Current Knowledge on Pathogenicity and Management of Stemphylium botryosum in Lentils (Lens culinaris ssp. culinaris Medik)
Abstract
1. Introduction
2. Emergence and Spread of SB
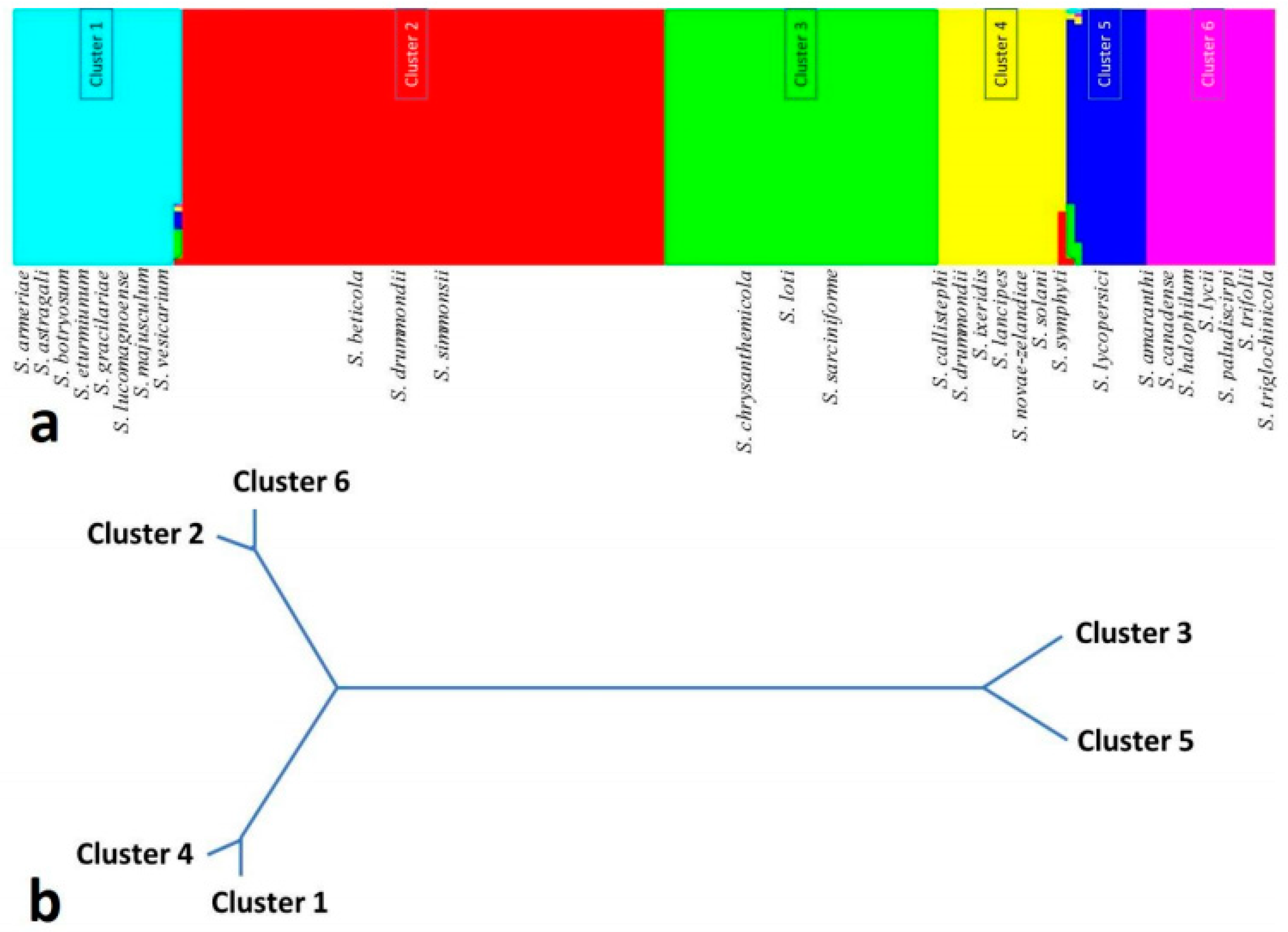
3. The Pathogen
4. Population Genetic Structure
5. Epidemiology
6. Symptoms and Disease Assessment
7. Secondary Metabolites and Pathogenicity
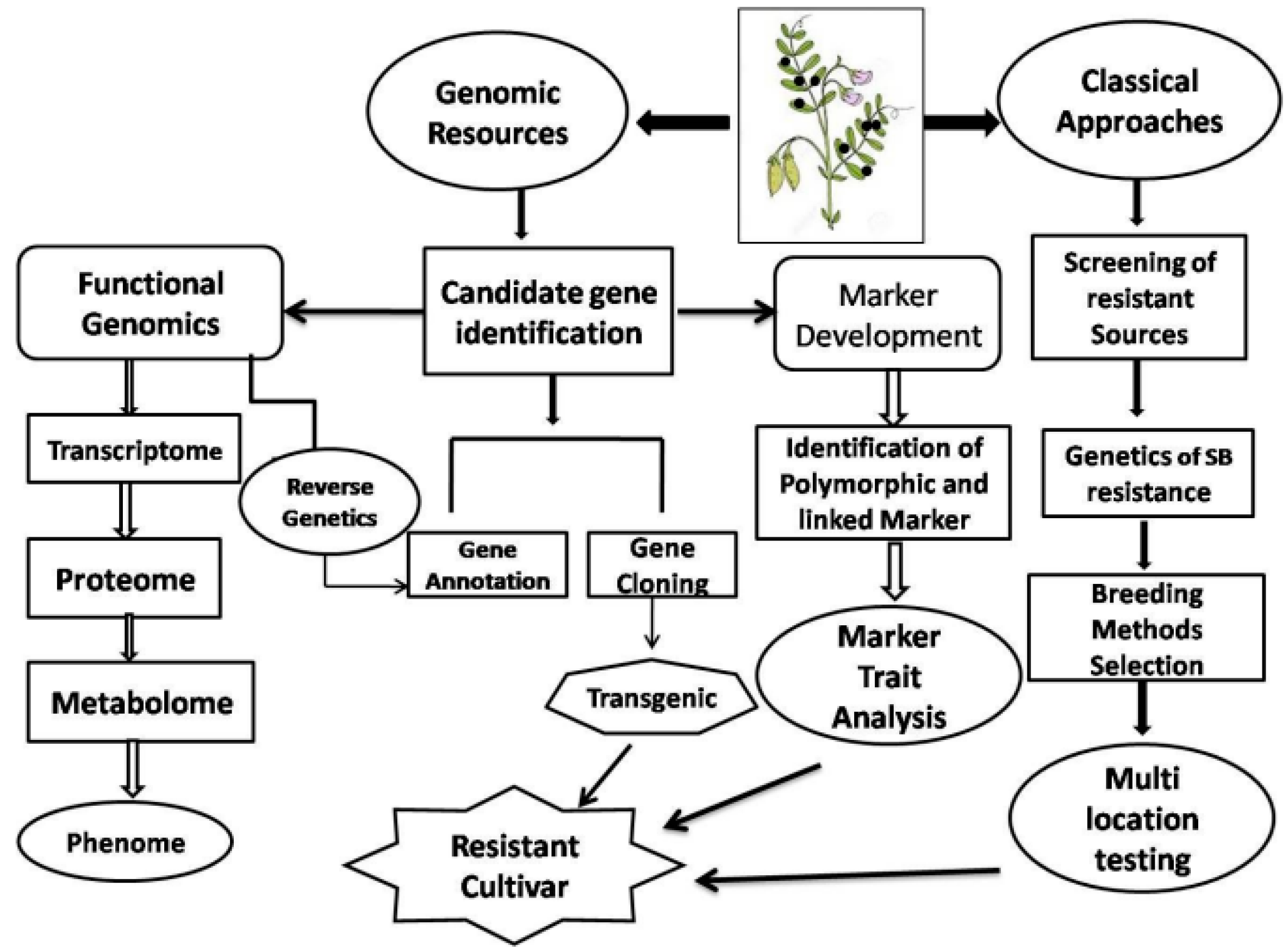
8. Disease Management
8.1. Host Plant Resistance (HPR)
8.2. Integrated Disease Management
9. Future Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAOSTAT 2017. Available online: http://faostatfaoorg/ (accessed on 15 March 2017).
- Raymond, J. World’s healthiest foods: Lentils (India). Health Mag. 2006. Available online: http://www.health.com/health/article/0,23414,1149140,00.html (accessed on 29 May 2019).
- Sen Gupta, D.; Thavarajah, D.; Knutson, P.; Thavarajah, P.; McGee, R.J.; Coyne, C.J.; Kumar, S. Lentils (Lens culinaris L.), a rich source of folates. J. Agric. Food Chem. 2013, 61, 7794–7799. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, G. Pulse Crops and Sustainability: A Framework to Evaluate Multiple Benefits. 2016. Available online: http://www.fao.org/pulses (accessed on 29 May 2019).
- Mwakutuya, E.; Banniza, S. Influence of temperature and wetness periods on the development of Stemphylium blight on lentil. Plant Dis. 2010, 94, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Morrall, R.A.A.; Carriere, B.; Pearse, C.; Schmeling, D.; Thomson, L. Seedborne pathogen of lentil in Saskatchewan in 2005. Can. Plant. Dis. Surv. 2006, 86, 104–106. [Google Scholar]
- Garvey, S. Stemphylium Blight Takes Hold. Farm Forum, Fall Issue. 2009. Available online: http://farmforum. ca/article/stemphylium-blight-takes-hold-2/ (accessed on 29 May 2019).
- Bakr, M.A.; Zahid, I. Stemphylium blights a new foliar disease of lentil in Bangladesh. Bangladesh J. Plant Pathol. 1986, 2, 69–70. [Google Scholar]
- Bakr, M.A.; Ahmed, F. Development of Stemphylium blight of lentil and its chemical control. Bangladesh J. Plant Pathol. 1992, 8, 39–40. [Google Scholar]
- Huq, M.I.; Khan, A. Epidemiology of Stemphylium blight of lentil. Bangladesh J. Sci. Ind. Res. 2008, 43, 513–520. [Google Scholar] [CrossRef][Green Version]
- Simay, E.I. Occurrence of Epicoccum and Stemphylium leaf spot of Lens culinaris Medik. in Hungary. Lens 1990, 17, 28–30. [Google Scholar]
- Sinha, J.N.; Singh, A.P. Stemphylium sarciniforme on Lens culinaris. Indian Phytopathol. 1991, 44, 421. [Google Scholar]
- Joshi, S. Review of important grain legume diseases and their management. In Proceedings of the National Workshop on Integrated Pest Management (IPM); Plant Protection Society of Nepal: Lalitpur, Nepal, 2006; pp. 100–116. [Google Scholar]
- Kant, P.; Materne, M.; Rodda, M.S.; Slater, A.T. Screening lentil germplasm for Stemphylium blight resistance. Australas. Plant Pathol. 2017, 46, 129–136. [Google Scholar] [CrossRef]
