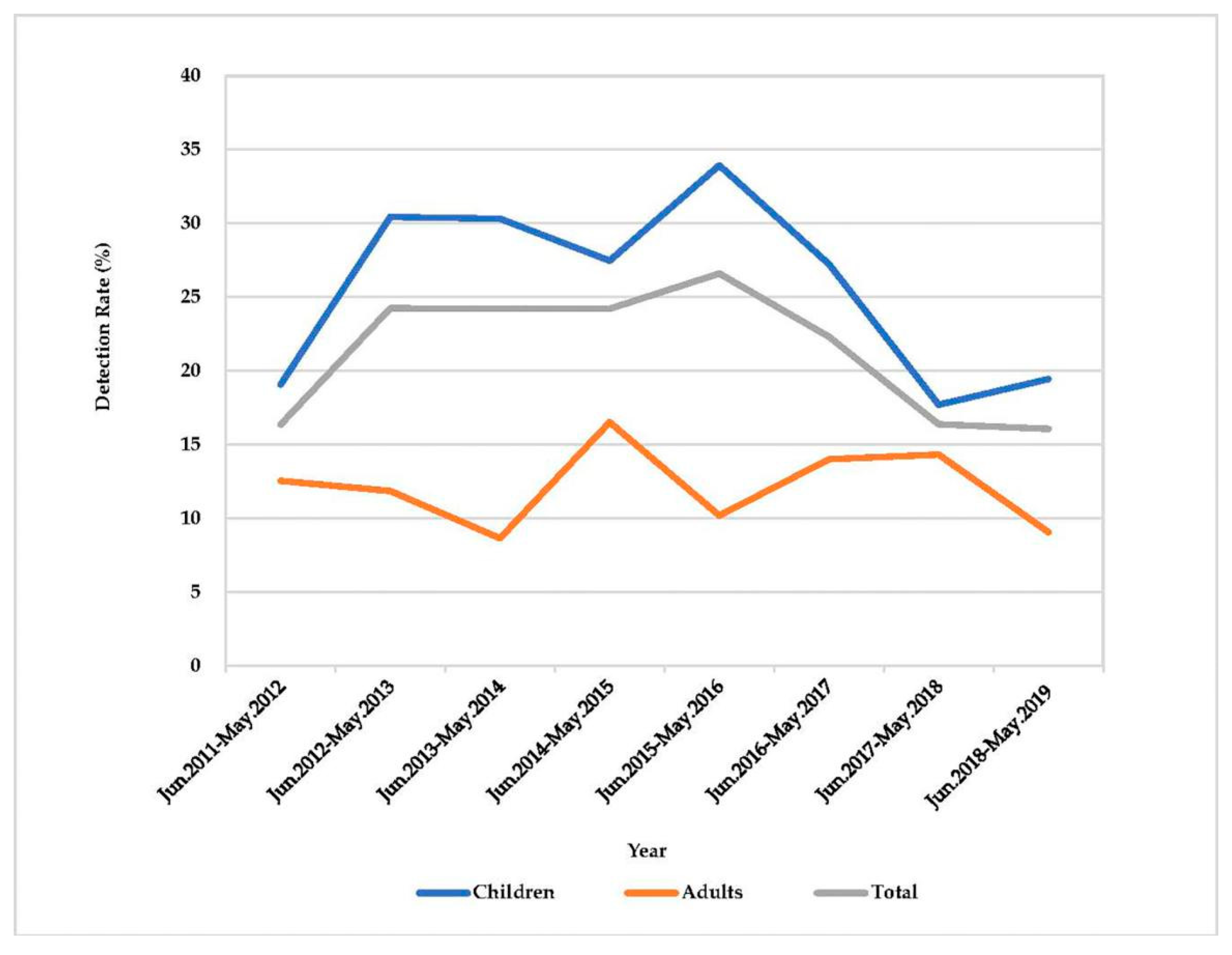
2.1. Detection of Rotavirus, VP7 and VP4 Genotyping
2.2. The Case of G12 RVA Infection
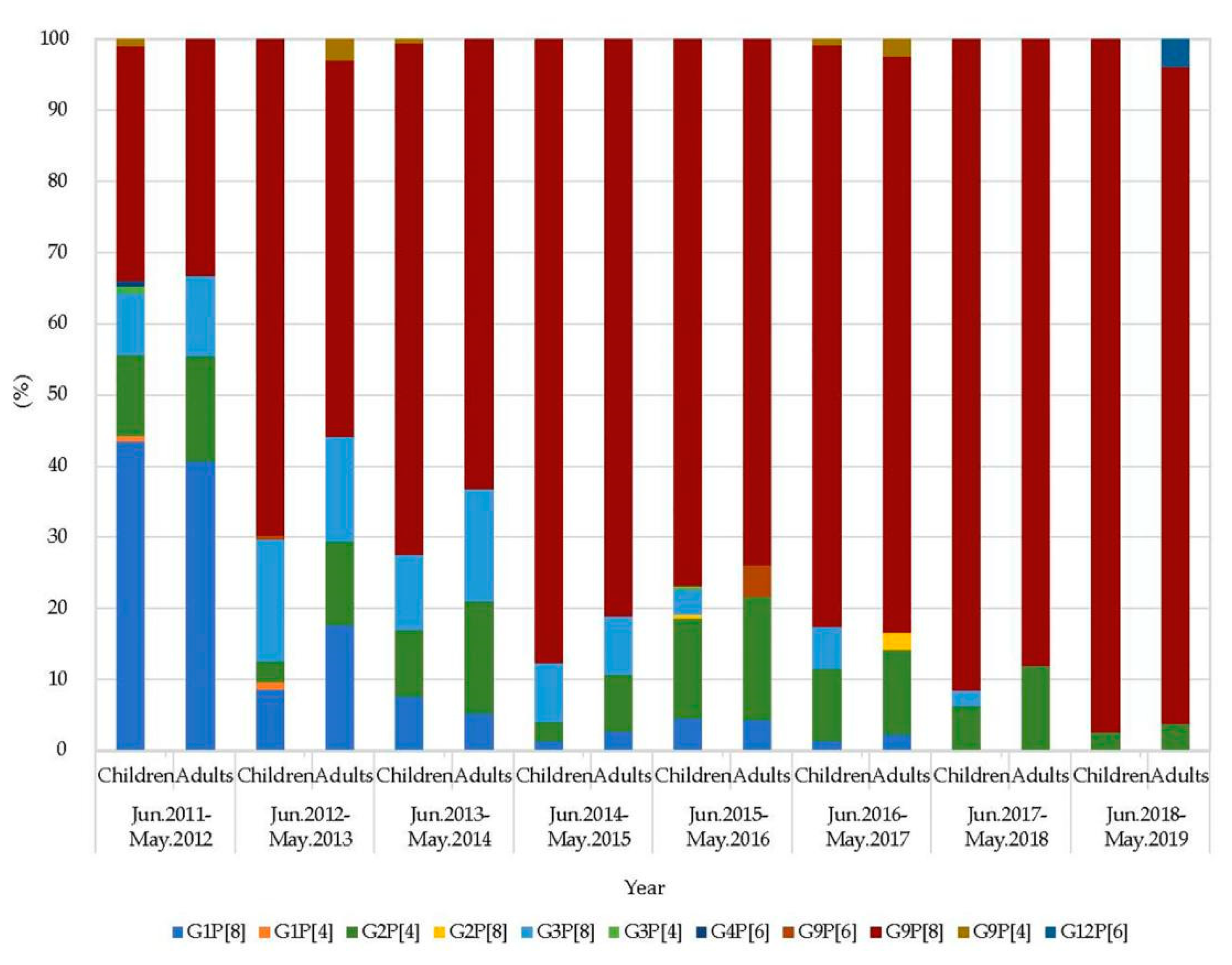
2.3. Genotype Constellation
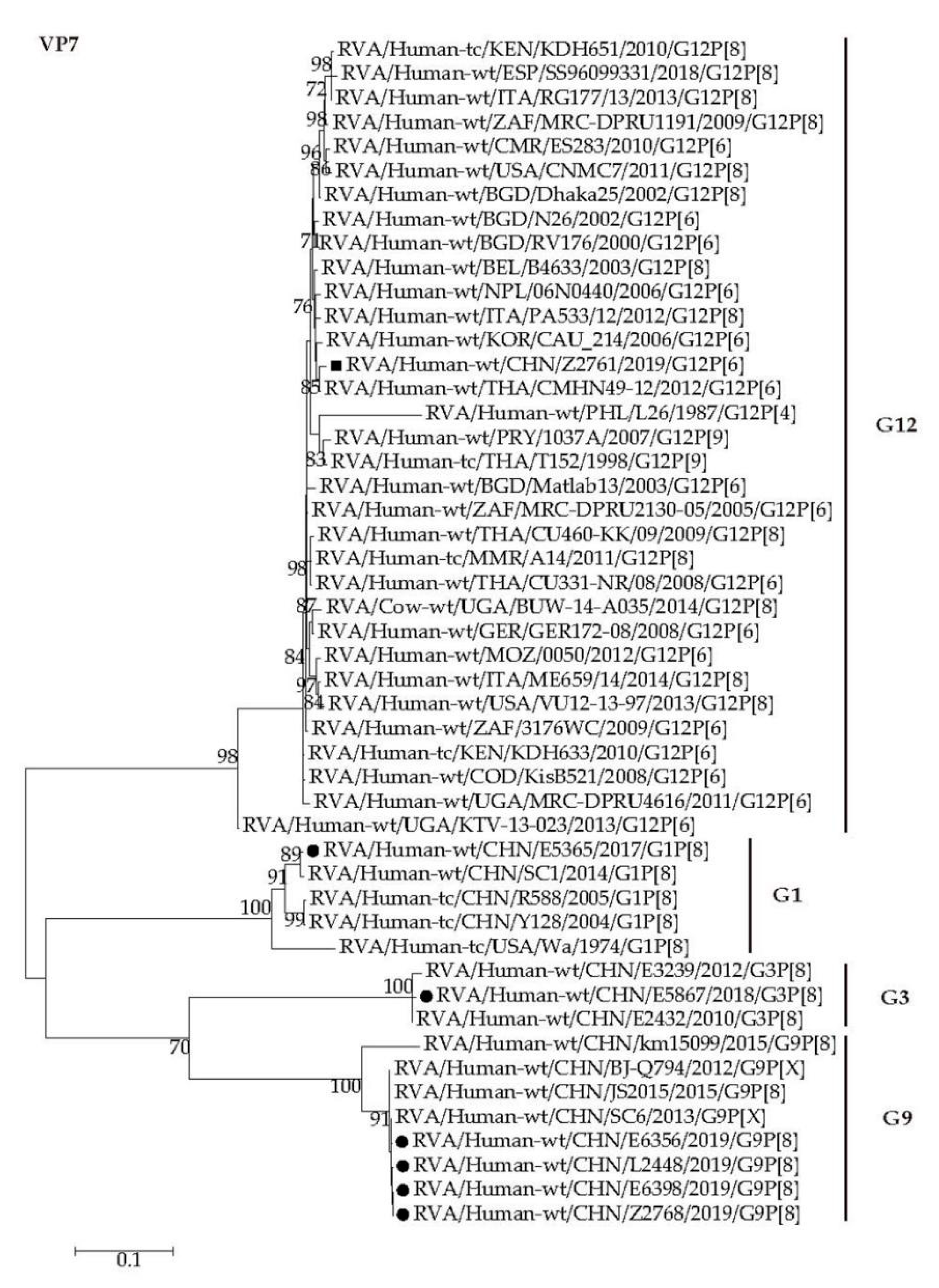
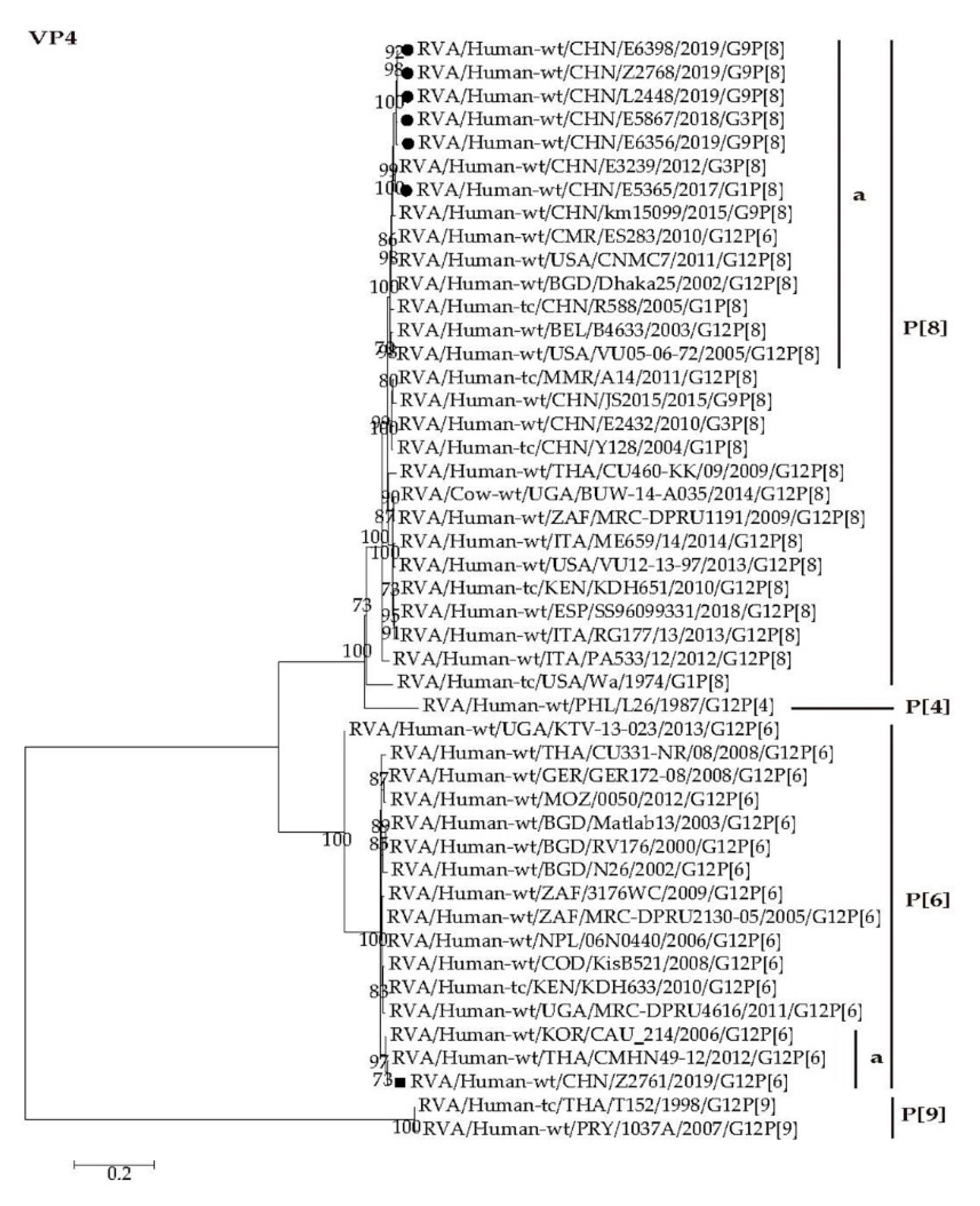
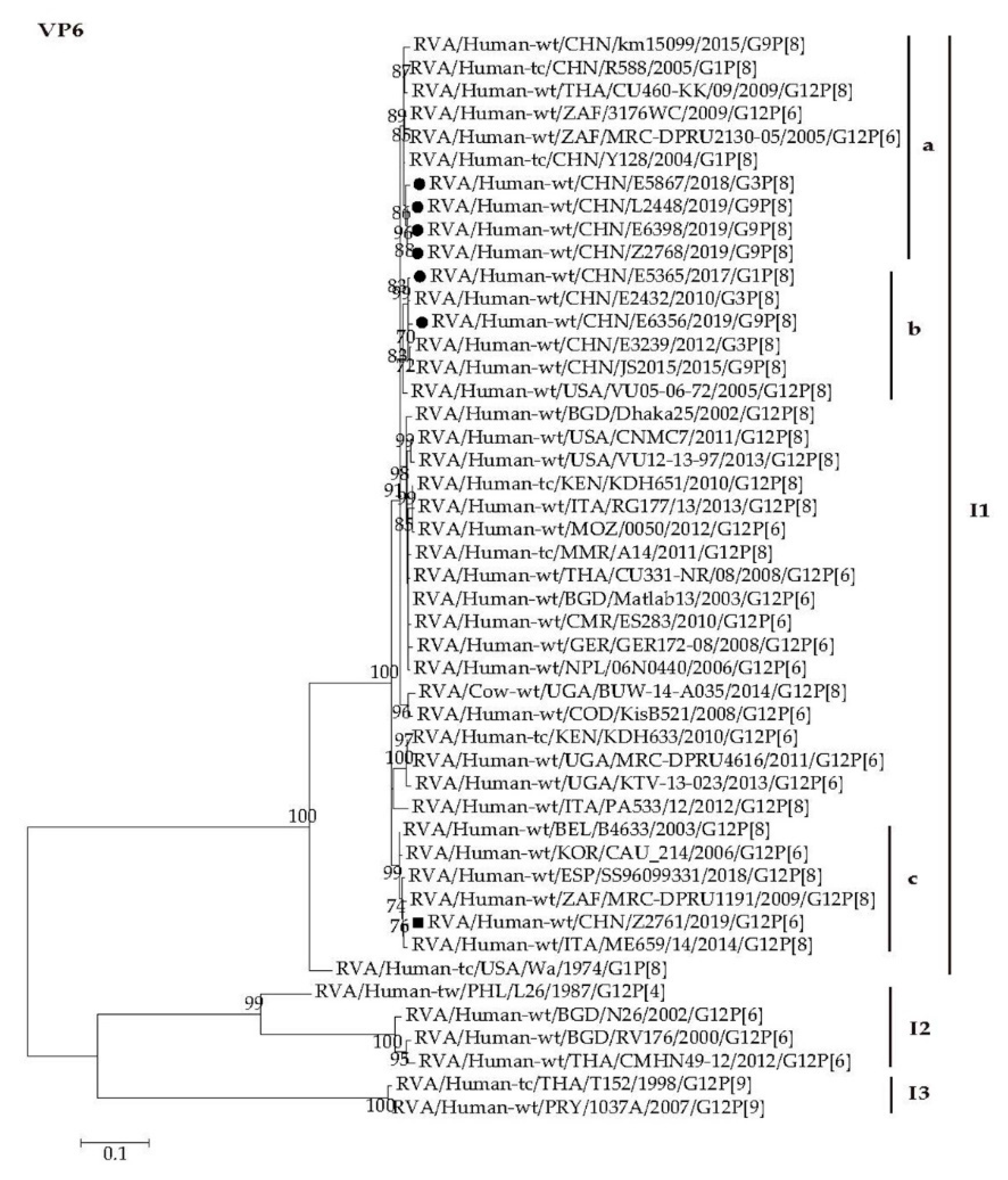
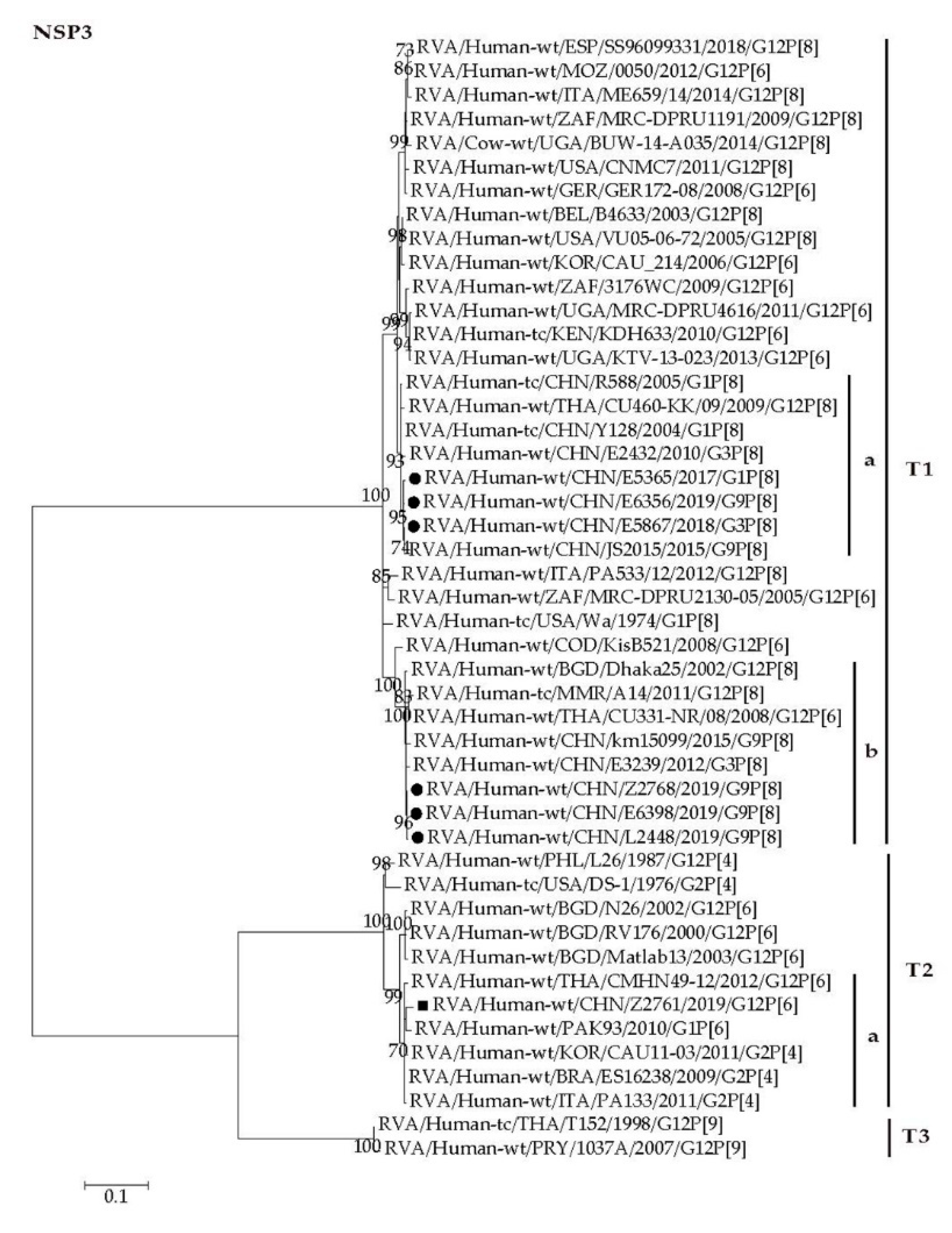
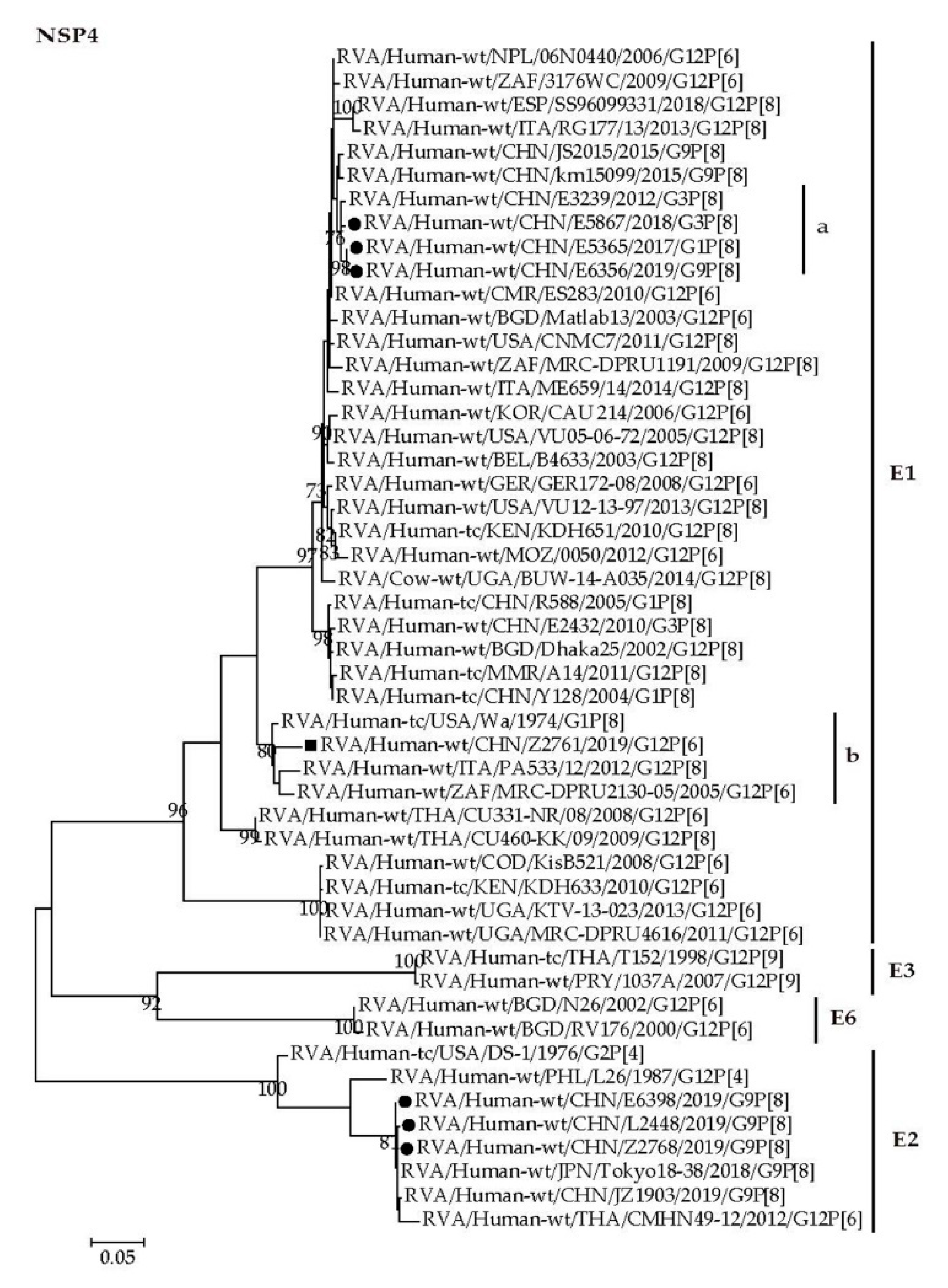
2.4. Phylogenetic Analysis
2.4.1. Structural Protein Genes
2.4.2. Nonstructural Protein Genes
3. Discussion
4. Materials and Methods
4.1. Specimens
