Bactericidal Effect of Clove Oil against Multidrug-Resistant Streptococcus suis Isolated from Human Patients and Slaughtered Pigs
Abstract
1. Introduction
2. Results
2.1. Chemical Compositions of Essential Oil
2.2. Antibacterial Activity of Clove Oil
2.3. Determination of MIC and MBC
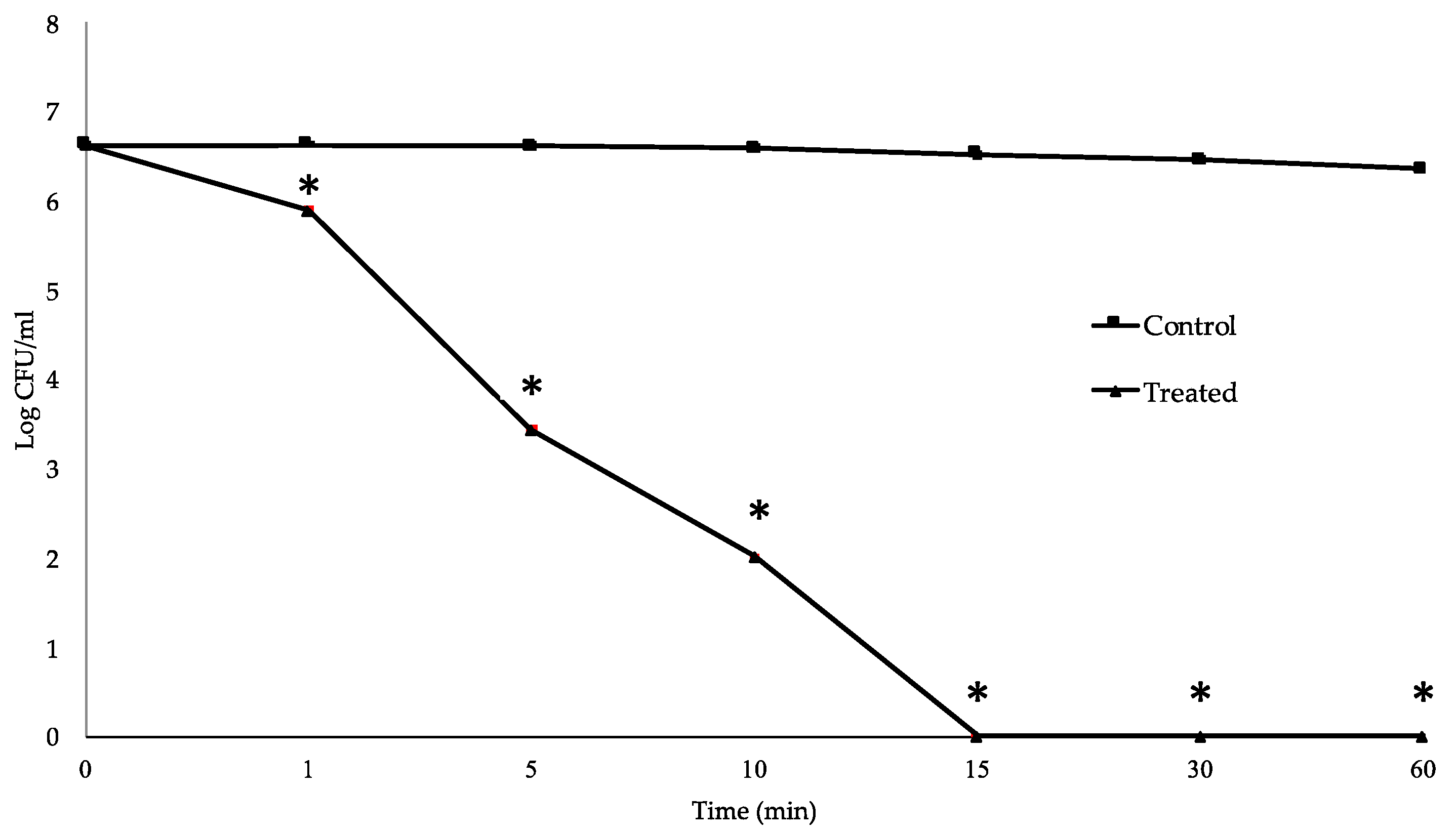
2.4. Time-Kill Study
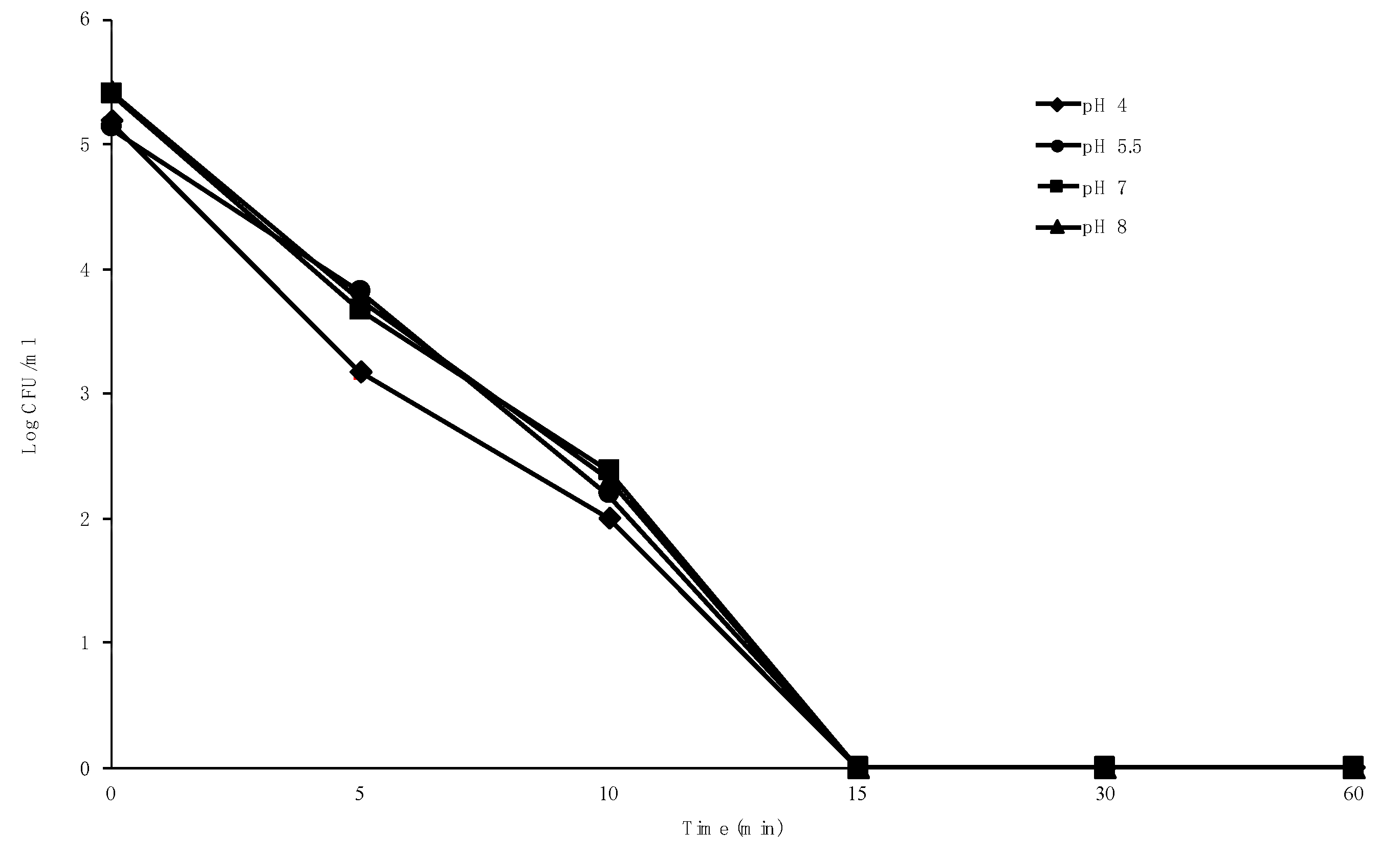
2.5. Effect of pH on Bactericidal Activity
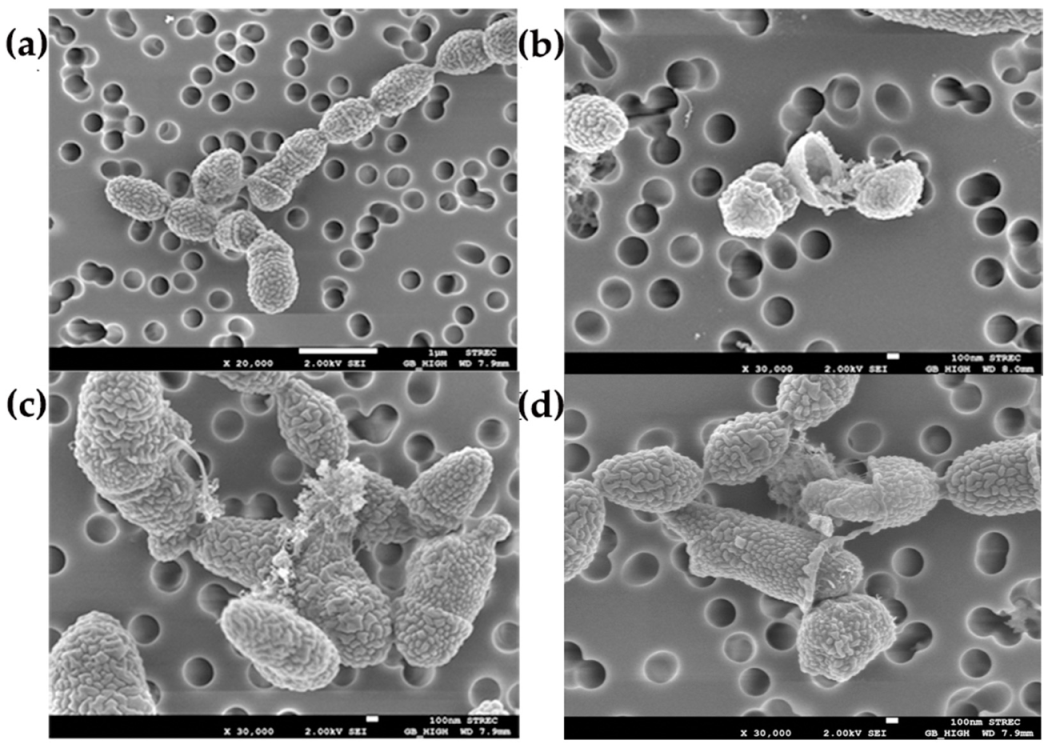
2.6. Effect of Clove Oil on Cell Surface of S. suis
3. Discussion
4. Materials and Methods
4.1. Bacteria and Culture Conditions
4.2. Essential Oil and Characterization
4.3. Preparation of Clove Oil Concentration
4.4. Determination of Antibacterial Activity
4.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.6. Time-Kill Study
4.7. Effect of pH on Bactericidal Activity
4.8. Scanning Electron Microscopy (SEM)
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takeuchi, D.; Kerdsin, A.; Pienpringam, A.; Loetthong, P.; Samerchea, S.; Luangsuk, P.; Khamisara, K.; Wongwan, N.; Areeratana, P.; Chiranairadul, P.; et al. Population-based study of Streptococcus suis infection in humans in Phayao province in Northern Thailand. PLoS ONE 2012, 7, e31265. [Google Scholar] [CrossRef] [PubMed]
- Wisselink, H.J.; Veldman, K.T.; Van den Eede, C.; Salmon, S.A.; Mevius, D.J. Quantitative susceptibility of Streptococcus suis strains isolated from diseased pigs in seven European countries to antimicrobial agents licenced in veterinary medicine. Vet. Microbiol. 2006, 113, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Mevius, D.J.; Schroeter, A.; Teale, C.; Jouy, E.; Butaye, P.; Franco, A.; Utinane, A.; Amado, A.; Moreno, M.; et al. Occurrence of antimicrobial resistance among bacterial pathogens and indicator bacteria in pigs in different European countries from YEAR 2002–2004: The ARBAO-II study. Acta Vet. Scand. 2008, 50, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ning, Y.; Zhang, Z.; Song, L.; Qiu, H.; Gao, H. In vitro antimicrobial susceptibility of Streptococcus suis strains isolated from clinically healthy sows in China. Vet. Microbiol. 2008, 131, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Princivalli, M.S.; Palmieri, C.; Magi, G.; Vignaroli, C.; Manzin, A.; Camporese, A.; Barocci, S.; Magistrali, C.; Facinelli, B. Genetic diversity of Streptococcus suis clinical isolates from pigs and humans in Italy (2003–2007). EuroSurveillance 2009, 14, 19310. [Google Scholar] [CrossRef] [PubMed]