- Sinha, J.N.; Singh, A.P. Effect of environment on the development and spread of stemphylium blight of lentil. Indian Phytopathol. 1993, 46, 252–253. [Google Scholar]
- Polfliet, M. Infection of Stemphylium increases every year. Fruitteelt 2002, 92, 16–17. [Google Scholar]
- Wang, Y.; Zhang, X.G. Three new species of Stemphylium from China. Mycotaxon 2006, 96, 77–81. [Google Scholar]
- Puig, M.; Ruz, L.; Montesinos, E.; Moragrega, C.; Llorente, I. Combined morphological and molecular approach for identification of Stemphylium vesicarium inoculum in pear orchards. Fungal Biol. 2015, 119, 136–144. [Google Scholar] [CrossRef]
- Koike, S.T.; Henderson, D.M.; Butler, E.E. Leaf spot disease of spinach in California caused by Stemphylium botryosum. Plant Dis. 2001, 85, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Everts, K.L.; Armentrout, D.K. Report of leaf spot of spinach caused by Stemphylium botryosum in Maryland and Delaware. Plant Dis. 2001, 85, 1209. [Google Scholar] [CrossRef]
- Cowling, W.A.; Gilchrist, D.G. Expression of pathogen virulence and host resistance during infection of Alfalfa with Stemphylium botryosum. Phytopathology 1982, 72, 36–42. [Google Scholar] [CrossRef]
- Rokaibah, A.A. Leaf blight, a new bacterial disease of alfalfa associated with Stemphylium leaf spot. Alexandria J. Agric Sci. 1996, 27, 47–55. [Google Scholar]
- Berg, C.C.; Leath, K.T. Responses of red clover cultivars to Stemphylium leaf spot. Crop Sci. 1996, 36, 71–73. [Google Scholar] [CrossRef]
- Camara, M.P.S.; O’Neill, N.R.; van Berkum, P. Phylogeny of Stemphylium spp. based on ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 2002, 94, 660–672. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, Y.; Pei, Y.F. Molecular and morphological description of two new species of Stemphylium from China and France. Mycologia 2010, 102, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, J.H.C.; Hanse, B.; Van Leeuwen, G.C.M.; Groenewald, J.Z.; Crous, P.W. Stemphylium revisited. Stud. Mycol. 2017, 87, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Graf, S.; Bohlen-Janssen, H.; Miessner, S.; Wichura, A.; Stammler, G. Differentiation of Stemphylium vesicarium from Stemphylium botryosum as causal agent of the purple spot disease on asparagus in Germany. Eur. J. Plant Pathol. 2016, 144, 411–418. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 117693430500100003. [Google Scholar] [CrossRef]
- Muñoz-Pajares, A.J. SIDIER: Substitution and indel distances to infer evolutionary relationships. Methods Ecol. Evol. 2013, 4, 1195–1200. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Strange, R.N.; Sabia, C.; Visconti, A. Production of a toxin stemphol by Stemphylium species. Nat. Toxins 1994, 2, 14–18. [Google Scholar] [CrossRef]
- Erskine, W.; Sarker, A. Lentil: The Bangladesh Breakthrough; ICARDA Caravan No. 6; ICARDA: Aleppo, Syria, 1997. [Google Scholar]
- Bakr, M.A. Plant protection of lentil in Bangladesh. In Proceedings of the Seminar on Lentil in South Asia, New Delhi, India, 11–15 March 1991. [Google Scholar]