4.2. Investigation on The Case Infected G12 Rotavirus
4.3. Detection of Rotavirus
4.4. Genotyping of RVA
4.5. Whole Genome Sequencing
4.6. Phylogenetic Analysis
4.7. Statistical Analysis
4.8. Accession Numbers of Nucleotide Sequences in Genbank
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Dennehy, P.H. Rotavirus vaccines: An overview. Clin. Microbiol. Rev. 2008, 21, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Steele, D.; Gentsch, J.R.; Wecker, J.; Glass, R.I.; Parashar, U.D. Real-world impact of rotavirus vaccination. Pediatr. Infect. Dis. J. 2011, 30, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- WHO. Meeting of the immunization Strategic Advisory Group of Experts, April 2008—conclusions and recommendations. Wkly. Epidemiol. Rec. 2008, 83, 193–208. [Google Scholar]
- Chandran, A.; Fitzwater, S.; Zhen, A.; Santosham, M. Prevention of rotavirus gastroenteritis in infants and children: Rotavirus vaccine safety, efficacy, and potential impact of vaccines. Biologics 2010, 4, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.S.; Li, Y.; Wang, S.M.; Zhang, X.J.; Hao, Z.Y.; Chen, Y.; Wang, D.; Zhang, Y.H.; Zhang, Z.Y.; Ma, J.C.; et al. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case-control design. Emerg. Microbes. Infect. 2015, 4, e64. [Google Scholar] [CrossRef]
- Estes, M.K. Rotaviruses and their replication. In Fields Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2001; Volume 4, pp. 1747–1785. [Google Scholar]
- Estes, M.K.; Kapikian, A.Z. Rotaviruses. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 5, pp. 1917–1974. [Google Scholar]
- Rotavirus Classification Working Group: RCWG. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 24 August 2020).