- Strangmann, E.; Fröleke, H.; Kohse, K.P. Septic shock caused by Streptococcus suis: Case report and investigation of a risk group. Int. J. Hyg. Environ. Health 2002, 205, 385–392. [Google Scholar] [CrossRef]
- Vela, A.I.; Moreno, M.A.; Cebolla, J.A.; González, S.; Latre, M.V.; Domínguez, L.; Fernández-Garayzábal, J.F. Antimicrobial susceptibility of clinical strains of Streptococcus suis isolated from pigs in Spain. Vet. Microbiol. 2005, 105, 143–147. [Google Scholar] [CrossRef]
- Hoa, N.T.; Chieu, T.T.B.; Nghia, H.D.T.; Mai, N.T.H.; Anh, P.H.; Wolbers, M.; Baker, S.; Campbell, J.I.; Chau, N.V.V.; Hien, T.T.; et al. The antimicrobial resistance patterns and associated determinants in Streptococcus suis Isolated from humans in Southern Vietnam, 1997–2008. BMC Infect. Dis. 2011, 11, 6. [Google Scholar] [CrossRef]
- Ye, C.; Bai, X.; Zhang, J.; Jing, H.; Zheng, H.; Du, H.; Cui, Z.; Zhang, S.; Jin, D.; Xu, Y.; et al. Spread of Streptococcus suis sequence type 7, China. Emerg. Infect. Dis. 2008, 14, 787–791. [Google Scholar] [CrossRef]
- Ma, E.; Chung, P.H.; So, T.; Wong, L.; Choi, K.M.; Cheung, D.T.; Kam, K.M.; Chuang, S.K.; Tsang, T.; Collaborative study group on Streptococcus suis infection in Hong Kong. Streptococcus suis infection in Hong Kong: An emerging infectious disease? Epidemiol. Infect. 2008, 136, 1691–1697. [Google Scholar] [CrossRef]
- Shneerson, J.M.; Chattopadhyay, B.; Murphy, M.F.; Fawcett, I.W. Permanent perceptive deafness due to Streptococcus suis type II infection. J. Laryngol. Otol. 1980, 94, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Teng, L.; Ho, S.W.; Hsueh, P.R. Streptococcus suis infection. J. Mirobiol. Immunol. Infect. 2005, 38, 306–313. [Google Scholar]
- Varela, N.P.; Gadbois, P.; Thibault, C.; Gottschalk, M.; Dick, P.; Wilson, J. Antimicrobial resistance and prudent drug use for Streptococcus suis. Anim. Health Res. Rev. 2013, 14, 68–77. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34, S93–S106. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Xu, J.; Calzas, C.; Segura, M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010, 5, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Lun, Z.-R.; Wang, Q.-P.; Chen, X.-G.; Li, A.-X.; Zhu, X.-Q. Streptococcus suis: An emerging zoonotic pathogen. Lancet Infect. Dis. 2007, 7, 201–209. [Google Scholar] [CrossRef]
