Persistent Listeria monocytogenes Isolates from a Poultry-Processing Facility Form More Biofilm but Do Not Have a Greater Resistance to Disinfectants than Sporadic Strains
Abstract
1. Introduction
2. Results
2.1. Capacity of L. monocytogenes to Form Biofilm
2.2. The Effect of Disinfectants on Biofilms
3. Discussion
3.1. Capacity of Strains of L. monocytogenes to Form Biofilm
3.2. The Effect of Disinfectants on Biofilms
4. Materials and Methods
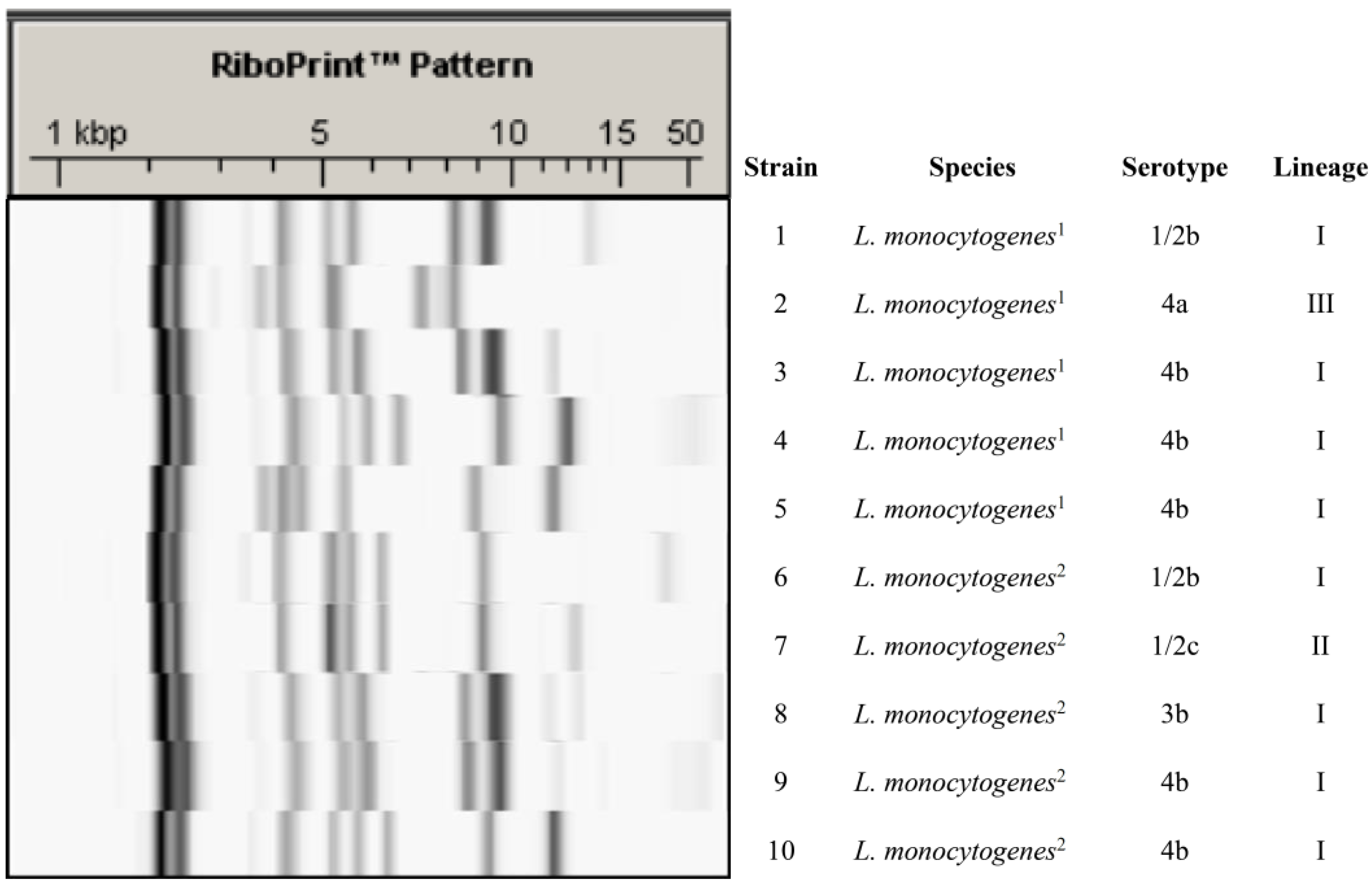
4.1. L. monocytogenes Strains
4.2. Ribotyping and Serotyping
4.3. Biofilm Determination
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Noordhout, C.M.; Devleesschauwer, B.; Angulo, F.J.; Verbeke, G.; Haagsma, J.; Kirk, M.; Havelaar, A.; Speybroeck, N. The global burden of listeriosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1073–1082. [Google Scholar] [CrossRef]
- Lanciotti, R.; Braschi, G.; Patrignani, F.; Gobbetti, M.; De Angelis, M. How Listeria monocytogenes shapes its proteome in response to natural antimicrobial compounds. Front. Microbiol. 2019, 10, 437. [Google Scholar] [CrossRef]
- CDC. Listeria (listeriosis). Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/listeria/index.html (accessed on 21 September 2019).
- Pohl, A.M.; Pouillot, R.; Van Doren, J.M. Changing US population demographics: What does this mean for listeriosis incidence and exposure? Foodborne Pathog. Dis. 2017, 14, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Stoller, A.; Stevens, M.J.A.; Stephan, R.; Guldimann, C. Characteristics of Listeria monocytogenes strains persisting in a meat processing facility over a 4-year period. Pathogens 2019, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- EFSA. Trends and sources of zoonoses and zoonotic agents and food-borne outbreaks in the European Union in 2008. EFSA J. 2010, 8, 1496. [Google Scholar]
- Leclerq, A.; Moura, A.; Vales, G.; Tessaud-Rita, N.; Aguilhon, C.; Lecuit, M. Listeria thailandensis sp. nov. Int. J. Syst. Evol. Microbiol. 2019, 69, 74–81. [Google Scholar] [CrossRef]
- Wagner, M.; McLauchlin, J. Biology in Handbook of Listeria monocytogenes; Dongyou, L., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 3–25. [Google Scholar]
- Jamshidi, A.; Zeinali, T. Significance and characteristics of Listeria monocytogenes in poultry products. Int. J. Food Sci. 2019, 2019, 7835253. [Google Scholar] [CrossRef]
- Ortiz, S.; López-Alonso, V.; Rodríguez, P.; Martínez-Suárez, J.V. The connection between persistent, disinfectant-resistant Listeria monocytogenes strains from two geographically separate Iberian pork processing plants: Evidence from comparative genome analysis. Appl. Environ. Microbiol. 2016, 82, 308–317. [Google Scholar] [CrossRef]
- Schmitz-Esser, S.; Müller, A.; Stessl, B.; Wagner, M. Genomes of sequence type 121 Listeria monocytogenes strains harbor highly conserved plasmids and prophages. Front. Microbiol. 2015, 6, 380. [Google Scholar] [CrossRef]