- Matthijnssens, J.; Ciarlet, M.; Rahman, M.; Attoui, H.; Bányai, K.; Estes, M.K.; Gentsch, J.R.; Iturriza-Gómara, M.; Kirkwood, C.D.; Martella, V.; et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008, 153, 1621–1629. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef]
- Gentsch, J.; Laird, A.; Bielfelt, B.; Griffin, D.; Bányai, K.; Ramachandran, M.; Jain, V.; Cunliffe, N.; Nakagomi, O.; Kirkwood, C.; et al. Serotype diversity and reassortment between human and animal rotavirus strains: Implications for rotavirus vaccine programs. J. Infect. Dis. 2005, 192, S146–S159. [Google Scholar] [CrossRef]
- Bányai, K.; László, B.; Duque, J.; Steele, A.D.; Nelson, E.A.S.; Gentsch, J.R.; Parashar, U.D. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: Insights for understanding the impact of rotavirus vaccination programs. Vaccine 2012, 30, A122–A130. [Google Scholar] [CrossRef]
- Rahman, M.; De Leener, K.; Goegebuer, T.; Wollants, E.; Van der Donck, I.; Van Hoovels, L.; Van Ranst, M. Genetic characterization of a novel, naturally occurring recombinant human G6P[6] rotavirus. J. Clin. Microbiol. 2003, 41, 2088–2095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matthijnssens, J.; Rahman, M.; Martella, V.; Yang, X.; De Vos, S.; De Leener, K.; Ciarlet, M.; Buonavoglia, C.; Van Ranst, M. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. J. Virol. 2006, 80, 3801–3810. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Rahman, M.; Yang, X.; Delbeke, T.; Arijs, I.; Kabue, J.P.; Muyembe, J.J.; Van Ranst, M. G8 rotavirus strains isolated in the democratic republic of Congo belong to the DS-1-like genogroup. J. Clin. Microbiol. 2006, 44, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gómara, M.; Maes, P.; Patton, J.T.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 82, 3204–3219. [Google Scholar] [CrossRef]
- Taniguchi, K.; Urasawa, T.; Kobayashi, N.; Gorziglia, M.; Urasawa, S. Nucleotide sequence of VP4 and VP7 genes of human rotaviruses with subgroup I specificity and long RNA pattern: Implication for new G serotype specificity. J. Virol. 1990, 64, 5640–5644. [Google Scholar] [CrossRef]
- Griffin, D.D.; Nakagomi, T.; Hoshino, Y.; Nakagomi, O.; Kirkwood, C.D.; Parashar, U.D.; Glass, R.I.; Gentsch, J.R. National Rotavirus Surveillance System. Characterization of nontypeable rotavirus strains from the United States: Identification of a new rotavirus reassortant (P2A[6],G12) and rare P3[9] strains related to bovine rotaviruses. Virology 2002, 294, 256–269. [Google Scholar] [CrossRef][Green Version]
- Castello, A.A.; Argüelles, M.H.; Rota, R.P.; Olthoff, A.; Jiang, B.; Glass, R.I.; Gentsch, J.R.; Glikmann, G. Molecular epidemiology of group A rotavirus diarrhea among children in Buenos Aires, Argentina, from 1999 to 2003 and emergence of the infrequent genotype G12. J. Clin. Microbiol. 2006, 44, 2046–2050. [Google Scholar] [CrossRef]
- Das, S.; Varghese, V.; Chaudhury, S.; Barman, P.; Mahapatra, S.; Kojima, K.; Bhattacharya, S.K.; Krishnan, T.; Ratho, R.K.; Chhotray, G.P.; et al. Emergence of novel human group A rotavirus G12 strains in India. J. Clin. Microbiol. 2003, 41, 2760–2762. [Google Scholar] [CrossRef]
- Wakuda, M.; Nagashima, S.; Kobayashi, N.; Pongsuwanna, Y.; Taniguchi, K. Serologic and genomic characterization of a G12 human rotavirus in Thailand. J. Clin. Microbiol. 2003, 41, 5764–5769. [Google Scholar] [CrossRef]
- Shinozaki, K.; Okada, M.; Nagashima, S.; Kaiho, I.; Taniguchi, K. Characterization of human rotavirus strains with G12 and P[9] detected in Japan. J. Med. Virol. 2004, 73, 612–616. [Google Scholar] [CrossRef]
- Samajdar, S.; Varghese, V.; Barman, P.; Ghosh, S.; Mitra, U.; Dutta, P.; Bhattacharya, S.K.; Narasimham, M.V.; Panda, P.; Krishnan, T.; et al. Changing pattern of human group A rotaviruses: Emergence of G12 as an important pathogen among children in eastern India. J. Clin. Virol. 2006, 36, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Pandey, B.D.; Sherchand, J.B.; Ahmed, K.; Yokoo, M.; Nakagomi, T.; Cuevas, L.E.; Cunliffe, N.A.; Hart, C.A.; Nakagomi, O. Molecular epidemiology of rotavirus diarrhea among children and adults in Nepal: Detection of G12 strains with P[6] or P[8] and a G11P[25] strain. J. Clin. Microbiol. 2006, 44, 3499–3505. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Ramani, S.; Primrose, B.; Iturriza-Gómara, M.; Gray, J.J.; Brown, D.W.; Kang, G. Modification of rotavirus multiplex RT-PCR for the detection of G12 strains based on characterization of emerging G12 rotavirus strains from South India. J. Med. Virol. 2007, 79, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Pun, S.B.; Nakagomi, T.; Sherchand, J.B.; Pandey, B.D.; Cuevas, L.E.; Cunliffe, N.A.; Hart, C.A.; Nakagomi, O. Detection of G12 human rotaviruses in Nepal. Emerg. Infect. Dis. 2007, 13, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Matthijnssens, J.; Yang, X.; Delbeke, T.; Arijs, I.; Taniguchi, K.; Iturriza-Gómara, M.; Iftekharuddin, N.; Azim, T.; Van Ranst, M. Evolutionary history and global spread of the emerging g12 human rotaviruses. J. Virol. 2007, 81, 2382–2390. [Google Scholar] [CrossRef]
