Seroprevalence and Molecular Detection of Bovine Anaplasmosis in Egypt
Abstract
1. Introduction
2. Materials and Methods
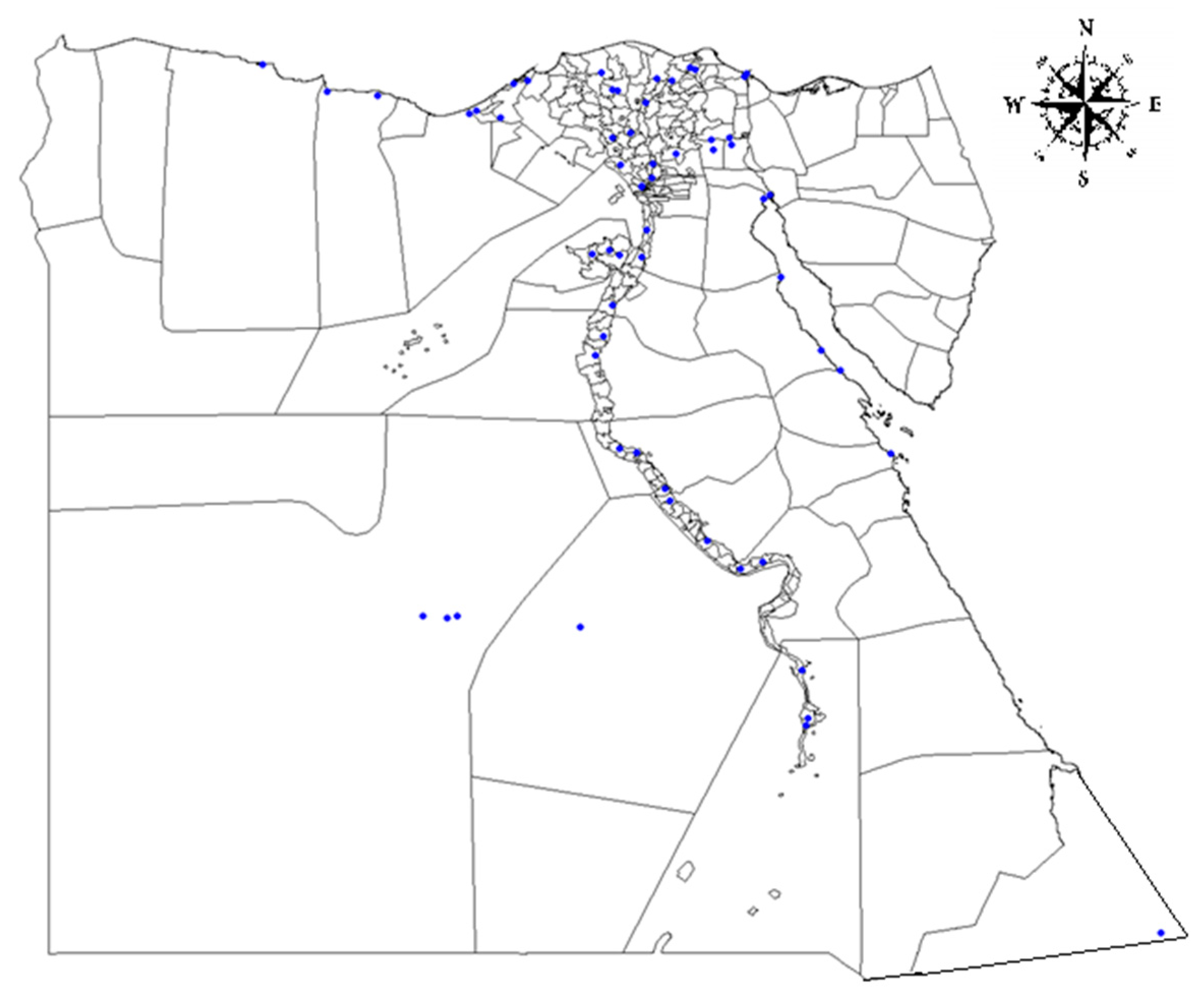
2.1. Study Area and Sample Information
2.2. Detection of Anaplasma spp.-Specific Antibodies Using cELISA
2.3. Detection of Anaplasma spp./Ehrlichia spp. DNA Using Real Time PCR
2.4. Statistical Analysis
2.5. Ethical Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Theiler, A. Gall-sickness of South Africa. (Anaplasmosis of Cattle.). J. Comp. Pathol. Therap. 1910, 23, 98–115. [Google Scholar] [CrossRef]
- Ueti, M.W.; Reagan, J.O.J.; Knowles, D.P.J.; Scoles, G.A.; Shkap, V.; Palmer, G.H. Identification of midgut and salivary glands as specific and distinct barriers to efficient tick-borne transmission of Anaplasma marginale. Infect. Immun. 2007, 75, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Rikihisa, Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Merck. The Veterinary Manual: Anaplasmosis. Available online: https://www.msdvetmanual.com/circulatory-system/blood-parasites/anaplasmosis (accessed on 31 July 2019).