- Hashemi, P.; Vandenberg, A.; Banniza, S. Developing a protocol for large scale inoculation of lentil germplasms with Stemphylium botryosum. Proc. Plant Can. 2005, 15–18. [Google Scholar]
- Kumar, P. Genetics of Resistance to Stemphylium Leaf Blight of Lentil (Lens culinaris) in the Cross Barimasur-4 × CDC Milestone. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2007. [Google Scholar]
- Saha, G.C.; Sarker, A.; Chen, W.; Vandemark, G.J.; Muehlbauer, F.J. Inheritance and linkage map positions of genes conferring resistance to stemphylium blight in lentil. Crop Sci. 2010, 50, 1831–1839. [Google Scholar] [CrossRef]
- Podder, R.; Banniza, S.; Vandenberg, A. Screening of wild and cultivated lentil germplasm for resistance to stemphylium blight. Plant Genet. Resour. 2013, 11, 26–35. [Google Scholar] [CrossRef]
- Das, R.; Nath, R.; Dikshit, H.K. Host resistance of lentil genotypes against Stemphylium blight caused by Stemphylium botryosum Wallr. in lower gangetic alluvial zone of West Bengal, India. J. Mycopathol. Res. 2017, 55, 169–172. [Google Scholar]
- Trigos, Á.; Mendoza, G.; Espinoza, C.; Salinas, A.; Fernández, J.J.; Norte, M. The role of macrosporin in necrotic spots. Phytochem. Lett. 2011, 4, 122–125. [Google Scholar] [CrossRef]
- Singh, P.; Bugiani, R.; Cavanni, P.; Nakajima, H.; Kodama, M.; Otani, H.; Kohmoto, K. Purification and biological characterization of host-specific SV-toxins from Stemphylium vesicarium causing brown spot of European pear. Phytopathology 1999, 89, 947–953. [Google Scholar] [CrossRef]
- Tanahashi, M.; Okuda, S.; Miyazaki, E.; Parada, R.Y.; Ishihara, A.; Otani, H.; Osaki-Oka, K. Production of Host-selective SV-toxins by Stemphylium sp. Causing Brown Spot of European Pear in Japan. J. Phytopathol. 2017, 165, 189–194. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Müller, W.E.; Totzke, F.; Zirrgiebel, U.; Schächtele, C.; Kubbutat, M.H.G.; Lin, W.H.; et al. Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J. Natl. Prod. 2009, 72, 626–631. [Google Scholar] [CrossRef]
- Liu, Y.; Marmann, A.; Abdel-Aziz, M.S.; Wang, C.Y.; Müller, W.E.; Lin, W.H.; Mándi, A.; Kurtán, T.; Daletos, G.; Proksch, P. Tetrahydroanthraquinone derivatives from the endophytic fungus Stemphylium globuliferum. Eur. J. Org. Chem. 2015, 2015, 2646–2653. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Edrada-Ebel, R.A.; Müller, W.E.G.; Kubbutat, M.H.G.; Wray, V.; Rainer, E.; Proksch, P. Protein kinase inhibitors and other cytotoxic metabolites from the fungal endophyte Stemphylium botryosum isolated from Chenopodium album. Mycosphere 2010, 1, 153–162. [Google Scholar]
- Kanamaru, S.; Miho, H.; Takanori, M.; Taro, T.; Shinji, K.; Kazuaki, T.; Ken-Ichi, N.; Tatsuo, N.; Masaru, H. Absolute stereochemistry of altersolanol A and alterporriols. Chirality 2012, 24, 137–146. [Google Scholar] [CrossRef]
- Olsen, K.J.K.; Rossman, A.; Andersen, B. Metabolite production by species of Stemphylium. Fungal Biol. 2018, 122, 172–181. [Google Scholar] [CrossRef]