- Ramani, S.; Banerjee, I.; Gladstone, B.P.; Sarkar, R.; Selvapandian, D.; Le Fevre, A.M.; Jaffar, S.; Iturriza-Gómara, M.; Gray, J.J.; Estes, M.K.; et al. Geographic information systems and genotyping in identification of rotavirus G12 infections in residents of an urban slum with subsequent detection in hospitalized children: Emergence of G12 genotype in South India. J. Clin. Microbiol. 2007, 45, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Sharma, S.; Agarwal, R.K.; Longmei, K.; Gentsch, J.R.; Paul, V.K.; Glass, R.I.; Bhan, M.K. First detection of G12 rotaviruses in newborns with neonatal rotavirus infection at all India Institute of Medical Sciences, New Delhi, India. J. Clin. Microbiol. 2007, 45, 3824–3827. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Le, V.P.; Kim, J.Y.; Cho, S.L.; Nam, S.W.; Lim, I.; Lee, H.J.; Kim, K.; Chung, S.I.; Song, W.; Lee, K.M.; et al. Detection of unusual rotavirus genotypes G8P[8] and G12P[6] in South Korea. J. Med. Virol. 2008, 80, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ray, P.; Gentsch, J.R.; Glass, R.I.; Kalra, V.; Bhan, M.K. Emergence of G12 rotavirus strains in Delhi, India, in 2000 to 2007. J. Clin. Microbiol. 2008, 46, 1343–1348. [Google Scholar] [CrossRef]
- Alam, M.M.; Malik, S.A.; Shaukat, S.; Naeem, A.; Sharif, S.; Angez, M.; Rana, M.S.; Khurshid, A.; Zaidi, S.Z. Genetic characterization of rotavirus subtypes in Pakistan-first report of G12 genotype from Pakistan under WHO-Eastern Mediterranean region. Virus Res. 2009, 144, 280–284. [Google Scholar] [CrossRef]
- Ogden, K.M.; Tan, Y.; Akopov, A.; Stewart, L.S.; McHenry, R.; Fonnesbeck, C.J.; Piya, B.; Carter, M.H.; Fedorova, N.B.; Halpin, R.A.; et al. Multiple introductions and antigenic mismatch with vaccines may contribute to increased predominance of G12P[8] rotaviruses in the United States. J. Virol. 2018, 93, e01476-18. [Google Scholar] [CrossRef] [PubMed]
- Japhet, M.O.; Famurewa, O.; Iturriza-Gómara, M.; Adesina, O.A.; Opaleye, O.O.; Niendorf, S.; Bock, C.T.; Mas Marques, A. Group A rotaviruses circulating prior to a national immunization programme in Nigeria: Clinical manifestations, high G12P[8] frequency, intra-genotypic divergence of VP4 and VP7. J. Med. Virol. 2018, 90, 239–249. [Google Scholar] [CrossRef]
- Wylie, K.M.; Stanley, K.M.; TeKippe, E.M.; Mihindukulasuriya, K.; Storch, G.A. Resurgence of rotavirus genotype G12 in St. Louis during the 2014–2015 rotavirus season. J. Pediatric. Infect. Dis. Soc. 2017, 6, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Sherchand, J.B.; Pokhrel, B.M.; Parajuli, K.; Shah, N.; Mishra, S.K.; Sharma, S.; Kattel, H.P.; Khadka, S.; Khatiwada, S.; et al. Molecular epidemiology of Rotavirus causing diarrhea among children less than five years of age visiting national level children hospitals, Nepal. BMC Pediatr. 2017, 17, 101. [Google Scholar] [CrossRef]
- Da Silva, M.F.; Fumian, T.M.; de Assis, R.M.; Fialho, A.M.; Carvalho-Costa, F.A.; da Silva Ribeiro de Andrade, J.; Leite, J.P. VP7 and VP8* genetic characterization of group A rotavirus genotype G12P[8]: Emergence and spreading in the Eastern Brazilian coast in 2014. J. Med. Virol. 2017, 89, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Tanmoy, A.M.; Ahmed, A.N.; Arumugam, R.; Hossain, B.; Marzan, M.; Saha, S.; Arifeen, S.E.; Baqui, A.H.; Black, R.E.; Kang, G.; et al. Rotavirus surveillance at a WHO-coordinated invasive bacterial disease surveillance site in Bangladesh: A feasibility study to integrate two surveillance systems. PLoS ONE 2016, 11, e0153582. [Google Scholar] [CrossRef]
- Langa, J.S.; Thompson, R.; Arnaldo, P.; Resque, H.R.; Rose, T.; Enosse, S.M.; Fialho, A.; de Assis, R.M.; da Silva, M.F.; Leite, J.P. Epidemiology of rotavirus A diarrhea in Chokwe, Southern Mozambique, from February to September, 2011. J. Med. Virol. 2016, 88, 1751–1758. [Google Scholar] [CrossRef]
- Rahman, M.; Sultana, R.; Ahmed, G.; Nahar, S.; Hassan, Z.M.; Saiada, F.; Podder, G.; Faruque, A.S.; Siddique, A.K.; Sack, D.A.; et al. Prevalence of G2P[4] and G12P[6] rotavirus, Bangladesh. Emerg. Infect. Dis. 2007, 13, 18–24. [Google Scholar] [CrossRef]
- Gómez, M.M.; Resque, H.R.; Volotão Ede, M.; Rose, T.L.; da Silva, M.F.; Heylen, E.; Zeller, M.; Matthijnssens, J.; Leite, J.P. Distinct evolutionary origins of G12P[8] and G12P[9] group A rotavirus strains circulating in Brazil. Infect. Genet. Evol. 2014, 28, 385–388. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.H.; Kobayashi, N.; Zhou, D.J.; Yang, Z.Q.; Zhou, X.; Peng, J.S.; Zhu, Z.R.; Zhao, D.F.; Liu, M.Q.; Gong, J. Molecular epidemiologic analysis of group A rotaviruses in adults and children with diarrhea in Wuhan city, China, 2000–2006. Arch. Virol. 2007, 152, 669–685. [Google Scholar] [CrossRef]