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S.A. The natural history of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef] [PubMed]
- OIE. Bovine Anaplasmosis. Available online: http://www.oie.int/standard-setting/terrestrial-manual/access-online/ (accessed on 17 June 2019).
- Aubry, P.; Geale, D.W. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Diseases of the Hemolymphatic and Immune Systems. In Veterinary Medicine, 11th ed.; Saunders, W.B., Ed.; Saunders Ltd.: London, UK, 2017. [Google Scholar] [CrossRef]
- Ioannou, I.; Chochlakis, D.; Kasinis, N.; Anayiotos, P.; Lyssandrou, A.; Papadopoulos, B.; Tselentis, Y.; Psaroulaki, A. Carriage of Rickettsia spp., Coxiella burnetii and Anaplasma spp. by endemic and migratory wild birds and their ectoparasites in Cyprus. Clin. Microbiol. Infect. 2009, 15, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Mărcuţan, I.D.; Sándor, A.D.; Mihalca, A.D.; Gherman, C.M.; Kalmár, Z.; D’Amico, G.; Dumitrache, M.O.; Cozma, V. Prevalence of Anaplasma phagocytophilum in ticks collected from migratory birds in Danube Delta, Romania. Parasites Vectors 2014, 7, P16–P16. [Google Scholar] [CrossRef]
- Ybañez, A.P.; Inokuma, H. Anaplasma species of veterinary importance in Japan. Vet. World 2016, 9, 1190–1196. [Google Scholar] [CrossRef][Green Version]
- Henniger, T.; Henniger, P.; Grossmann, T.; Distl, O.; Ganter, M.; von Loewenich, F.D. Congenital infection with Anaplasma phagocytophilum in a calf in northern Germany. Acta Vet. Scand. 2013, 55, 38. [Google Scholar] [CrossRef]
- Silaghi, C.; Santos, A.S.; Gomes, J.; Christova, I.; Matei, I.A.; Walder, G.; Domingos, A.; Bell-Sakyi, L.; Sprong, H.; von Loewenich, F.D.; et al. Guidelines for the Direct Detection of Anaplasma spp. in Diagnosis and Epidemiological Studies. Vector Borne Zoonotic Dis. 2017, 17, 12–22. [Google Scholar] [CrossRef]
- McDaniel, C.J.; Cardwell, D.M.; Moeller, R.B.J.; Gray, G.C. Humans and cattle: A review of bovine zoonoses. Vector Borne Zoonotic Dis. 2014, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Annen, K.; Friedman, K.; Eshoa, C.; Horowitz, M.; Gottschall, J.; Straus, T. Two cases of transfusion-transmitted Anaplasma phagocytophilum. Am. J. Clin. Pathol. 2012, 137, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Jereb, M.; Pecaver, B.; Tomazic, J.; Muzlovic, I.; Avsic-Zupanc, T.; Premru-Srsen, T.; Levicnik-Stezinar, S.; Karner, P.; Strle, F. Severe Human Granulocytic Anaplasmosis Transmitted by Blood Transfusion. Emerg. Infect. Dis. 2012, 18, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Matthew Waxman, M.D. Anaplasma phagocytophilum Transmitted Through Blood Transfusion-Minnesota 2007. Ann. Emerg. Med. 2009, 53, 643–645. [Google Scholar] [CrossRef]