- Chowdhury, A.M.; Ahmed, A.; Zaman, M. Studies on the defence structural factors of some susceptible and resistant varieties of lentil plants. J. Mycol. Res. 1997, 35, 35–39. [Google Scholar]
- Rahman, M.M.; Ahmed, M.I.M.H.B.; Rahman, M.M. Performance of advanced lentil genotypes in different pulse growing regions of Bangladesh. Eco-Friendly Agric. J. 2015, 8, 116–120. [Google Scholar]
- Mondal, D.; Bhattacharyya, P.K.; Das, R. Disease Reaction of Lentil Genotypes against Stemphylium Blight caused by Stemphylium botryosum Wallr. in West Bengal. J. Agroecol. Nat. Res. Manag. 2017, 4, 149–152. [Google Scholar]
- Mondal, D.; Bhattacharyya, P.K.; Das, R.; Bhattacharyya, S. Diversity analysis by SSR markers and morphological markers among Stemphylium blight tolerance genotypes with high yielding cultivars of Lentil (Lens culinaris Medik). Legume Res. 2018. [Google Scholar] [CrossRef]
- Naik, M.K.; Prasad, Y.; Bhat, K.V.; Devika Rani, G.S. Morphological, physiological, pathogenic and molecular variability among isolates of Alternaria solani from tomato. Indian Phytopathol. 2010, 63, 168–173. [Google Scholar]
- Subedi, S.; Shrestha, S.M.; Khatri-Chhetri, G.B.; Thapa, R.B.; Ghimire, S.K.; Ghatri, D.B.; Neupane, S. Influence of sowing date of sowing on stemphylium blight disease severity and yield performance of lentil at Rampur, Chitwan, Nepal. J. Inst. Agric. Anim. Sci. 2015, 33, 129–136. [Google Scholar] [CrossRef]
- Menzies, S.A.; Broadhurst, P.G.; Triggs, C.M. Stemphylium disease of asparagus (Asparagus officinalis L.) in New Zealand. N. Z. J. Crop Hortic. Sci. 1992, 20, 427–433. [Google Scholar] [CrossRef]
- Basallote, M.J.; Prados, A.M.; Perez de Algaba, A.; Melero Vara, J.M. First report in Spain of two leaf spots of garlic caused by Stemphylium vesicarium. Plant Dis. 1993, 77, 952. [Google Scholar] [CrossRef]
- Meyer, M.P.; Hausbeck, M.K.; Podolsky, R. Optimal fungicide management of purple spot of asparagus and impact on yield. Plant Dis. 2000, 84, 525–530. [Google Scholar] [CrossRef]
- Montesinos, E.; Vilardell, P. Evaluation of FAST as a forecasting system for scheduling fungicide sprays for control of Stemphylium vesicarium on pear. Plant Dis. 1992, 76, 1221–1226. [Google Scholar] [CrossRef]
- Llorente, I.; Vilardell, P.; Bugiani, R.; Gherardi, I.; Montesinos, E. Evaluation of BSP cast disease warning system in reduced fungicide use programs for management of brown spot of pear. Plant Dis. 2000, 84, 631–637. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salam, M.U.; Day, T.K.; Ahmed, A.U.; Nessa, N.; Haque, A.H.M.M.; Subedi, S.; Malik, A.I.; Rahman, M.M.; Erskine, W. Stempedia: A weather-based model to explore and manage the risk of lentil Stemphylium blight disease. Australas. Plant Pathol. 2016, 45, 499–507. [Google Scholar] [CrossRef]
- Rashid, M.H.; Uddin, M.J.; Islam, Q.M.S. Development of Integrated Management Package for Stemphylium blight and Rust Disease of Lentil Project, PRC, BARI, Ishurdi, Pabna; Government of the People’s Republic of Bangladesh, Bangladesh Secretariat: Dhaka, Bangladesh, 2009; p. 46.