- Nowak, J.; Cruz, C.D.; Tempelaars, M.; Abee, T.; van Vliet, A.M.H.; Fletcher, G.C.; Hedderley, D.; Palmer, J.; Flint, S. Persistent Listeria monocytogenes strains isolated from mussel production facilities form more biofilm but are not linked to specific genetic markers. Int. J. Food Microbiol. 2019, 256, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Melcón, C.; Capita, R.; Rodríguez-Jerez, J.J.; Martínez-Suárez, J.V.; Alonso-Calleja, C. Effect of low doses of disinfectants on the biofilm-forming ability of Listeria monocytogenes. Foodborne Path. Dis. 2019, 16, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Melcón, C.; Riesco-Peláez, F.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Susceptibility of Listeria monocytogenes planktonic cultures and biofilms to sodium hypochlorite and benzalkonium chloride. Food Microbiol. 2019, 82, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Quingping, W.; Jumei, Z.; Moutong, C.; Zéan, Y. Prevalence, antibiotic resistance and genetic diversity of Listeria monocytogenes isolated from retail ready-to-eat foods in China. Food Control 2015, 47, 340–347. [Google Scholar] [CrossRef]
- Meloni, D.; Galluzzo, P.; Mureddu, A.; Piras, F.; Griffiths, M.; Mazette, R. Listeria monocytogenes in RTE foods marketed in Italy: Prevalence and automated EcoRI ribotyping of the isolates. Int. J. Food Microbiol. 2009, 129, 166–173. [Google Scholar] [CrossRef]
- Vasilev, V.; Japheth, R.; Breuer, R.; Andom, N.; Abraham, R.B.; Yoni, Y.; Valinsky, L.; Agmon, V. A survey of Listeria monocytogenes strains, isolated from ready-to-eat foods in Israel over a period of 10 years, 1998–2007. Food Control 2010, 21, 1179–1181. [Google Scholar] [CrossRef]
- Martins, E.A.; Gernmano, P.M.L. Listeria monocytogenes in ready-to-eat, sliced, cooked ham and salami products, marketed in the city of São Paulo, Brazil: Occurrence, quantification, and serotyping. Food Control 2011, 22, 297–302. [Google Scholar] [CrossRef]
- Fallah, A.A.; Saei-Dehkordi, S.S.; Rahnama, M.; Tahmasby, H.; Mahzounieh, M. Prevalence and antimicrobial resistance patterns of Listeria species isolated from poultry products marketed in Iran. Food Control 2012, 28, 327–332. [Google Scholar] [CrossRef]
- Notification details—2019.2989. Foodborne outbreak caused by Listeria monocytogenes (>1.5x10E4 CFU/g) in chilled pork products from Spain. Available online: https://webgate.ec.europa.eu/rasff-window/portal/?event=notificationDetail&NOTIF_REFERENCE=2019.2989 (accessed on 20 September 2019).
- Junta de Andalucía. Consejería de Salud y Familias. Available online: https://www.juntadeandalucia.es/organismos/saludyfamilias/actualidad/noticias/detalle/220344.html (accessed on 20 September 2019).
- Korsak, D.; Borek, A.; Daniluk, S.; Grabowska, A.; Pappelbaum, K. Antimicrobial susceptibilities of Listeria monocytogenes strains isolated from food and food processing environment in Poland. Int. J. Food Microbiol. 2012, 158, 203–208. [Google Scholar] [CrossRef]