- Huang, J.; Ma, J.; Shang, K.; Hu, X.; Liang, Y.; Li, D.; Wu, Z.; Dai, L.; Chen, L.; Wang, L. Evolution and diversity of the antimicrobial resistance associated mobilome in Streptococcus suis: A probable mobile genetic elements reservoir for other streptococci. Front. Cell. Infect. Microbiol. 2016, 6, 118. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, E.H.; Hong, S.H.; Song, H.J.; Shin, M.K.; Kim, S.H.; Shin, T.Y. Effect of Syzygium aromaticum extract on immediate hypersensitivity in rats. J. Ethnopharmacol. 1998, 60, 125–131. [Google Scholar] [CrossRef]
- Shapiro, S.; Meier, A.; Guggenheim, B. The antimicrobial activity of essential oils and essential oil components towards oral bacteria. Oral Microbiol. Immunol. 1994, 9, 202–208. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.B.; Mafra, J.F.; da Rocha Bispo, A.S.; Ferreira, M.A.; de Lima Silva, F.; Rodrigues, A.V.N.; Evangelista-barreto, N.S. combination of chitosan coating and clove essential oil reduces lipid oxidation and microbial growth in frozen stored tambaqui (Colossoma macropomum) fillets. LWT 2019, 116, 108546. [Google Scholar] [CrossRef]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Zobayed, S.; Afreen, F.; Kozai, T. Necessity and production of medicinal plants under controlled environments. Environ. Control Biol. 2005, 43, 243–252. [Google Scholar] [CrossRef]
- Myint, S.; Wan Daud, W.R.; Mohamad, A.B.; Kadhum, A.A.H. Gas chromatographic determination of eugenol in ethanol extract of cloves. J. Chromatogr. B Biomed. Sci. Appl. 1996, 679, 193–195. [Google Scholar] [CrossRef]
- Siddiqua, S.; Anusha, B.A.; Ashwini, L.S.; Negi, P.S. Antibacterial Activity of Cinnamaldehyde and Clove Oil: Effect on Selected Foodborne Pathogens in Model Food Systems and Watermelon Juice. J. Food Sci. Technol. 2015, 52, 5834–5841. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2013, 92, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Nascimento Gislene, G.F.; Locatelli, J.; Freitas Paulo, C.; Silva Giuliana, L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Nzeako, B.C.; Al-Kharousi, Z.S.; Al-Mahrooqui, Z. Antimicrobial activities of clove and thyme extracts. Sultan Qaboos Univ. Med. J. 2006, 6, 33–39. [Google Scholar] [PubMed]