- Quinn, P.J. Rickettsiales and Coxiella burnetii. In Concise Review of Veterinary Microbiology, 2nd ed.; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Chung, C.; Wilson, C.; Bandaranayaka-Mudiyanselage, C.B.; Kang, E.; Adams, D.S.; Kappmeyer, L.S.; Knowles, D.P.; McElwain, T.F.; Evermann, J.F.; Ueti, M.W.; et al. Improved diagnostic performance of a commercial Anaplasma antibody competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5–glutathione S-transferase fusion protein as antigen. J. Vet. Diagn. Investig. 2013, 26, 61–71. [Google Scholar] [CrossRef]
- Kocan, K.M.; Blouin, E.F.; Barbet, A.F. Anaplasmosis control. Past, present, and future. Ann. N. Y. Acad. Sci. 2000, 916, 501–509. [Google Scholar] [CrossRef]
- Kopáček, P.; Hajdušek, O.; Burešová, V.; Daffre, S. Tick Innate Immunity. In Invertebrate Immunity; Söderhäll, K., Ed.; Springer: Boston, MA, USA, 2010; pp. 137–162. [Google Scholar] [CrossRef]
- Merino, O.; Alberdi, P.; Pérez de la Lastra, J.M.; de la Fuente, J. Tick vaccines and the control of tick-borne pathogens. Front. Cell. Infect. Microbiol. 2013, 3, 30–30. [Google Scholar] [CrossRef]
- Hove, P.; Khumalo, Z.T.H.; Chaisi, M.E.; Oosthuizen, M.C.; Brayton, K.A.; Collins, N.E. Detection and Characterisation of Anaplasma marginale and A. centrale in South Africa. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Okafor, C.C.; Collins, S.L.; Daniel, J.A.; Harvey, B.; Sun, X.; Coetzee, J.F.; Whitlock, B.K. Factors associated with Seroprevalence of Anaplasma marginale in Kentucky cattle. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 212–219. [Google Scholar] [CrossRef]
- Marcondes, C.B. Anaplasmosis. In Arthropod Borne Diseases; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- CAPMAS. Animal Diseases. Available online: https://www.capmas.gov.eg/ (accessed on 17 June 2019).
- El-Ashker, M.; Hotzel, H.; Gwida, M.; El-Beskawy, M.; Silaghi, C.; Tomaso, H. Molecular biological identification of Babesia, Theileria, and Anaplasma species in cattle in Egypt using PCR assays, gene sequence analysis and a novel DNA microarray. Vet. Parasitol. 2015, 207, 329–334. [Google Scholar] [CrossRef]
- El-Ashker, M.; Salama, M.; El-Sebaei, M.; Risha, E.; Abdelhamid, F.; El-Diasty, M.; El-Fadle, E. Significance of clinical variables and selected biochemical markers in predicting the outcome of bovine anaplasmosis. Veterinární Med. 2016, 60, 301–308. [Google Scholar] [CrossRef]
- Radwan, M.E.I.; Ali, A.; Abd elhamied, O. Epidemiological Studies, Molecular Diagnosis of Anaplasma marginale in Cattle and Biochemical Changes Associated with it in Kaliobia Governorate. Am. J. Infect. Dis. Microbiol. 2013, 1, 46–49. [Google Scholar] [CrossRef][Green Version]
- Abdel Hamid, O.M.; Radwan, M.E.I.; Ali, A. Biochemical changes associated with Anaplasma infection in cattle. Glob. J. Biotechnol. Biochem. 2014, 9, 19–23. [Google Scholar] [CrossRef]