- Prencipe, V.A.; Rizzi, V.; Acciari, V.; Iannetti, L.; Giovannini, A.; Serraino, A.; Calderone, D.; Rossi, A.; Morelli, D.; Marino, L.; et al. Listeria monocytogenes prevalence, contamination levels and strains characterization throughout the Parma ham processing chain. Food Control 2012, 25, 150–158. [Google Scholar] [CrossRef]
- Kramarenko, T.; Roasto, M.; Meremae, K.; Kuningas, M.; Poltsama, P.; Elias, T. Listeria monocytogenes prevalence and serotype diversity in various foods. Food Control 2013, 30, 24–29. [Google Scholar] [CrossRef]
- Martín, B.; Perich, A.; Gómez, D.; Yangüela, J.; Rodríguez, A.; Garriga, M.; Aymerich, T. Diversity and distribution of Listeria monocytogenes in meat processing plants. Food Microbiol. 2014, 44, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jiang, X. Prevalence and characterization of Listeria monocytogenes isolated from retail food in Henan, China. Food Control 2014, 37, 228–231. [Google Scholar] [CrossRef]
- Ebner, R.; Stephan, R.; Althaus, D.; Brisse, S.; Maury, M.; Tasara, T. Phenotypic and genotypic characteristics of Listeria monocytogenes strains isolated during 2011–2014 from different food matrices in Switzerland. Food Control 2015, 57, 321–326. [Google Scholar] [CrossRef]
- Kérouanton, A.; Marault, M.; Petit, L.; Grout, J.; Dao, T.T.; Brisabois, A. Evaluation of a multiplex PCR assay as an alternative method for Listeria monocytogenes serotyping. J. Microbiol. Meth. 2010, 80, 134–137. [Google Scholar] [CrossRef]
- Lannetti, L.; Acciari, V.A.; Antoci, S.; Addante, N.; Bardasi, L.; Bilei, S.; Calistri, P.; Cito, F.; Cogoni, P.; D’Aurelio, R.; et al. Listeria monocytogenes in ready-to-eat foods in Italy: Prevalence of contamination at retail and characterization of strains from meat products and cheese. Food Control 2016, 68, 55–61. [Google Scholar] [CrossRef]
- Paul, D.; Steele, C.; Donalson, J.R.; Banes, M.M.; Kumar, R.; Bridges, S.M.; Arick, M.; Lawrence, M. Genome comparison of Listeria monocytogenes serotype 4a strain HCC23 with selected lineage I and lineage II L. monocytogenes strains and other Listeria strains. Genom. Data 2014, 2, 219–225. [Google Scholar] [CrossRef]
- Orsi, R.H.; Den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef]
- Tsai, Y.-H.L.; Maron, S.B.; McGann, P.; Nightingale, K.K.; Wiedmann, M.; Orsi, R.H. Recombination and positive selection contributed to the evolution of Listeria monocytogenes lineages III and IV, two distinct and well supported uncommon L. monocytogenes lineages. Infect. Gen. Evol. 2011, 11, 1881–1890. [Google Scholar] [CrossRef]