- Abdullah, B.H.; Hatem, S.F.; Jumaa, W. A comparative study of the antibacterial activity of clove and rosemary essential oils on multidrug resistant bacteria. UK J. Pharm. Biosci. 2015, 3, 18–22. [Google Scholar] [CrossRef]
- Revati, S.; Bipin, C.; Chitra, P.B.; Minakshi, B. In vitro antibacterial activity of seven Indian spices against high level gentamicin resistant strains of enterococci. Arch. Med. Sci. AMS 2015, 11, 863–868. [Google Scholar] [CrossRef]
- Dhara, L.; Tripathi, A. Antimicrobial activity of eugenol and cinnamaldehyde against extended spectrum beta lactamase producing Enterobacteriaceae by in vitro and molecular docking analysis. Eur. J. Integr. Med. 2013, 5, 527–536. [Google Scholar] [CrossRef]
- de Aguiar, F.C.; Solarte, A.L.; Tarradas, C.; Luque, I.; Maldonado, A.; Galán-Relaño, Á.; Huerta, B. Antimicrobial activity of selected essential oils against Streptococcus suis isolated from pigs. Microbiologyopen 2018, 7, e00613. [Google Scholar] [CrossRef]
- Perugini Biasi-Garbin, R.; Saori Otaguiri, E.; Morey, A.T.; Fernandes da Silva, M.; Belotto Morguette, A.E.; Armando Contreras Lancheros, C.; Kian, D.; Perugini, M.R.E.; Nakazato, G.; Durán, N.; et al. Effect of eugenol against Streptococcus agalactiae and synergistic interaction with biologically produced silver nanoparticles. Evid.-based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Ananda Baskaran, S.; Kazmer, G.W.; Hinckley, L.; Andrew, S.M.; Venkitanarayanan, K. Antibacterial effect of plant-derived antimicrobials on major bacterial mastitis pathogens in vitro. J. Dairy Sci. 2009, 92, 1423–1429. [Google Scholar] [CrossRef]
- Bidlas, E.; Du, T.; Lambert, R.J.W. An explanation for the effect of inoculum size on MIC and the growth/no growth interface. Int. J. Food Microbiol. 2008, 126, 140–152. [Google Scholar] [CrossRef]
- Smith, K.P.; Kirby, J.E. The inoculum effect in the era of multidrug resistance: minor differences in inoculum have dramatic effect on MIC determination. Antimicrob. Agents Chemother. 2018, 62, e00433–e00518. [Google Scholar] [CrossRef] [PubMed]