- Elhariri, M.D.; Elhelw, R.A.; Hamza, D.A.; Soliman, D.E. Molecular detection of Anaplasma marginale in the Egyptian water bufaloes (Bubuloes bubalis) based on major surface protein 1α. J. Egyp. Soc. Parasitol. 2017, 47, 247–252. [Google Scholar]
- El-Naga, T.R.; Barghash, S.M. Blood Parasites in Camels (Camelus dromedarius) in Northern West Coast of Egypt. J. Bacteriol. Parasitol. 2016, 7. [Google Scholar] [CrossRef]
- Fereig, R.M.; Mohamed, S.G.A.; Mahmoud, H.; AbouLaila, M.R.; Guswanto, A.; Nguyen, T.T.; Ahmed Mohamed, A.E.; Inoue, N.; Igarashi, I.; Nishikawa, Y. Seroprevalence of Babesia bovis, B. bigemina, Trypanosoma evansi, and Anaplasma marginale antibodies in cattle in southern Egypt. Ticks Tick Borne Dis. 2017, 8, 125–131. [Google Scholar] [CrossRef] [PubMed]
- El-Naga, T.R.; Mahmoud, M.A.; Osman., W.A.; Goda, A.S.A. Serological survey of Anaplasma marginale (Rickettsia) antibodies in animal by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. Suez Canal Vet. Med. J. (SCVMJ) 2009, 19, 309–319. [Google Scholar]
- Younis, E.E.; Hegazy, N.A.M.; El-Deeb, W.; El-Khatib, R.M. Epidemiological and biochemical studies on bovine anaplamosis in dakahlia and demiatta governorates in Egypt. Bull. Anim. Health Prod. Afr. 2009, 57. [Google Scholar] [CrossRef]
- Salm, F.F.; Younis, E.E.; Hegazy, N.M.; El-Sawalhy, A.A. Epidemiological studies on bovine anaplasmosis. In Bulletin of Animal Health and Production in Africa; Inter-African Bureau for Animal Resources: Nairobi, Kenya, 2011; pp. 179–189. [Google Scholar]
- Loftis, A.D.; Reeves, W.K.; Szumlas, D.E.; Abbassy, M.M.; Helmy, I.M.; Moriarity, J.R.; Dasch, G.A. Rickettsial agents in Egyptian ticks collected from domestic animals. Exp. Appl. Acarol. 2006, 40, 67–81. [Google Scholar] [CrossRef]
- VMRD. Product Catalog. Available online: https://www.vmrd.com/ and https://www.vmrd.com/core/files/vmrd/uploads/files/VMRD%20Catalog_4_2_18.pdf (accessed on 17 July 2019).
- Stuen, S.; Nevland, S.; Moum, T. Fatal cases of Tick-borne fever (TBF) in sheep caused by several 16S rRNA gene variants of Anaplasma phagocytophilum. Ann. N. Y. Acad. Sci. 2003, 990, 433–434. [Google Scholar] [CrossRef]
- Kim, H.Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 2017, 42, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Funk, S.; Salathé, M.; Jansen, V.A.A. Modelling the influence of human behaviour on the spread of infectious diseases: A review. J. R. Soc. Interface 2010, 7, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, J.F.; Grace, D. The consequences of human actions on risks for infectious diseases: A review. Infect. Ecol. Epidemiol. 2015, 5, 30048. [Google Scholar] [CrossRef]