- Orsi, R.H.; Bowen, B.M.; Wiedmann, M. Homopolymeric tracts represent a general regulatory mechanism in prokaryotes. BMC Genom. 2010, 11, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, W.B.; Cutter, C.N. An ecological perspective of Listeria monocytogenes biofilms in food processing facilities. Crit. Rev. Food Sci. Nutr. 2013, 53, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Eskhan, A.O.; Abu-Lail, N.I. Cellular and molecular investigations of the adhesion and mechanics of Listeria monocytogenes lineages’ I and II environmental and epidemic strains. J. Coll. Interface Sci. 2013, 394, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, G.T.; Bruce, J.L.; McDonough, P.L.; Scarlett, J.; Boor, K.J.; Wiedmann, M. Comparative genetic characterization of Listeria monocytogenes isolates from human and animal listeriosis cases. Microbiology 2001, 147, 1095–1104. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenyed, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review in biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- González-Machado, C.; Capita, R.; Riesco-Peláez, F.; Alonso-Calleja, C. Visualization and quantification of the cellular and extracellular components of Salmonella Agona biofilms at different stages of development. PLoS ONE 2018, 13, e0200011. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Riesco-Peláez, F.; Carballo, J.; García-Fernández, C.; Capita, R.; Alonso-Calleja, C. Structure and viability of 24- and 72-h-old biofilms formed by four pathogenic bacteria on polystyrene and glass contact surfaces. Food Microbiol. 2018, 76, 513–517. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; dos Santos, L.R.; Tagliari, V.Z.; Rizzo, N.N.; Trenhago, G.; de Oliveira, A.P.; Goetz, F.; do Nascimento, V.P. Quantification of biofilm production on polystyrene by Listeria, Escherichia coli and Staphylococcus aureus isolated from a poultry slaughterhouse. Braz. J. Microbiol. 2010, 41, 1082–1085. [Google Scholar] [CrossRef]
- Kadam, S.R.; Den Besten, H.M.W.; Dan Der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef]
- Harvey, J.; Keenan, K.P.; Gilmour, A. Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiol. 2007, 24, 380–392. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Camino, R.; Magalhães, R.; Ferreira, V.; Santos, I.; Silva, J.; Almeida, G.; Teixeira, P. Biofilm formation among clinical and food isolates of Listeria monocytogenes. Int. J. Microbiol. 2013, 2013, 524975. [Google Scholar] [CrossRef] [PubMed]
- Chavant, P.; Martinie, B.; Meylheuc, T.; Bellon-Fontaine, M.-N.; Hebraud, M. Listeria monocytogenes LO28: Surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl. Environ. Microbiol. 2002, 68, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Torlak, E.; Sert, D. Combined effect of benzalkonium chloride and ultrasound against Listeria monocytogenes biofilm on plastic surface. Lett. Appl. Microbiol. 2013, 57, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, M.; Piveteau, P.; Desvaux, M.; Brisse, S.; Briandet, R. Exploring the diversity of Listeria monocytogenes biofilm architecture by high-throughput confocal laser scanning microscopy and the predominance of the honeycomb-like morphotype. Appl. Environ Microbiol. 2015, 81, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Borucki, M.K.; Peppin, J.D.; White, D.; Loge, F.; Call, D.R. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7336–7342. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.E.; Ross, T.; Bowman, J.P. Variability in biofilm production by Listeria monocytogenes correlated to strain origin and growth conditions. Int. J. Food Microbiol. 2011, 150, 14–24. [Google Scholar] [CrossRef]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef]
