Changes in Antioxidant System during Grain Development of Wheat (Triticum aestivum L.) and Relationship with Protein Composition under FHB Stress
Abstract
1. Introduction
2. Results and Discussion
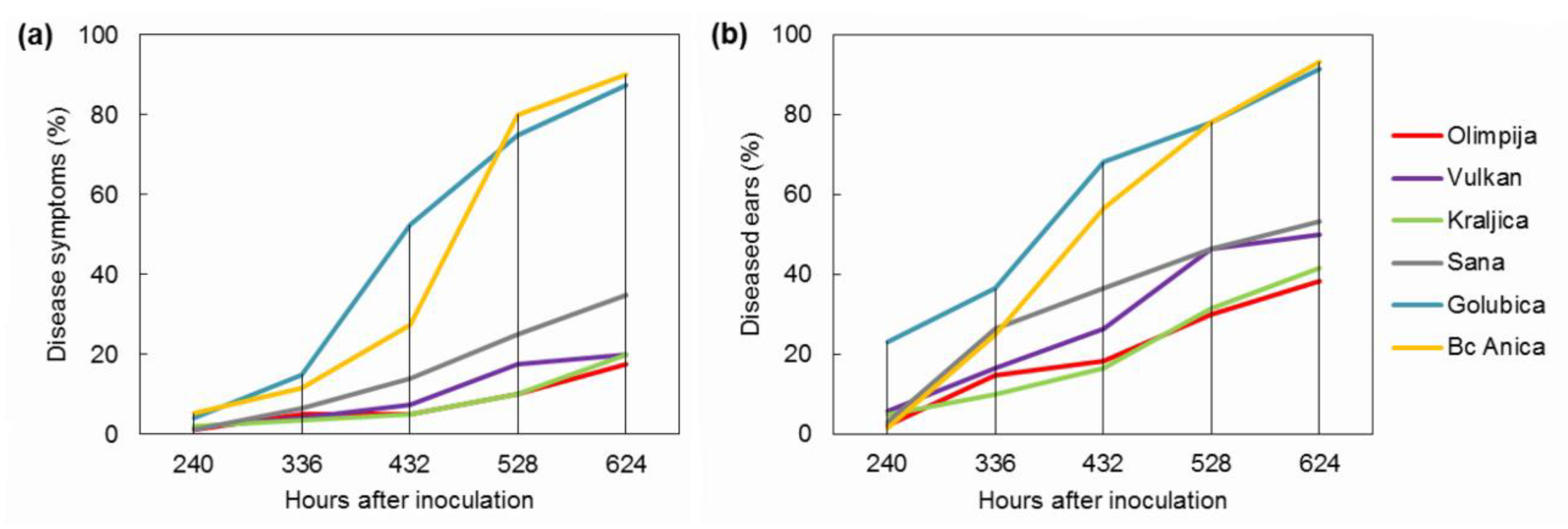
2.1. FHB Severity and Incidence
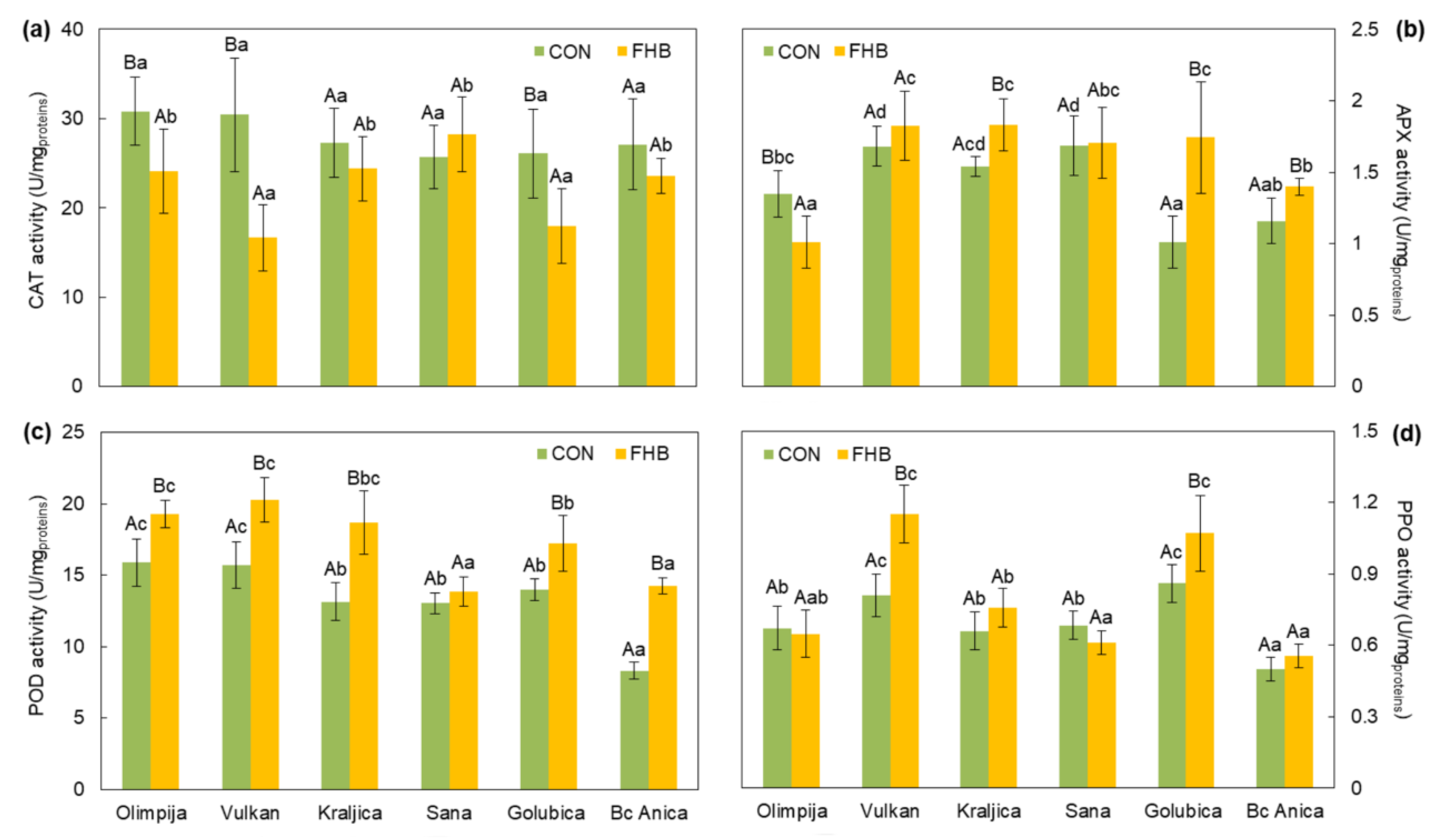
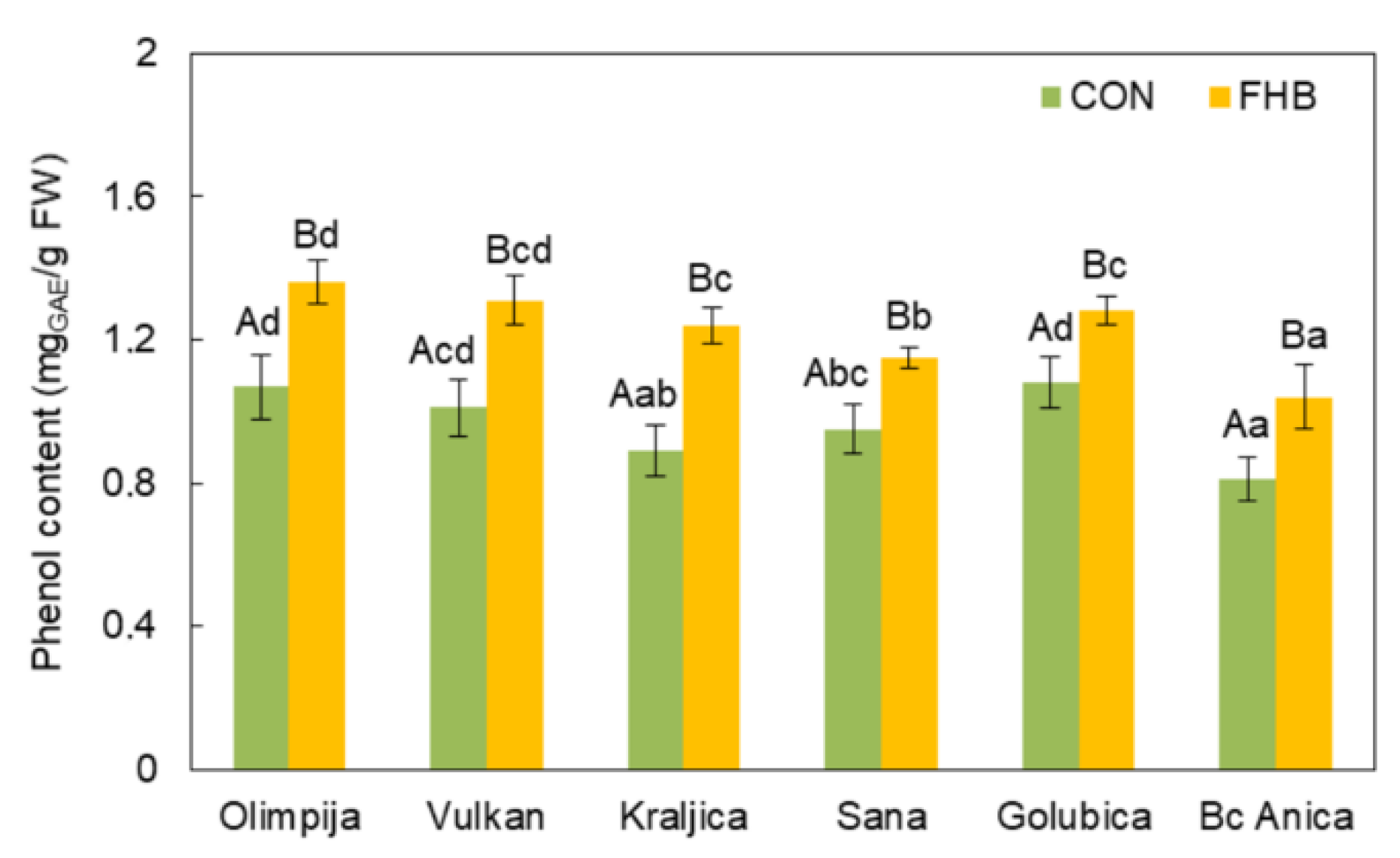
2.2. Enzymatic and Non-Enzymatic Components of Antioxidant Defense System
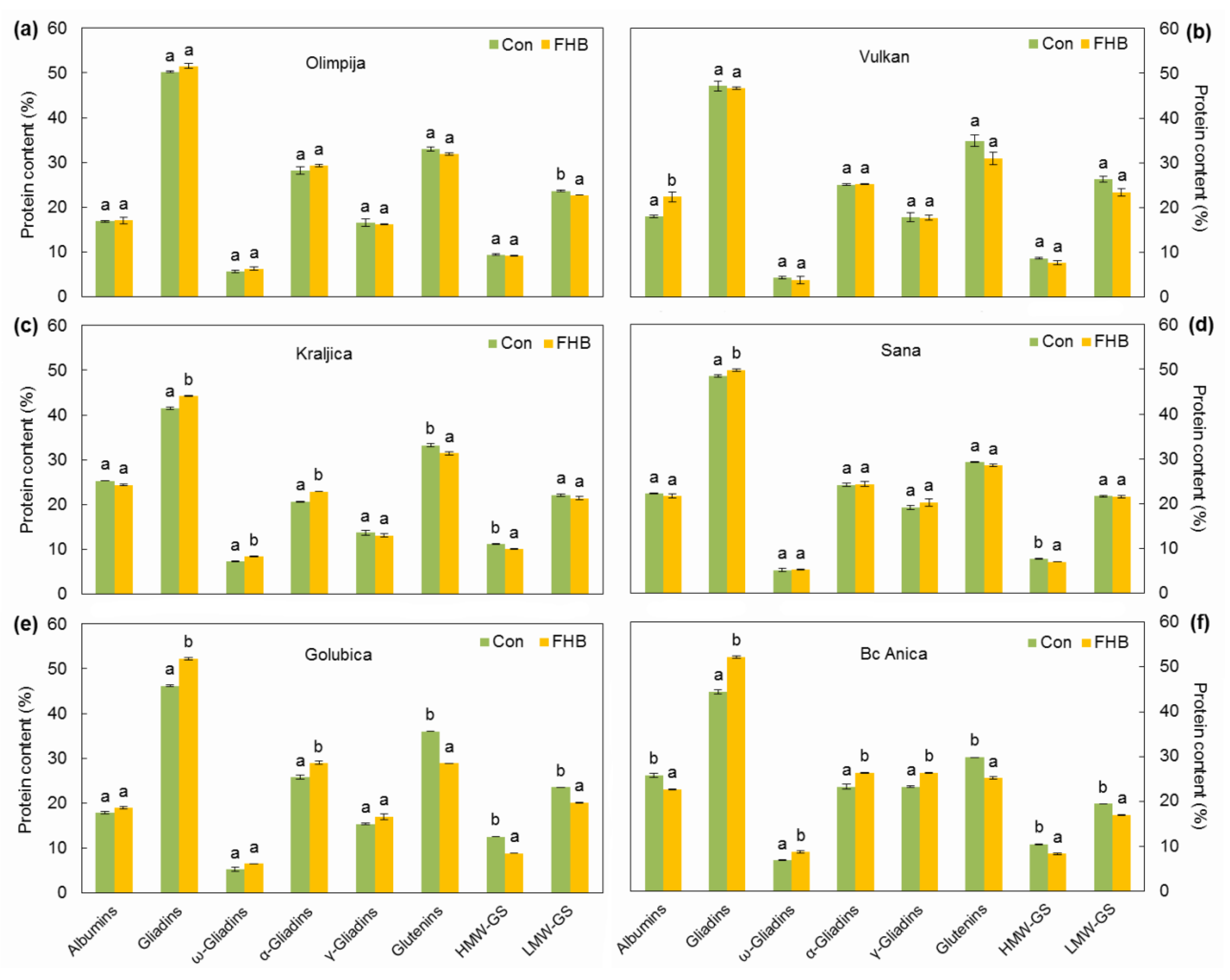
2.3. Protein Composition
3. Materials and Methods
3.1. Inoculum Production
3.2. Field Trials
3.3. Enzyme Activity
Measurements of Enzyme Activities
3.4. Determination of MDA Content and H2O2 Concentration
3.5. Determination of Phenol Content
3.6. Protein Extraction and Characterization
3.7. Deoxinivalenol (DON) Analyses
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goswami, R.; Kistler, H. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Ribichich, K.F.; Lopez, S.E.; Vegetti, A.C. Histopathological Spikelet Changes Produced by Fusarium graminearum in Susceptible and Resistant Wheat Cultivars. Plant Dis. 2000, 84, 794–802. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Deoxynivalenol. In WHO Food Additives Series 47. FAO Food and Nutrition Paper; World Health Organization: Anderlecht, Belgium, 2001; Volume 74, pp. 419–556. [Google Scholar]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A Major Player in the Multifaceted Response of Fusarium to Its Environment. Toxins 2014, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Shaner, G. Scab in wheat: Prospects for control. Plant Dis. 1994, 78, 760–766. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar] [CrossRef]
- Gorinstein, S.; Lojek, A.; Ciz, M.; Pawelzik, E.; Delgado-Licon, E.; Medina, O.J.; Moreno, M.; Arnao Salas, I.; Goshev, I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int. J. Food Sci. Technol. 2008, 43, 629–637. [Google Scholar] [CrossRef]