- Wang, Y.H.; Kobayashi, N.; Zhou, X.; Nagashima, S.; Zhu, Z.R.; Peng, J.S.; Liu, M.Q.; Hu, Q.; Zhou, D.J.; Watanabe, S.; et al. Phylogenetic analysis of rotaviruses with predominant G3 and emerging G9 genotypes from adults and children in Wuhan, China. J. Med. Virol. 2009, 81, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Zhou, X.; Ghosh, S.; Zhou, D.J.; Pang, B.B.; Peng, J.S.; Hu, Q.; Kobayashi, N. Prevalence of human rotavirus genotypes in Wuhan, China, during 2008–2011: Changing trend of predominant genotypes and emergence of strains with the P[8]b subtype of the VP4 gene. Arch. Virol. 2011, 156, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, N.; Antoni, S.; Mwenda, J.M.; Weldegebriel, G.; Biey, J.N.M.; Cheikh, D.; Fahmy, K.; Teleb, N.; Ashmony, H.A.; Ahmed, H.; et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–2016: Findings from the Global Rotavirus Surveillance Network. Lancet. Glob. Health 2019, 7, e893–e903. [Google Scholar] [CrossRef]
- Lestari, F.B.; Vongpunsawad, S.; Wanlapakorn, N.; Poovorawan, Y. Rotavirus infection in children in Southeast Asia 2008–2018: Disease burden, genotype distribution, seasonality, and vaccination. J. Biomed. Sci. 2020, 27, 66. [Google Scholar] [CrossRef]
- Anderson, E.J.; Weber, S.G. Rotavirus infection in adults. Lancet. Infect. Dis. 2004, 4, 91–99. [Google Scholar] [CrossRef]
- Svenungsson, B.; Lagergren, A.; Ekwall, E. Enteropathogens in adult patients with diarrhea and healthy control subjects: A 1-year prospective study in a Swedish clinic for infectious diseases. Clin. Infect. Dis. 2000, 30, 770–778. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Chuchaona, W.; Lestari, F.B.; Pasittungkul, S.; Klinfueng, S.; Wanlapakorn, N.; Vongpunsawad, S.; Chirathaworn, C.; Poovorawan, Y. High prevalence of DS-1-like rotavirus infection in Thai adults between 2016 and 2019. PLoS ONE 2020, 15, e0235280. [Google Scholar] [CrossRef]
- Xiao, N.; Wu, J.Y.; Zhou, Y.; Sun, M.S.; Li, H.J. Epidemiological and clinical studies of rotavirus-induced diarrhea in China from 1994–2013. Hum. Vaccin. Immunother. 2014, 10, 3672–3680. [Google Scholar]
- Yu, J.; Lai, S.; Geng, Q.; Ye, C.; Zhang, Z.; Zheng, Y.; Wang, L.; Duan, Z.; Zhang, J.; Wu, S.; et al. Prevalence of rotavirus and rapid changes in circulating rotavirus strains among children with acute diarrhea in China, 2009–2015. J. Infect. 2019, 78, 66–74. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Jia, L.; Payne, D.C.; Hall, A.J.; Xu, Z.; Gao, Z.; Chang, Z.; Jiang, B.; Parashar, U.D.; et al. Active, population-based surveillance for rotavirus gastroenteritis in Chinese children: Beijing Municipality and Gansu Province, China. Pediatr. Infect. Dis. J. 2015, 34, 40–46. [Google Scholar] [CrossRef]
- Tian, Y.; Chughtai, A.A.; Gao, Z.; Yan, H.; Chen, Y.; Liu, B.; Huo, D.; Jia, L.; Wang, Q.; MacIntyre, C.R. Prevalence and genotypes of group A rotavirus among outpatient children under five years old with diarrhea in Beijing, China, 2011–2016. BMC Infect Dis. 2018, 18, 497. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fu, J.; Ai, J.; Zhang, J.; Liu, C.; Huo, X.; Bao, C.; Zhu, Y. Phylogenetic analysis of human G9P[8] rotavirus strains circulating in Jiangsu, China between 2010 and 2016. J. Med. Virol. 2018, 90, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Liu, P. Clinical and molecular epidemiologic trends reveal the important role of rotavirus in adult infectious gastroenteritis, in Shanghai, China. Infect. Genet. Evol. 2016, 47, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Shen, X.J.; Xia, S.L.; Zhang, B.F.; Mu, Y.J.; Huang, X.Y.; Xu, B.L. Infection status, clinical symptoms and gene type transition of group A rotavirus in children, less than five years-of-age, with diarrhea in sentinel hospitals of Henan Province, China. Zhonghua Yu Fang Yi Xue Za Zhi 2017, 51, 82–86. [Google Scholar]
- Kang, Y.; Cai, Y. Epidemiology and Genetic Diversity of Rotavirus in Kunming, China, in 2015. Intervirology 2018, 61, 9–13. [Google Scholar] [CrossRef]
- Wu, B.S.; Huang, Z.M.; Weng, Y.W.; Chen, F.Q.; Zhang, Y.L.; Lin, W.D.; Yu, T.T. Prevalence and genotypes of rotavirus A and human adenovirus among hospitalized children with acute gastroenteritis in Fujian, China, 2009–2017. Biomed. Environ. Sci. 2019, 32, 210–214. [Google Scholar]
- Zeng, Y.; Li, T.; Zhao, B.; Lai, F.; Tang, X.; Qiao, Y.; Chen, W.; Yu, F.; Zhang, S.; Wang, Y.; et al. Molecular epidemiology of group A rotavirus in outpatient diarrhea infants and children in Chongqing, China, 2011-2015. J. Med. Virol. 2019, 91, 1788–1796. [Google Scholar] [CrossRef]
- Sadiq, A.; Bostan, N.; Yinda, K.C.; Naseem, S.; Sattar, S. Rotavirus: Genetics, pathogenesis and vaccine advances. Rev. Med. Virol. 2018, 28, e2003. [Google Scholar] [CrossRef]
- Shintani, T.; Ghosh, S.; Wang, Y.H.; Zhou, X.; Zhou, D.J.; Kobayashi, N. Whole genomic analysis of human G1P[8] rotavirus strains from different age groups in China. Viruses 2012, 4, 1289–1304. [Google Scholar] [CrossRef]
- Wang, Y.H.; Pang, B.B.; Ghosh, S.; Zhou, X.; Shintani, T.; Urushibara, N.; Song, Y.W.; He, M.Y.; Liu, M.Q.; Tang, W.F.; et al. Molecular epidemiology and genetic evolution of the whole genome of G3P[8] human rotavirus in Wuhan, China, from 2000 through 2013. PLoS ONE 2014, 9, e88850. [Google Scholar] [CrossRef]