- Lunden, J.M.; Miettinen, M.K.; Autio, T.J.; Korkeala, H.J. Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. J. Food Prot. 2000, 63, 1204–1207. [Google Scholar] [CrossRef]
- Norwood, D.E.; Gilmour, A. Adherence of Listeria monocytogenes strains to stainless steel coupons. J. Appl. Microbiol. 1999, 86, 576–582. [Google Scholar] [CrossRef]
- Norwood, D.E.; Gilmour, A. The differential adherence capabilities of two Listeria monocytogenes strains in monoculture and multispecies biofilms as a function of temperature. Lett. Appl. Microbiol. 2001, 33, 320–324. [Google Scholar] [CrossRef]
- Nakamura, H.; Takakura, K.-I.; Sone, Y.; Itano, Y.; Nishikawa, Y. Biofilm formation and resistance to benzalkonium chloride in Listeria monocytogenes isolated from a fish processing plant. J. Food Prot. 2013, 76, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Folsom, J.P.; Siragusa, G.R.; Frank, J.F. Formation of biofilm at different nutrient levels by various genotypes of Listeria monocytogenes. J. Food Prot. 2006, 69, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Breidt, F.; Gorski, L. Synergistic effects of sodium chloride, glucose, and temperature on biofilm formation by Listeria monocytogenes serotype 1/2a and 4b strains. Appl. Environ. Microbiol. 2010, 76, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.M.; Samir, A.; Abo-Shama, U.H.; Mohamed, E.A.; Orabi, A.; Zolnikov, T. Determination of virulence and antibiotic resistance pattern of biofilm producing Listeria species isolated from retail raw milk. BMC Microbiol. 2016, 16, 263. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, T.; Jacques, M.; Quessy, S.; Fravalo, P. Impact of nutrient restriction on the structure of Listeria monocytogenes biofilm grown in a microfluidic system. Front. Microbiol. 2017, 8, 864. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Piccolomini, R.; Paludi, D.; D’Orio, V.; Vergara, A.; Conter, M.; Ianieri, A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: Relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. [Google Scholar] [CrossRef]
- Combrouse, T.; Sadovskaya, I.; Faille, C.; Kol, O.; Guérardel, Y.; Midelet-Bourdin, G. Quantification of the extracellular matrix of the Listeria monocytogenes biofilms of different phylogenic lineages with optimization of culture conditions. J. Appl. Microbiol. 2013, 114, 1120–1131. [Google Scholar] [CrossRef]
- Colagiorgi, A.; Bruini, I.; Di Ciccio, P.A.; Zanardi, E.; Ghidini, S.; Ianeri, A. Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens 2017, 6, 41. [Google Scholar] [CrossRef]