- Meloni, S.; Perra, N.; Arenas, A.; Gómez, S.; Moreno, Y.; Vespignani, A. Modeling human mobility responses to the large-scale spreading of infectious diseases. Sci. Rep. 2011, 1, 62. [Google Scholar] [CrossRef] [PubMed]
| Domain | Western Domain | Nile Delta | Eastern Domain | Total | |
|---|---|---|---|---|---|
| Cattle | 334 (44.06%) | 283 (37.33%) | 135 (18.2%) | 758 | |
| Animal age | ≤4 years | 175 (57.94%) | 73 (24.17%) | 54 (17.81%) | 302 (39.84%) |
| >4 years | 162 (35.52%) | 210 (46.05%) | 84 (18.42%) | 456 (60.16%) | |
| Animal husbandry | Stable/Stationary | No samples | 280 (67.63%) | 134 (32.36%) | 414 (54.61%) |
| Nomadic | 303 (97.74%) | 3 (0.96%) | 4 (1.29%) | 310 (40.89%) | |
| Nomadic & Pasture | 34 | (-) | (-) | 34 (4.48%) | |
| Tick infestation | 193 (45.62%) | 149 (36.69%) | 81 (19.14%) | 423 (55.8%) | |
| Cattle kept in spatial separate | (-) | 280 (68.96%) | 126 (31.03%) | 406 (53.56%) | |
| Others animal species living on farm | 8 (32%) | 15 (60%) | 2 (8%) | 25 (3.29%) | |
| Domain | Governorate | No. of Animals Tested | No. of Farms (Positive) | Prevalence No. (%) | Co-detection of Coxiella and Anaplasma | |
|---|---|---|---|---|---|---|
| cELISA | PCR | |||||
| Western Area | Matrouh | 167 | 4 (4) | 25 (15) | 7 (4.2) | 3 |
| New valley | 170 | 6 (5) | 36 (21.6) | 9 (5.3) | 8 | |
| Eastern Area | Red Sea | 138 | 4 (3) | 25 (18.5) | 4 (2.9) | 10 |
| Nile Valley and Delta Area | Alexandria | 9 | 3 (1) | 1 (11.1) | 0 | 0 |
| Assiut | 33 | 2 (2) | 10 (30.3) | 2 (6.1) | 2 | |
| Aswan | 58 | 3 (1) | 3 (5.2) | 2 (3.4) | 2 | |
| Cairo | 12 | 2 (1) | 2 (16.7) | 0 | 0 | |
| Dakahlia | 11 | 2 (1) | 2 (18.2) | 1 (9.1) | 0 | |
| Damietta | 12 | 2 (2) | 3 (25) | 3 (25) | 0 | |
| Fayoum | 9 | 3 (2) | 2 (22.2) | 0 | 0 | |
| Gharbia | 2 | 1 (1) | 2 (100) | 2 (100) | 0 | |
| Ismailia | 7 | 4 (2) | 2 (28.5) | 0 | 0 | |
| Minya | 12 | 2 (1) | 1 (8.3) | 1 (8.3) | 0 | |
| Port Said | 12 | 2 (2) | 4 (33.3) | 3 (25) | 0 | |
| Qena | 22 | 3 (2) | 11 (50) | 3 (13.6) | 0 | |
| Sohag | 21 | 2 (1) | 1 (4.8) | 0 | 1 | |
| Suez | 12 | 2 (2) | 10 (83.3) | 3 (25) | 4 | |
| Beheira | 1 | 1 (0) | 0 | 0 | 0 | |
| Beni-Suef | 22 | 2 (0) | 0 | 0 | 0 | |
| Giza | 9 | 3 (0) | 0 | 0 | 0 | |
| Kafr El Sheikh | 7 | 3 (0) | 0 | 0 | 0 | |
| Menoufia | 9 | 3 (0) | 0 | 0 | 0 | |
| Qualyubia | 1 | 1 (0) | 0 | 0 | 0 | |
| Sharkia | 2 | 1 (0) | 0 | 0 | 0 | |
| Total | 758 | 61 (33%) | 140 (18.5%) | 40 (5.3%) | 30 (4%) | |
| c ELISA | Real Time PCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Factor | No. of Positive Animals (No. of Suspicious Samples) | Seropositive | Odds Ratio | 95% Confidence Interval (CI) Pos. (Pos. plus Suspicious) | Chi Square (df) (p-Value) | No. of Positive Animals (Suspicious) | DNA Positive Samples | 95% Confidence Interval (CI) | Chi Square (df) (p-Value) | |||