- Spanic, V.; Horvat, D.; Drezner, G.; Zdunic, Z. Changes in Protein Composition in the Grain and Malt after Fusarium Infection Dependently of Wheat Resistance. Pathogens 2019, 8, 112. [Google Scholar] [CrossRef]
- Anjum, F.M.; Khan, M.R.; Din, A.; Saeed, M.; Pasha, I.; Arshad, M.U. Wheat gluten: High molecular weight glutenin subunits-structure, genetics, and relation to dough elasticity. J. Food Sci. 2007, 72, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Horvat, D.; Spanic, V.; Dvojkovic, K.; Simic, G.; Magdic, D.; Nevistic, A. The Influence of Fusarium Infection on Wheat (Triticum aestivum L.) Proteins Distribution and Baking Quality. Cereal Res. Commun. 2015, 43, 61–71. [Google Scholar] [CrossRef]
- Martin, C.T.; Schöneberg, T.; Vogelgsang, S.; Vincenti, J.; Bertossa, M.; Mauch-Mani, B.; Mascher, F. Factors of wheat grain resistance to Fusarium head blight. Phytopathol. Mediterr. 2017, 56, 154–166. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Nepovimova, E.; Wang, Y.; Yang, H.; Li, L.; Zhang, X.; Kuca, K. Antioxidant agents against trichothecenes: New hints for oxidative stress treatment. Oncotarget 2017, 8, 110708–110726. [Google Scholar] [CrossRef]
- Jansen, C.; Von Wettstein, D.; Schäfer, W.; Kogel, K.H.; Felk, A.; Maier, F.J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar] [CrossRef]
- Yang, W.; Yu, M.; Fu, J.; Bao, W.; Wang, D.; Hao, L.; Yao, P.; Nüssler, A.K.; Yan, H.; Liu, L. Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food Chem. Toxicol. 2014, 64, 383–396. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant Secondary Metabolites in Cereals: Potential Involvement in Resistance to Fusarium and Mycotoxin Accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef]