- Pujol, I.; Guarro, J.; Sala, J.; Riba, M.D. Effects of incubation temperature, inoculum size, and time of reading on broth microdilution susceptibility test results for amphotericin B against Fusarium. Antimicrob. Agents Chemother. 1997, 41, 808–811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yadav, M.K.; Park, S.-W.; Chae, S.-W.; Song, J.-J.; Kim, H.C. Antimicrobial activities of Eugenia caryophyllata extract and its major chemical constituent eugenol against Streptococcus pneumoniae. APMIS 2013, 121, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Bennis, S.; Chami, F.; Chami, N.; Rhayour, K.; Tantaoui-Elaraki, A.; Remmal, A. Eugenol induces damage of bacterial and fungal envelope. Moroc. J. Biol. 2004, 1, 33–39. [Google Scholar]
- Kovács, J.K.; Felső, P.; Makszin, L.; Pápai, Z.; Horváth, G.; Ábrahám, H.; Palkovics, T.; Böszörményi, A.; Emődy, L.; Schneider, G. Antimicrobial and virulence-modulating effects of clove essential oil on the foodborne pathogen Campylobacter jejuni. Appl. Environ. Microbiol. 2016, 82, 6158–6166. [Google Scholar] [CrossRef] [PubMed]
- Hoquea, M.M.; Barib, M.L.; Junejac, V.K.; Kawamotob, S. Antimicrobial activity of cloves and cinnamon extracts against food borne pathogens and spoilage bacteria, and inactivation of Listeria monocytogenes in ground chicken meat with their essential oils. Rep. Nat’l. Food Res. Inst. 2008, 72, 9–21. [Google Scholar]
- Knight, K.P.; McKellar, R.C. Influence of cinnamon and clove essential oils on the D- and z-Values of Escherichia coli O157:H7 in apple cider. J. Food Prot. 2007, 70, 2089–2094. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018. [Google Scholar]
- Wongsawan, K.; Gottschalk, M.; Tharavichitkul, P. Serotype- and virulence-associated gene profile of Streptococcus suis isolates from pig carcasses in Chiang Mai province, Northern Thailand. J. Vet. Med. Sci. 2015, 77, 233–236. [Google Scholar] [CrossRef]
- Ohno, T.; Kita, M.; Yamaoka, Y.; Imamura, S.; Yamamoto, T.; Mitsufuji, S.; Kodama, T.; Kashima, K.; Imanishi, J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter 2003, 8, 207–215. [Google Scholar] [CrossRef]

| Peak | Retention Time | Phytoconstituents | Molecular Formula | Molecular Weight | Area (%) |