- Takahashi, H.; Miya, S.; Igarashi, K.; Suda, T.; Kuramoto, S.; Kimura, B. Biofilm formation ability of Listeria monocytogenes isolates from raw ready-to-eat seafood. J. Food Prot. 2009, 72, 1476–1480. [Google Scholar] [CrossRef]
- Smoot, L.M.; Pierson, M.D. Effect of environmental stress on the ability of Listeria monocytogenes Scott A to attach to food contact surfaces. J. Food Prot. 1998, 61, 1293–1298. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Ray, A.J.; Hammons, S.R.; Oliver, H.F. Persistent and transient Listeria monocytogenes strains from retail deli environments vary in their ability to adhere and form biofilms and rarely have inlA premature stop codons. Foodborne Pathog. Dis. 2015, 12, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Beuchat, L.R.; Montville, T.M. Food Microbiology: Fundamentals and Frontiers; ASM Press: Washington, DC, USA, 1997. [Google Scholar]
- Ochiai, Y.; Yamada, F.; Mochizuki, M.; Takano, T.; Hondo, R.; Ueda, F. Biofilm formation under different temperature conditions by a single genotype of persistent Listeria monocytogenes strains. J. Food Prot. 2014, 77, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Bartolotti, L.; Brito, L.; Civera, T. Biofilm formation and disinfectant susceptibility of persistent and nonpersistent Listeria monocytogenes isolates from Gorgonzola cheese processing plants. Foodborne Pathog. Dis. 2016, 13, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Sashara, K.; Zottola, E.A. Biofilm formation by Listeria monocytogenes utilizes a primary colonizing microorganism in flowing systems. J. Food Prot. 1993, 56, 1022–1028. [Google Scholar] [CrossRef]
- Carpentier, B.; Chassaing, D. Interactions in biofilms between Listeria monocytogenes and resident microorganisms from food industry premises. Int. J. Food Microbiol. 2004, 97, 111–122. [Google Scholar] [CrossRef]
- Capita, R.; Fernández-Pérez, S.; Buzón-Durán, L.; Alonso-Calleja, C. Effect of sodium hypochlorite and benzalkonium chloride on the structural parameters of the biofilms formed by ten Salmonella enterica serotypes. Pathogens 2019, 8, 154. [Google Scholar] [CrossRef]
- Capita, R.; Riesco-Peláez, F.; Alonso-Hernando, A.; Alonso-Calleja, C. Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl. Environ. Microbiol. 2014, 80, 1268–1280. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Alonso-Calleja, C.; Riesco-Peláez, F.; Capita, R. Effect of sub-inhibitory concentrations of biocides on the architecture and viability of MRSA biofilms. Food Microbiol. 2017, 65, 294–301. [Google Scholar] [CrossRef]
- Capita, R.; Buzón-Durán, L.; Riesco-Peláez, F.; Alonso-Calleja, C. Effect of sub-lethal concentrations of biocides on the structural parameters and viability of the biofilms formed by Salmonella Typhimurium. Foodborne Path. Dis. 2017, 14, 350–356. [Google Scholar] [CrossRef]