- Giordano, D.; Beta, T.; Reyneri, A.; Blandino, M. Changes in the Phenolic Acid Content and Antioxidant Activity during Kernel Development of Corn (Zea mays L.) and Relationship with Mycotoxin Contamination. Cereal Chem. 2017, 94, 315–324. [Google Scholar] [CrossRef]
- Miedaner, T.; Heinrich, N.; Schneider, B.; Oettler, G.; Rohde, S.; Rabenstein, F. Estimation of deoxynivalenol (DON) content by symptom rating and exoantigen content for resistance selection in wheat and triticale. Euphytica 2004, 139, 123–132. [Google Scholar] [CrossRef]
- Lanubile, A.; Maschietto, V.; De Leonardis, S.; Battilani, P.; Paciolla, C.; Marocco, A. Defense Responses to Mycotoxin-Producing Fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in Kernels of Susceptible and Resistant Maize Genotypes. Mol. Plant Microbe Interact. 2015, 28, 546–557. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Hashem, H.A.; Fahmy, A.H.; Ibrahim, A.S.; El Shihy, O.M.; Qaid, E.A. Protection Against Fusarium Head Blight: Important Defense Mechanisms Studied in Three Regenerated Egyptian Wheat Cultivars. J. Stress Physiol. Biochem. 2016, 12, 18–43. [Google Scholar]
- Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Nawraca, J.; Tomkowiak, A.; Perkowski, J. Phenolic acid content in wheat grain (Triticum spp.) of different genotypes. Rev. Fac. Cienc. Agrar. UNCUYO 2016, 48, 1–7. [Google Scholar]
- Rajeswari, P. Role of Phenols and Antioxidant Enzymes in Biocontrol of Fusarium oxysporum Causing Fusarium Wilt of Arachis hypogeae. L (Groundnut). Int. J. Agric. Sci. Res. 2014, 4, 95–104. [Google Scholar]
- Spanic, V.; Viljevac Vuletic, M.; Abicic, I.; Marcek, T. Early response of wheat antioxidant system with special reference to Fusarium head blight stress. Plant Physiol. Biochem. 2017, 115, 34–43. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Boyacioǧlu, D.; Hettiarachchy, N.S. Changes in some biochemical components of wheat grain that was infected with Fusarium graminearum. J. Cereal Sci. 1995, 21, 57–62. [Google Scholar] [CrossRef]