- Dian, Z.; Wang, B.; Fan, M.; Dong, S.; Feng, Y.; Zhang, A.M.; Liu, L.; Niu, H.; Li, Y.; Xia, X. Completely genomic and evolutionary characteristics of human-dominant G9P[8] group A rotavirus strains in Yunnan, China. J. Gen. Virol. 2017, 98, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chen, F.; Zhou, T.; Chen, J.Y.; Zheng, T.L.; Xu, X.; Pei, X.F. Prevalence and clinical profile of rotavirus A infection among diarrhoeal children and phylogenetic analysis with vaccine strains in Chengdu, West China, 2009–2014. Trop. Med. Int. Health 2018, 23, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, H.; Li, W.; Wang, X.; Tao, X.; Dou, L.; Dong, Y.; Wu, N.; Li, Y.G. Characterization of a G9 group A rotavirus reassortant strain detected in Jinzhou, China, in 2018–2019. Arch. Virol. 2020, 165, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Oda, M.; Somura, Y.; Shinkai, T. Molecular characteristics of novel mono-reassortant G9P[8] rotavirus A strains possessing the NSP4 gene of the E2 genotype detected in Tokyo, Japan. Jpn. J. Infect. Dis. 2020, 73, 26–35. [Google Scholar] [CrossRef]
- Kobayashi, N.; Lintag, I.C.; Urasawa, T.; Taniguchi, K.; Saniel, M.C.; Urasawa, S. Unusual human rotavirus strains having subgroup I specificity and “long” RNA electropherotype. Arch. Virol. 1989, 109, 11–23. [Google Scholar] [CrossRef]
- Herring, A.J.; Inglis, N.F.; Ojeh, C.K.; Snodgrass, D.R.; Menzies, J.D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J. Clin. Microbiol. 1982, 16, 473–477. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef]
- Iturriza-Gómara, M.; Kang, G.; Gray, J. Rotavirus genotyping: Keeping up with an evolving population of human rotaviruses. J. Clin. Virol. 2004, 31, 259–265. [Google Scholar] [CrossRef]
- Nagashima, S.; Kobayashi, N.; Paul, S.K.; Ghosh, S.; Chawla-Sarkar, M.; Hossain, M.A.; Krishnan, T. Identification of P[8]b subtype in OP354-like human rotavirus strains by a modified RT-PCR method. Jpn. J. Infect. Dis. 2010, 63, 208–211. [Google Scholar]
- Maes, P.; Matthijnssens, J.; Rahman, M.; Van Ranst, M. RotaC: A web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol. 2009, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
| Strains | Collection Date (YYYYMM) | Gender (F/M) | Age (Y/M/D) | Genotype 1 |
|---|---|---|---|---|
| VP7-VP4-VP6-VP1-VP2-VP3-NSP1-NSP2-NSP3-NSP4-NSP5 (Genbank Accession Numbers) | ||||
| RVA/Human-tc/USA/Wa/1974/G1P[8] | 1974 | - | - | G1-P[8]-I1-R1-C1-M1-A1-N1-T1-E1-H1 |
| (JX406747-JX406757) | ||||
| RVA/Human-tc/USA/DS-1/1976/G2P[4] | 1976 | - | - | G2-P[4]-I2-R2-C2-M2-A2-N2-T2-E2-H2 |
| (HQ650116-HQ650126) | ||||
| RVA/Human-wt/CHN/Z2761/2019/G12P[6] | 20190307 | F | 59y | G12-P[6]-I1-R1-C1-M1-A1-N1-T2-E1-H1 |
| (MN106117, MN106124, MN106131, MN106138, MN106145, MN106152, MN106159, MN106166, MN106173, MN106180, MN106187) | ||||
| RVA/Human-wt/CHN/E5365/2017/G1P[8] | 20170306 | M | 9m21d | G1-P[8]-I1-R1-C1-M1-A1-N1-T1-E1-H1 |
| (MN106111, MN106118, MN106125, MN106132, MN106139, MN106146, MN106153, MN106160, MN106167, MN106174, MN106181) | ||||
| RVA/Human-wt/CHN/E5867/2018/G3P[8] | 20180403 | M | 5m25d | G3-P[8]-I1-R1-C1-M1-A1-N1-T1-E1-H1 |
| (MN106112, MN106119, MN106126, MN106133, MN106140, MN106147, MN106154, MN106161, MN106168, MN106175, MN106182) | ||||
| RVA/Human-wt/CHN/E6356/2019/G9P[8] | 20190118 | M | 1y7m | G9-P[8]-I1-R1-C1-M1-A1-N1-T1-E1-H1 |
| (MN106113, MN106120, MN106127, MN106134, MN106141, MN106148, MN106155, MN106162, MN106169, MN106176, MN106183) | ||||
| RVA/Human-wt/CHN/E6398/2019/G9P[8] | 20190308 | M | 1y8m | G9-P[8]-I1-R1-C1-M1-A1-N1-T1-E2-H1 |
| (MN106114, MN106121, MN106128, MN106135, MN106142, MN106149, MN106156, MN106163, MN106170, MN106177, MN106184) | ||||
| RVA/Human-wt/CHN/L2448/2019/G9P[8] | 20190218 | F | 30y | G9-P[8]-I1-R1-C1-M1-A1-N1-T1-E2-H1 |
| (MN106115, MN106122, MN106129, MN106136, MN106143, MN106150, MN106157, MN106164, MN106171, MN106178, MN106185) | ||||
| RVA/Human-wt/CHN/Z2768/2019/G9P[8] | 20190318 | F | 55y | G9-P[8]-I1-R1-C1-M1-A1-N1-T1-E2-H1 |
| (MN106116, MN106123, MN106130, MN106137, MN106144, MN106151, MN106158, MN106165, MN106172, MN106179, MN106186) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wang, Y.-H.; Pang, B.-B.; Chen, N.; Kobayashi, N. Surveillance of Human Rotavirus in Wuhan, China (2011–2019): Predominance of G9P[8] and Emergence of G12. Pathogens 2020, 9, 810. https://doi.org/10.3390/pathogens9100810
Zhou X, Wang Y-H, Pang B-B, Chen N, Kobayashi N. Surveillance of Human Rotavirus in Wuhan, China (2011–2019): Predominance of G9P[8] and Emergence of G12. Pathogens. 2020; 9(10):810. https://doi.org/10.3390/pathogens9100810
Chicago/Turabian StyleZhou, Xuan, Yuan-Hong Wang, Bei-Bei Pang, Nan Chen, and Nobumichi Kobayashi. 2020. "Surveillance of Human Rotavirus in Wuhan, China (2011–2019): Predominance of G9P[8] and Emergence of G12" Pathogens 9, no. 10: 810. https://doi.org/10.3390/pathogens9100810
APA StyleZhou, X., Wang, Y.-H., Pang, B.-B., Chen, N., & Kobayashi, N. (2020). Surveillance of Human Rotavirus in Wuhan, China (2011–2019): Predominance of G9P[8] and Emergence of G12. Pathogens, 9(10), 810. https://doi.org/10.3390/pathogens9100810