- Gharti, D.B.; Joshi, S.; Darai, R.; Ghimire, T.N.; Chadaro, M.B. Identifying sources of resistance to major diseases of lentil. In Proceedings of the 28th National Winter Crops Research Workshop held at RARS, Lumle, Kaski, Falgun, 9–10 March 2011; pp. 25–26. [Google Scholar]
- Islam, S.; Ariful, M. Search for Resistance and Chemical Control against Stemphylium Blight Disease of Lentil. Ph.D. Thesis, Sher –E-Bangla Agricultural University, Dhaka, Bangladesh, 2015. [Google Scholar]
- Bhadauria, V.; Ramsay, L.; Bett, K.E.; Banniza, S. QTL mapping reveals genetic determinants of fungal disease resistance in the wild lentil species Lens ervoides. Sci. Rep. 2017, 7, 3231. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.K.; Ghimire, S.K.; Shrestha, S.M.; Sah, B.P.; Sarker, A.; Sah, S.K. Source of resistant against Fusarium wilt and Stemphylium blight in lentil (Lens culinaris Medikus). Int. J. Appl. Sci. Biotechnol. 2017, 5, 102–107. [Google Scholar] [CrossRef]
- Razzak, M.A.; Islam, M.A.; Rahman, M.H.; Sathi, M.A.; Atikuzzamman, M. Screening of lentil germplasm against stemphylium blight by observing disease reaction in three different stages. Reason 2018, 45, 3. [Google Scholar] [CrossRef]
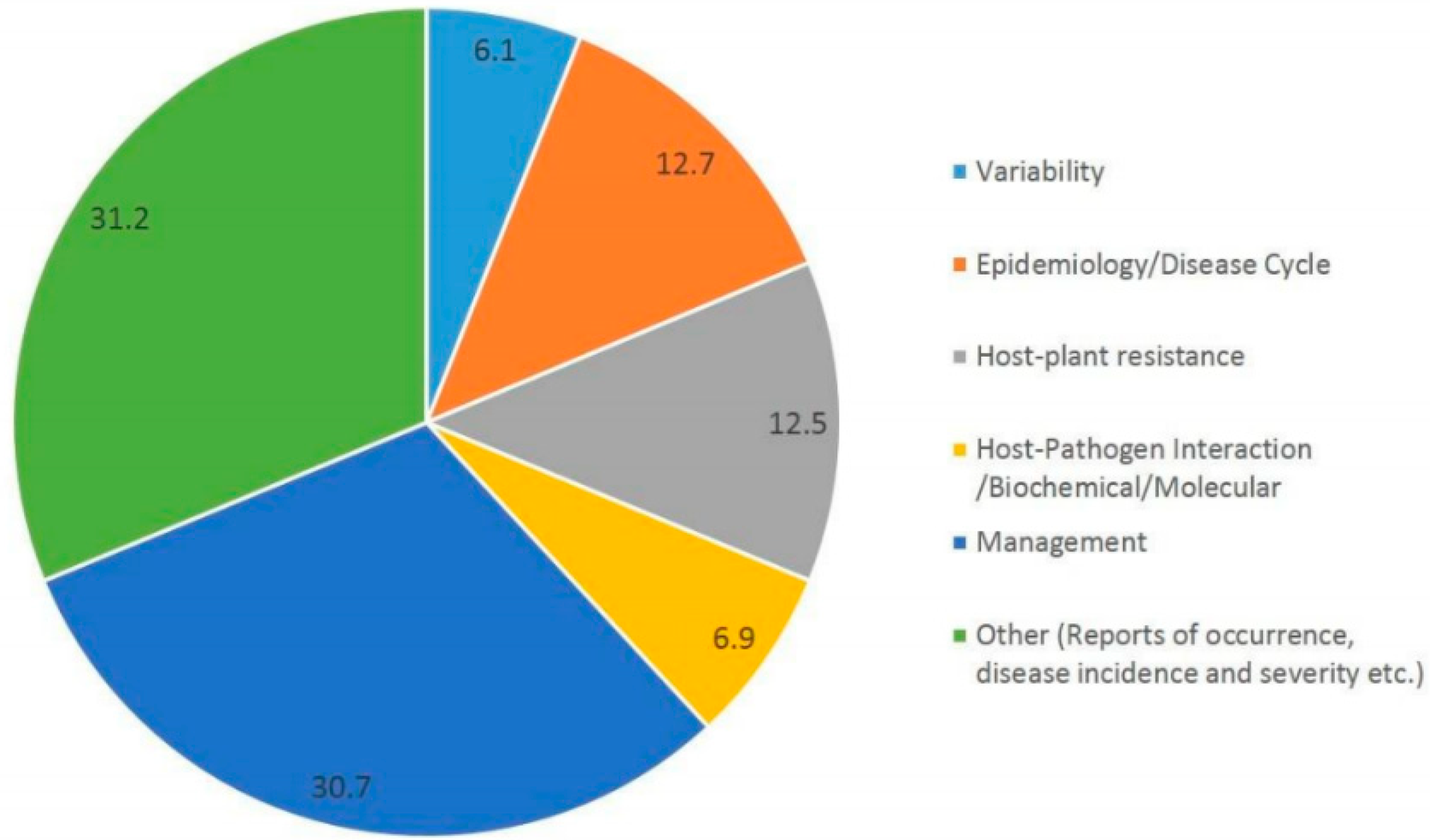
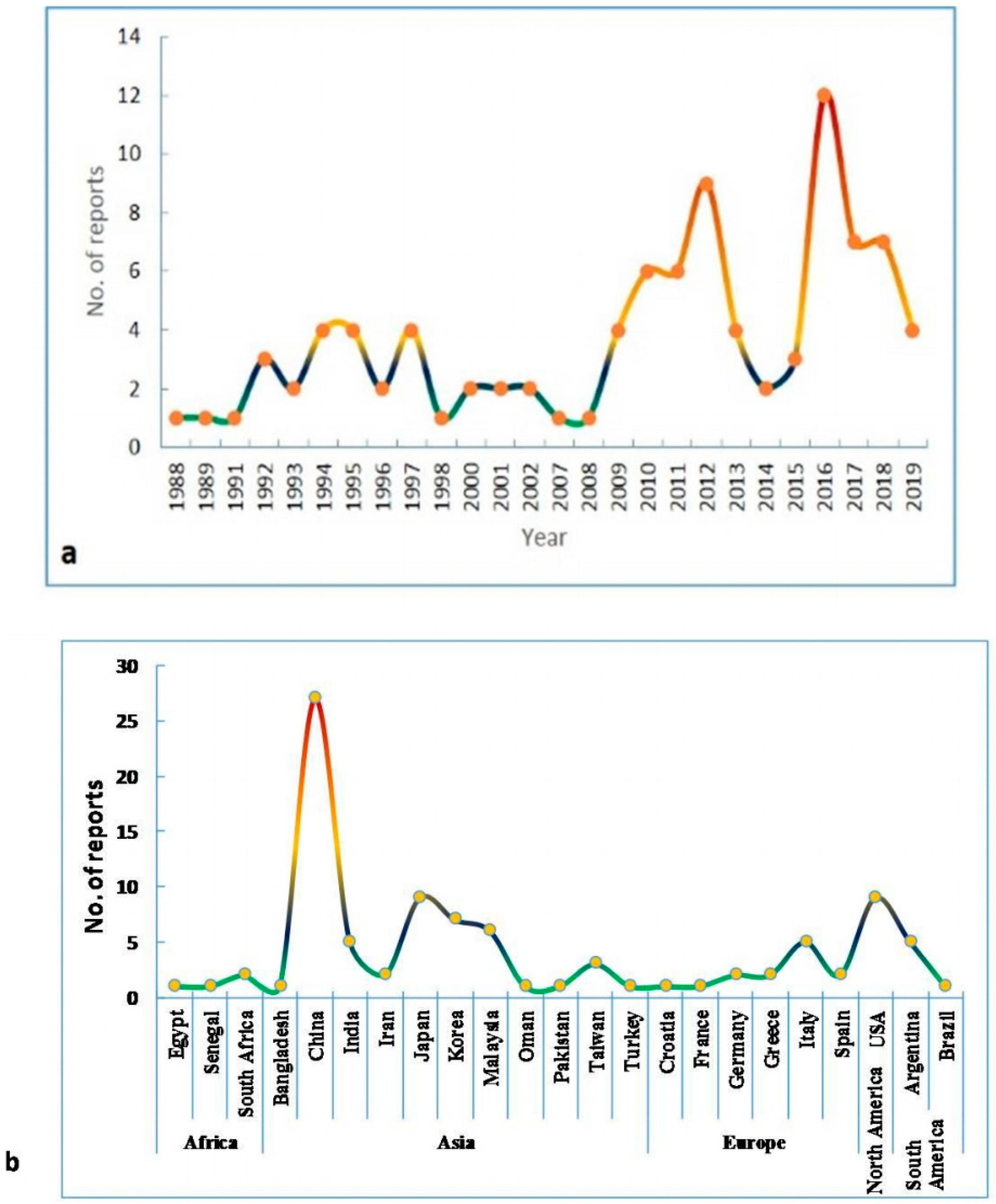
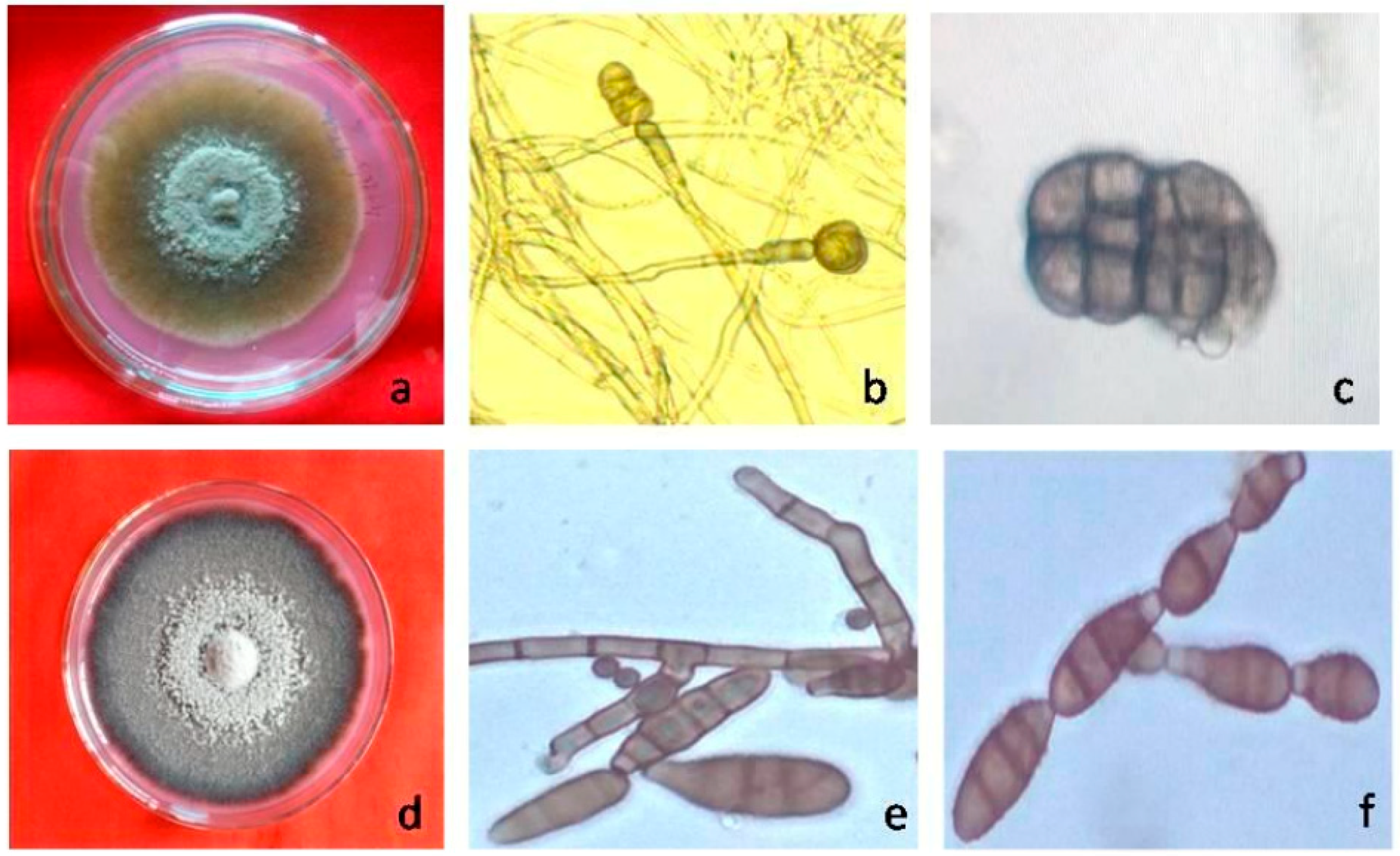
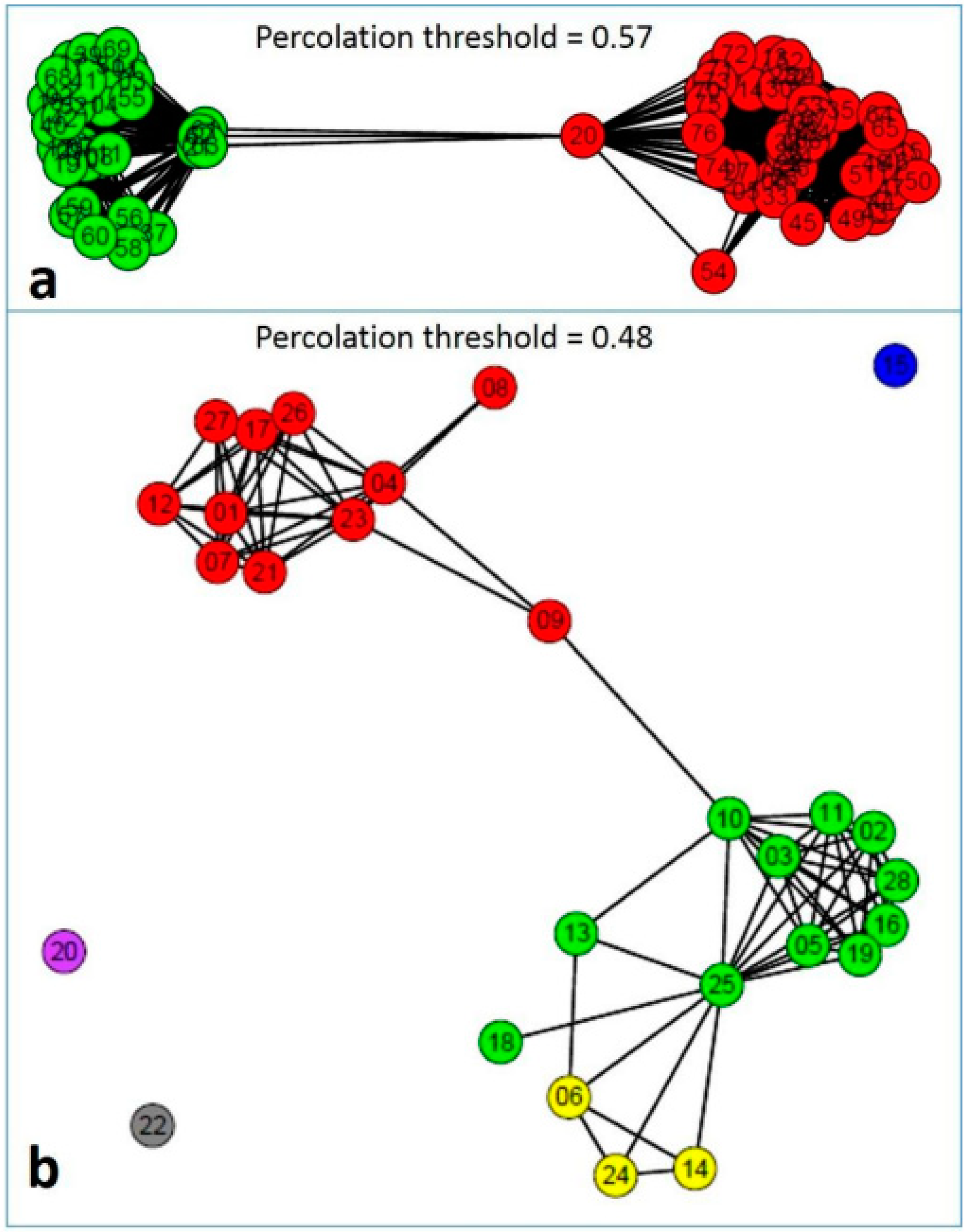

| Genera | Alternaria | Stemphylium | Pithomyces | Epicoccum | Ulocladium |
|---|---|---|---|---|---|
| Colony character | Olivaceous to gray to black woolly colonies | Velvety to cottony brown or black colony | Brown to black in color | Colony fast growing, with a strong yellow to orange-brown diffusible pigment. | Distinctive yellow to orange-brown color colony with brown diffusing pigment |