- Aase, B.; Sundheim, G.; Langsrud, S.; Rørvik, L.M. Occurrence of and a posible mechanism for resistance to a quaternary ammonium compound in Listeria monocytogenes. Int. J. Food Microbiol. 2000, 62, 57–63. [Google Scholar] [CrossRef]
- Fox, E.M.; Leonard, N.; Jordan, K. Physiological and transcriptional characterization of persistent and non persistent Listeria monocytogenes isolates. Appl. Environ. Microbiol. 2011, 77, 6559–6569. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Suárez, J.V.; Ortiz, S.; López-Alonso, V. Potential impact of the resistance to quaternary ammonium disinfectants on the persistence of Listeria monocytogenes in food processing environments. Front. Microbiol. 2016, 7, 638. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Schirmer, B.C.T.; Heir, E.; Fagerlund, A.; Hjemli, P.; Langsrud, S. Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int. J. Food Microbiol. 2017, 241, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Holah, J.T.; Taylor, J.H.; Dawson, D.J.; Hall, K.E. Biocide use in the food industry and the disinfectant resistance of persistent strains of Listeria monocytogenes and Escherichia coli. J. Appl. Microbiol. 2002, 92, 111S–220S. [Google Scholar] [CrossRef] [PubMed]
- Kastbjerg, V.G.; Gram, L. Model systems allowing quantification of sensitivity to disinfectants and comparison of disinfectant susceptibility of persistent and presumed nonpersistent Listeria monocytogenes. J. Appl. Microbiol. 2009, 106, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Heir, E.; Lindstedt, B.A.; Røtterud, O.J.; Vardund, T.; Kapperud, G.; Nesbakken, T. Molecular epidemiology and disinfectant susceptibility of Listeria monocytogenes from meat processing plants and human infections. Int. J. Food Microbiol. 2004, 96, 85–96. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C.; Prieto, M. Prevalence of Salmonella enterica serovars and genovars from chicken carcasses in slaughterhouses in Spain. J. Appl. Microbiol. 2007, 103, 1366–1375. [Google Scholar] [CrossRef]
- Díez-García, M.; Capita, R.; Alonso-Calleja, C. Influence of serotype on the growth kinetics and the ability to form biofilms of Salmonella isolates from poultry. Food Microbiol. 2012, 31, 173–180. [Google Scholar] [CrossRef]

| Strain (Serotype) | Treatment (Five Minutes) | ||||||
|---|---|---|---|---|---|---|---|
| CONTROL | Sodium Hypochlorite (10% of Active Chlorine) | Benzalkonium Chloride | |||||
| Without Treatment | 10,000 ppm | 25,000 ppm | 50,000 ppm | 2500 ppm | 10,000 ppm | 25,000 ppm | |
| 1 (1/2b) | 0.318 ± 0.053 abccd | 0.261 ± 0.191 aba | 0.237 ± 0.187 aba | 0.207 ± 0.049 aa | 0.493 ± 0.075 dd | 0.436 ± 0.072 cdb | 0.372 ± 0.090 bcdbc |
| 2 (4a) | 0.361 ± 0.108 abcd | 0.284 ± 0.200 abca | 0.218 ± 0.194 aba | 0.201 ± 0.074 aa | 0.457 ± 0.146 ccd | 0.444 ± 0.106 cb | 0.388 ± 0.087 bcc |
| 3 (4b) | 0.244 ± 0.108 ababcd | 0.166 ± 0.044 aba | 0.184 ± 0.044 aba | 0.148 ± 0.061 aa | 0.273 ± 0.132 ba | 0.258 ± 0.085 aba | 0.233 ± 0.057 aba |