|---|---|---|---|---|---|
| 1 | 22.5156 | Eugenol | C10H12O2 | 164.20 | 97.7573 |
| 2 | 24.4184 | Caryophyllene | C15H24 | 204.35 | 1.1647 |
| 3 | 25.7996 | Gamma-humulene | C15H24 | 204.35 | 0.3944 |
| 4 | 30.9152 | Caryophyllene oxide | C15H24O | 220.35 | 0.7836 |
| S. suis Isolates | Inhibition Zone (mm) | MIC (% v/v) | MBC (% v/v) | ||||
|---|---|---|---|---|---|---|---|
| Penicillin 10 µg | Clove Oil | ||||||
| 1% | 5% | 10% | 15% | ||||
| PCM01 | 33.20 ± 0.24 | 0.00 ± 0.00 | 0.00 ± 0.00 | 7.00 ± 0.00 | 13.00 ± 0.00 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| PCM02 | 15.00 ± 0.00 | 0.00 ± 0.00 | 7.13 ± 0.23 | 10.10 ± 0.08 | 15.00 ± 0.00 | 0.10 ± 0.00 | 0.1 ± 0.00 |
| PCM04 | 17.30 ± 0.47 | 0.00 ± 0.00 | 5.07 ± 0.12 | 6.03 ± 0.05 | 8.00 ± 0.00 | 0.10 ± 0.00 | 0.1 ± 0.00 |
| PCM06 | 32.01 ± 0.09 | 0.00 ± 0.00 | 8.00 ± 0.00 | 6.00 ± 0.00 | 11.07 ± 0.05 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| PLP03 | 15.00 ± 0.00 | 0.00 ± 0.00 | 7.00 ± 0.00 | 7.00 ± 0.00 | 15.03 ± 0.05 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| PLP06 | 45.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 7.07 ± 0.05 | 14.00 ± 0.00 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| PCM05 | 45.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.03 ± 0.05 | 12.10 ± 0.08 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| MNCM06 | 34.01 ± 0.09 | 0.00 ± 0.00 | 8.00 ± 0.00 | 12.00 ± 0.00 | 15.00 ± 0.00 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| MNCM07 | 35.00 ± 0.00 | 0.00 ± 0.00 | 7.23 ± 0.21 | 10.13 ± 0.09 | 15.00 ± 0.00 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| MNCM10 | 46.01 ± 0.05 | 0.00 ± 0.00 | 13.07 ± 0.12 | 12.23 ± 0.17 | 14.00 ± 0.00 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| MNCM43 | 33.00 ± 0.00 | 0.00 ± 0.00 | 5.00 ± 0.00 | 9.93 ± 0.09 | 10.00 ± 0.00 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| MNCM21 | 35.00 ± 0.00 | 0.00 ± 0.00 | 7.00 ± 0.00 | 9.00 ± 0.00 | 15.07 ± 0.05 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| MNCM25 | 40.00 ± 0.01 | 0.00 ± 0.00 | 8.13 ± 0.12 | 11.07 ± 0.05 | 15.00 ± 0.00 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| LPH5 | 39.00 ± 0.02 | 0.00 ± 0.00 | 8.00 ± 0.00 | 14.03 ± 0.05 | 11.03 ± 0.05 | 0.05 ± 0.00 | 0.1 ± 0.00 |