| Proportion in Positive Animals (Suspicious) | Proportion in Total Animals (Suspicious) | |||||||||||
| Domain | Western Domain | 61 (22) | 43.57% (55%) | 61/337 = 18.10% (6.52%) | 18.10% | 1.09 | 14.1–22.6% | χ2(4) = 2.23; p = 0.69 | 16 (9) | 4.74% | 2.7–7.6% | χ2(6) = 9.01; p = 0.17 |
| Nile Delta | 54 (11) | 38.57% (27.5%) | 54/283 = 19.08% (3.88%) | 19.08% | 0.92 | 14.7–24.2% | 20 (1) | 7.1% | 4.4–10.7% | |||
| Eastern Domain | 25 (7) | 17.85 (17.5%) | 25/138 = 18.11% (5.07%) | 18.11% | 0.99 | 12.1–25.6% | 4 (3) | 2.9% | 0.8–7.3% | |||
| Total | 140 (40) | 140/758 = 18.46% (5.27%) | 18.5% | ND | 15.8–21.4% | 40 (13) | 5.3% | 3.8–7.1% | ||||
| Animal age group | ≤4 years | 56 (17) | 40% (42.5%) | 18.54% (5.62%) | 18.54% | 1.02 | 14.3–23.4% | χ2(2) = 0.144; p = 0.93 | 19 (7) | 6.3% | 3.8–9.7% | χ2(3) = 2.57; p = 0.46 |
| >4 years | 84 (23) | 60% (57.5%) | 18.42% (5.04%) | 18.42% | 0.98 | 15.0–22.3% | 21 (6) | 4.60% | 2.9–7% | |||
| Animal husbandry | Stable/Stationary | 79 (18) | 56.42% (45%) | 19.08% (4.34%) | 19.1% | 0.96 | 15.4–23.2% | χ2(6) = 8.30; p = 0.21 | 24 (4) | 5.8% | 3.7–8.5% | χ2(9) = 8.82; p = 0.69 |
| Nomadic | 51 (22) | 36.42% (55%) | 16.45% (7.09%) | 16.5% | 0.98 | 12.5–21.1% | 13 (8) | 4.2% | 2.3–7.1% | |||
| Nomadic & Pasture | 10 | 7.14% | 29.41% | 29.41% | 1.34 | 15.1–47.5% | 3 (1) | 8.8% | 1.9–23.7% | |||
| Tick infestation | 91 (27) | 65% (67.5%) | 21.51% (6.38%) | 19.45% | 1.71 | 17.7–25.7% | χ2(2) = 9.36; p = 0.009a | 26 (11) | 6.1% | 4.1–8.9% | χ2(3) = 11.74; p = 0.45 | |
| Animals kept separate | 79 (18) | 56.42% (45%) | 19.45% (4.43%) | 19.5% | 1.02 | 15.7–23.6% | χ2(2) = 1.64; p = 0.44 | 24 (4) | 5.9% | 3.8–8.7% | χ2(4) = 3.38; p = 0.33 | |
| Another animal species living on farm | 6 (1) | 25% (4%) | 24% (4%) | 24% | ND | 9.4–45.1% | ND | 1 (2) | 4% | 0.1–20.4% | ND | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvizi, O.; El-Adawy, H.; Melzer, F.; Roesler, U.; Neubauer, H.; Mertens-Scholz, K. Seroprevalence and Molecular Detection of Bovine Anaplasmosis in Egypt. Pathogens 2020, 9, 64. https://doi.org/10.3390/pathogens9010064
Parvizi O, El-Adawy H, Melzer F, Roesler U, Neubauer H, Mertens-Scholz K. Seroprevalence and Molecular Detection of Bovine Anaplasmosis in Egypt. Pathogens. 2020; 9(1):64. https://doi.org/10.3390/pathogens9010064
Chicago/Turabian StyleParvizi, Omid, Hosny El-Adawy, Falk Melzer, Uwe Roesler, Heinrich Neubauer, and Katja Mertens-Scholz. 2020. "Seroprevalence and Molecular Detection of Bovine Anaplasmosis in Egypt" Pathogens 9, no. 1: 64. https://doi.org/10.3390/pathogens9010064
APA StyleParvizi, O., El-Adawy, H., Melzer, F., Roesler, U., Neubauer, H., & Mertens-Scholz, K. (2020). Seroprevalence and Molecular Detection of Bovine Anaplasmosis in Egypt. Pathogens, 9(1), 64. https://doi.org/10.3390/pathogens9010064