| Conidium shape | Large, dark muriform with beak | Large, dark muriform | Large, dark muriform | Large, dark muriform | Large, dark verrucose muriform |
| Conidium formation | Conidia formed in chains or singly | Conidia formed singly | Conidia formed singly | Conidia formed singly on densely compacted, non-specialized, determinant | Conidia formed singly |
| Conidium arrangement | Lacks percurrent proliferation (Conidia produced through nodes on conidiophores | Percurrent proliferation present | Lacks percurrent proliferation and geniculate conidiophores | Conidial production restricted to sporodochia areas | Conidia formed in a sympodial fashion from geniculate conidiophores |
| Conidiophore | Erect, septate, and geniculate | Short, arise singly or in whorls, septate and swollen at the apex. | Short, peg like lateral branches from the vegetative hyphae | Nonspecialized, determinant; branches repeatedly and visible as dense masses in sporodochia | Simple or branched, smooth, strongly geniculate |
| Locus | #ind | NS | s | K | π | #h | Hd | Fs | D (p Value) |
|---|---|---|---|---|---|---|---|---|---|
| ITS | 157 | 518 | 53 | 6.44 | 0.0133 ± 0.0008 | 27 | 0.85 ± 0.02 | −4.493 (0.21) | −1.215 (0.09) |
| gpd | 157 | 516 | 151 | 24.57 | 0.0495 ± 0.0017 | 43 | 0.92 ± 0.02 | 3.368 (0.82) | −0.180 (0.50) |
| Calmodulin | 157 | 664 | 206 | 42.43 | 0.0704 ± 0.0019 | 49 | 0.95 ± 0.01 | 8.950 (0.94) | 0.476 (0.74) |
| 28S rRNA | 22 | 796 | 50 | 4.91 | 0.0062 ± 0.0045 | 7 | 0.67 ± 0.09 | −21.560 (0.00) | −2.535 (0.00) |
| ATPase | 47 | 684 | 183 | 38.38 | 0.0598 ± 0.0069 | 22 | 0.93 ± 0.02 | −17.938 (0.00) | −0.308 (0.45) |