- Sorahinobar, M.; Niknam, V.; Ebrahimzade, H.; Soltanloo, H. Differential antioxidative responses of susceptible and resistant wheat cultivars against Fusarium head blight. Int. J. Farming Allied Sci. 2015, 4, 239–243. [Google Scholar]
- Spanic, V.; Viljevac Vuletic, M.; Drezner, G.; Zdunic, Z.; Horvat, D. Performance Indices in Wheat Chlorophyll a Fluorescence and Protein Quality Influenced by FHB. Pathogens 2017, 6, 59. [Google Scholar] [CrossRef]
- Dupont, F.M.; Altenbach, S.B. Molecular and biochemical impacts of environmental factors on wheat grain development and protein yield. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- Eggert, K.; Rawel, H.M.; Pawelzik, E. In vitro degradation of wheat gluten fractions by Fusarium graminearum proteases. Eur. Food Res. Technol. 2011, 233, 697–705. [Google Scholar] [CrossRef]
- Eggert, K.; Wieser, H.; Pawelzik, E. The influence of Fusarium infection and growing location on the quantitative protein composition of (part I) emmer (Triticum dicoccum). Eur. Food Res. Technol. 2010, 230, 837–847. [Google Scholar] [CrossRef]
- Prange, A.; Birzele, B.; Krämer, J.; Meier, A.; Modrow, H.; Köhler, P. Fusarium-inoculated wheat: Deoxynivalenol contents and baking quality in relation to infection time. Food Control 2005, 16, 739–745. [Google Scholar] [CrossRef]
- Wang, J.H.; Wieser, H.; Pawelzik, E.; Weinert, J.; Keutgen, J.; Wolf, G. Impact of the fungal protease produced by Fusarium culmorum on the protein quality and breadmaking properties of winter wheat. Eur. Food Res. Technol. 2005, 220, 552–559. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Siegel, B.Z.; Galston, W. The isoperoxidases of Pisum sativum. Plant Physiol. 1967, 42, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Raymond, J.; Rakariyatham, N.; Azanza, J. Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochemistry 1993, 34, 927–931. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Leads toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Wieser, H.; Antes, S.; Selmeier, W. Quantitative determination of gluten protein types in wheat flour by reverse-phase high preformance liquid chromatography. Cereal Chem. 1998, 75, 644–650. [Google Scholar] [CrossRef]
- Spanic, V.; Marcek, T.; Abicic, I.; Sarkanj, B. Effects of Fusarium Head Blight on Wheat Grain and Malt Infected by Fusarium culmorum. Toxins 2018, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Spanic, V.; Zdunic, Z.; Drezner, G.; Sarkanj, B. The Pressure of Fusarium Disease and Its Relation with Mycotoxins in the Wheat Grain and Malt. Toxins 2019, 11, 198. [Google Scholar] [CrossRef]

| Variety | AUDPC for General Resistance | AUDPC for Type I Resistance | Resistance/Susceptibility | DON µg kg−1 |
|---|---|---|---|---|
| Kraljica | 75.5 | 199.1 | R | 1424 |
| Olimpija | 72.5 | 199.9 | R | 588 |
| Vulkan | 90.8 | 280.8 | R | 755 |
| Sana | 145.3 | 333.3 | MS | 2205 |
| Golubica | 430.0 | 647.5 | S | 3308 |
| Bc Anica | 358.3 | 471.6 | S | 2299 |
| Df | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source of Variation | POD | APX | CAT | PPO | MDA | H2O2 | Proteins | Phenols | |
| Variety (V) | 5 | 64.60 *** | 0.6070 *** | 43.45 ns | 0.33974 *** | 14.066 *** | 27,360 *** | 9.591 *** | 0.11683 *** |
| Treatment (T) | 1 | 229.67 *** | 0.4950 *** | 437.51 *** | 0.15504 *** | 58.885 *** | 18,891 *** | 13.039 *** | 1.04280 *** |
| V*T | 5 | 8.57 *** | 0.3092 *** | 76.18 ** | 0.05877 *** | 6.549 *** | 1950 *** | 4.850 *** | 0.00831 ns |