| MNCM50 | 34.00 ± 0.03 | 0.00 ± 0.00 | 7.40 ± 0.35 | 8.10 ± 0.08 | 11.00 ± 0.00 | 0.10 ± 0.00 | 0.1 ± 0.00 |
| Clove Oil Concentration (%v/v) | MIC Distribution by the Number of Isolates | MBC Distribution by the Number of Isolates | MIC50/MBC50 | MIC90/MBC90 |
|---|---|---|---|---|
| 0.0125 | 0 | 0 | 0.05/0.1 | 0.1/0.1 |
| 0.025 | 0 | 0 | ||
| 0.05 | 12 | 0 | ||
| 0.1 | 3 | 15 | ||
| 0.2 | 0 | 0 |
| S. suis Isolates | Serotypes ** | ST † | Genotype ‡ | Sources § |
|---|---|---|---|---|
| mrp/epf/sly | ||||
| PCM01 | 2 | 104 | +/-/- | healthy pig |
| PCM02 | 7 | 373 | +/-/+ | healthy pig |
| PCM04 | 7 | 89 | -/-/+ | healthy pig |
| PCM06 | 9 | 16 | -/-/- | healthy pig |
| PLP03 | 16 | 374 | -/-/- | healthy pig |
| PLP06 | 16 | 375 | -/-/- | healthy pig |
| PCM05 | 16 | 376 | -/-/- | healthy pig |
| MNCM06 | 2 | 1 | +/+/+ | human |
| MNCM07 | 14 | 11 | +/*/+ | human |
| MNCM10 | 2 | 25 | +/-/- | human |
| MNCM43 | 2 | 28 | +/-/- | human |
| MNCM21 | 2 | 101 | -/-/+ | human |
| MNCM25 | 2 | 102 | +/-/- | human |
| LPH5 | 2 | 103 | +/-/- | human |
| MNCM50 | 2 | 104 | -/-/+ | human |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongsawan, K.; Chaisri, W.; Tangtrongsup, S.; Mektrirat, R. Bactericidal Effect of Clove Oil against Multidrug-Resistant Streptococcus suis Isolated from Human Patients and Slaughtered Pigs. Pathogens 2020, 9, 14. https://doi.org/10.3390/pathogens9010014
Wongsawan K, Chaisri W, Tangtrongsup S, Mektrirat R. Bactericidal Effect of Clove Oil against Multidrug-Resistant Streptococcus suis Isolated from Human Patients and Slaughtered Pigs. Pathogens. 2020; 9(1):14. https://doi.org/10.3390/pathogens9010014
Chicago/Turabian StyleWongsawan, Kanruethai, Wasana Chaisri, Sahatchai Tangtrongsup, and Raktham Mektrirat. 2020. "Bactericidal Effect of Clove Oil against Multidrug-Resistant Streptococcus suis Isolated from Human Patients and Slaughtered Pigs" Pathogens 9, no. 1: 14. https://doi.org/10.3390/pathogens9010014
APA StyleWongsawan, K., Chaisri, W., Tangtrongsup, S., & Mektrirat, R. (2020). Bactericidal Effect of Clove Oil against Multidrug-Resistant Streptococcus suis Isolated from Human Patients and Slaughtered Pigs. Pathogens, 9(1), 14. https://doi.org/10.3390/pathogens9010014