| 4 (4b) | 0.308 ± 0.103 bbcd | 0.229 ± 0.092 aba | 0.225 ± 0.048 aba | 0.172 ± 0.054 aa | 0.317 ± 0.119 bab | 0.319 ± 0.121 bab | 0.294 ± 0.092 babc |
| 5 (4b) | 0.274 ± 0.092 bcabcd | 0.181 ± 0.087 aba | 0.163 ± 0.072 aba | 0.153 ± 0.050 aa | 0.306 ± 0.126 cab | 0.263 ± 0.099 abca | 0.276 ± 0.065 bcab |
| 6 (1/2b) | 0.193 ± 0.075 abab | 0.161 ± 0.035 aa | 0.151 ± 0.034 aa | 0.146 ± 0.057 aa | 0.271 ± 0.131 ba | 0.247 ± 0.094 aba | 0.226 ± 0.095 aba |
| 7 (1/2c) | 0.178 ± 0.104 abca | 0.156 ± 0.061 aba | 0.185 ± 0.095 abca | 0.147 ± 0.049 aa | 0.300 ± 0.081 dab | 0.278 ± 0.090 cda | 0.250 ± 0.051 bcda |
| 8 (3b) | 0.188 ± 0.107 aba | 0.211 ± 0.074 aba | 0.218 ± 0.094 aba | 0.141 ± 0.046 aa | 0.347 ± 0.112 cabc | 0.284 ± 0.105 bca | 0.257 ± 0.078 abca |
| 9 (4b) | 0.176 ± 0.069 aa | 0.188 ± 0.079 aa | 0.157 ± 0.045 aa | 0.186 ± 0.077 aa | 0.288 ± 0.141 aa | 0.229 ± 0.150 aa | 0.231 ± 0.119 aa |
| 10 (4b) | 0.205 ± 0.072 aabc | 0.181 ± 0.060 aa | 0.187 ± 0.083 aa | 0.150 ± 0.025 aa | 0.343 ± 0.134 babc | 0.266 ± 0.134 aba | 0.232 ± 0.081 aba |
| Group of Strains | Treatment (Five Minutes) | ||||||
|---|---|---|---|---|---|---|---|
| CONTROL | Sodium Hypochlorite (10% of Active Chlorine) | Benzalkonium Chloride | |||||
| Without Treatment | 10,000 ppm | 25,000 ppm | 50,000 ppm | 2500 ppm | 10,000 ppm | 25,000 ppm | |
| Persistent | 0.301 ± 0.097 ba | 0.224 ± 0.136 aa | 0.206 ± 0.122 aa | 0.177 ± 0.059 aa | 0.369 ± 0.145 ca | 0.344 ± 0.123 bca | 0.313 ± 0.095 bca |
| Sporadic | 0.188 ± 0.082 ab | 0.180 ± 0.062 aa | 0.180 ± 0.073 aa | 0.154 ± 0.052 aa | 0.310 ± 0.117 ca | 0.260 ± 0.110 bb | 0.239 ± 0.082 bb |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Campos, D.; Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Persistent Listeria monocytogenes Isolates from a Poultry-Processing Facility Form More Biofilm but Do Not Have a Greater Resistance to Disinfectants than Sporadic Strains. Pathogens 2019, 8, 250. https://doi.org/10.3390/pathogens8040250
Rodríguez-Campos D, Rodríguez-Melcón C, Alonso-Calleja C, Capita R. Persistent Listeria monocytogenes Isolates from a Poultry-Processing Facility Form More Biofilm but Do Not Have a Greater Resistance to Disinfectants than Sporadic Strains. Pathogens. 2019; 8(4):250. https://doi.org/10.3390/pathogens8040250
Chicago/Turabian StyleRodríguez-Campos, Daniel, Cristina Rodríguez-Melcón, Carlos Alonso-Calleja, and Rosa Capita. 2019. "Persistent Listeria monocytogenes Isolates from a Poultry-Processing Facility Form More Biofilm but Do Not Have a Greater Resistance to Disinfectants than Sporadic Strains" Pathogens 8, no. 4: 250. https://doi.org/10.3390/pathogens8040250
APA StyleRodríguez-Campos, D., Rodríguez-Melcón, C., Alonso-Calleja, C., & Capita, R. (2019). Persistent Listeria monocytogenes Isolates from a Poultry-Processing Facility Form More Biofilm but Do Not Have a Greater Resistance to Disinfectants than Sporadic Strains. Pathogens, 8(4), 250. https://doi.org/10.3390/pathogens8040250