| Error | 48 | 1.83 | 0.0415 | 18.33 | 0.00801 | 0.673 | 195 | 0.774 | 0.00435 |
| AUDPC for GR | AUDPC for T1 | DON Accumulation | ||
|---|---|---|---|---|
| AUDPC for GR | 1 | |||
| AUDPC for T1 | 0.94 ** | 1 | ||
| DON Accumulation | 0.83 * | 0.77 | 1 | |
| Varieties | Origin | Year of Release |
|---|---|---|
| Kraljica | HR, AIO | 2010 |
| Olimpija | HR, AIO | 2009 |
| Vulkan | HR, AIO | 2009 |
| Sana | HR, BC | 1983 |
| Bc Anica | HR, BC | 2010 |
| Golubica | HR, AIO | 1997 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanic, V.; Viljevac Vuletic, M.; Horvat, D.; Sarkanj, B.; Drezner, G.; Zdunic, Z. Changes in Antioxidant System during Grain Development of Wheat (Triticum aestivum L.) and Relationship with Protein Composition under FHB Stress. Pathogens 2020, 9, 17. https://doi.org/10.3390/pathogens9010017
Spanic V, Viljevac Vuletic M, Horvat D, Sarkanj B, Drezner G, Zdunic Z. Changes in Antioxidant System during Grain Development of Wheat (Triticum aestivum L.) and Relationship with Protein Composition under FHB Stress. Pathogens. 2020; 9(1):17. https://doi.org/10.3390/pathogens9010017
Chicago/Turabian StyleSpanic, Valentina, Marija Viljevac Vuletic, Daniela Horvat, Bojan Sarkanj, Georg Drezner, and Zvonimir Zdunic. 2020. "Changes in Antioxidant System during Grain Development of Wheat (Triticum aestivum L.) and Relationship with Protein Composition under FHB Stress" Pathogens 9, no. 1: 17. https://doi.org/10.3390/pathogens9010017
APA StyleSpanic, V., Viljevac Vuletic, M., Horvat, D., Sarkanj, B., Drezner, G., & Zdunic, Z. (2020). Changes in Antioxidant System during Grain Development of Wheat (Triticum aestivum L.) and Relationship with Protein Composition under FHB Stress. Pathogens, 9(1), 17. https://doi.org/10.3390/pathogens9010017