| EF-1 | 51 | 861 | 323 | 47.97 | 0.0786 ± 0.0149 | 21 | 0.93 ± 0.02 | −11.313 (0.00) | −1.085 (0.12) |
| Histidine kinase | 9 | 1187 | 4 | 1.39 | 0.0007 ± 0.0001 | 5 | 0.89 ± 0.07 | −10.848 (0.00) | −0.229 (0.41) |
| Sources of Variation | Sum of Squares | Variance Components | Percentage Variation |
|---|---|---|---|
| Among populations | 7003.487 | 49.68865 | 96.78650 |
| Within populations | 212.819 | 1.64976 | 3.21350 |
| Total | 7216.306 | 51.33841 | |
| FST | 0.96786 (p < 0.0001) | ||
| Serial | Genotypes | Remark | References |
|---|---|---|---|
| 1. | Barimasur-4 | Resistant | [33] |
| 2. | Eston and IG-72815 | Resistant | [36] |
| 3. | 10/P8406-122, FLIP-92- 52LX, LR-9-135, LR-9-130, LR-9-179, LR-9-69, LR-9-69, LR-9-100, LR-9-118, LR-9-28, LR-9-25, Procoz, LR-9-57, LR-9-107, LR-9-105, LR-9-48, LR-9-62, LR-9-25, 10/P11X955-135, 10/P2 FLIP-92-52LX955-167(4), and 10/P8405-23 | Resistant | [60] |
| 4. | ILL-7164, ILL-6458, ILL-1704, ILL-9927, ILL-8006(BM-4), ILL-1672, X94s43, ILL-2573, ILL-9992, ILL-6025, Aarial, ILL-8093, ILL-9976, ILL-6256, IL-1, ILL-6818, ILL-2700-1, X94s29, ILL- 9931, ILL-9996, ILL-5787, and ILL -8191 | Moderately Resistant | [61] |
| 5. | IG-72803, IG-116033, L-01-827, IG-72548, IG-72551, IG-72553, IG-72557, IG-72713, IG-72843, IG-136645, IG-72829, IG-72643, IG-72606, IG-72537, IG-72552, and IG-110809 | Resistant | [38] |
| 6. | BLX-06004-12, BLX-06004-2, and BLX-05001-6 | Moderately resistant | [62] |
| 7. | LL-1370, VL-151, LL-1375, RLG-195, L-4727, L-4769, LL-1397, DL-14-2, VL-526, VL-126, RKL- 14-20, IPL-334, L-4710, PL-210, and Precoz | Moderately resistant with 30% of foliage affected | [50] |
| 8. | P-3235, LL-1122, and ILL-10832 | Immune | [39] |
| 9. | L01-827A and IG-72815 | Lens ervoides accessions showing multiple resistance | [63] |
| 10. | ILL-0426, ILL- 0427, ILL-0215, ILL-6408, ILL-0133, ILL-0379, ILL-0365, and ILL-0192 | Resistant to moderately resistant | [14] |
| 11. | RL-13, RL-21, ILL-6468, ILL-9996, ILL-6024, ILL-6811, ILL-7164, Arun, and Maheswar Bharti, | Multiple Resistant | [64] |
| 12. | BD-3921, BD-3930, BD-3931, and BARI Masur-7 | Highly Resistant | [65] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.; Dutta, S.; Jash, S.; Barman, A.R.; Das, R.; Kumar, S.; Gupta, S. Current Knowledge on Pathogenicity and Management of Stemphylium botryosum in Lentils (Lens culinaris ssp. culinaris Medik). Pathogens 2019, 8, 225. https://doi.org/10.3390/pathogens8040225
Das A, Dutta S, Jash S, Barman AR, Das R, Kumar S, Gupta S. Current Knowledge on Pathogenicity and Management of Stemphylium botryosum in Lentils (Lens culinaris ssp. culinaris Medik). Pathogens. 2019; 8(4):225. https://doi.org/10.3390/pathogens8040225
Chicago/Turabian StyleDas, Arpita, Subrata Dutta, Subhendu Jash, Ashis Roy Barman, Raju Das, Shiv Kumar, and Sanjeev Gupta. 2019. "Current Knowledge on Pathogenicity and Management of Stemphylium botryosum in Lentils (Lens culinaris ssp. culinaris Medik)" Pathogens 8, no. 4: 225. https://doi.org/10.3390/pathogens8040225
APA StyleDas, A., Dutta, S., Jash, S., Barman, A. R., Das, R., Kumar, S., & Gupta, S. (2019). Current Knowledge on Pathogenicity and Management of Stemphylium botryosum in Lentils (Lens culinaris ssp. culinaris Medik). Pathogens, 8(4), 225. https://doi.org/10.3390/pathogens8040225





