Direct-Acting Antiviral Agents for HCV-Associated Glomerular Disease and the Current Evidence
Abstract
1. Introduction
2. HCV-Associated Kidney Disease: Histology
3. HCV and Kidney-Updated Evidence
4. Treatment of HCV-Related Glomerular Disease: Historical Perspective
5. Antiviral Treatment of HCV with DAAs and Renal Impairment
6. Antiviral Treatment of HCV (DAAs) for HCV-Related Glomerular Disease
7. Immunosuppressive Agents for Treatment of HCV-Related Glomerular Disease
8. Rituximab for Treatment of HCV-Related Glomerular Disease
9. Non-Selective Immunosuppression for HCV-Related Glomerular Disease
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ACEIs | Angiotensin-converting enzyme inhibitors |
| AEs | Adverse events |
| ARBs | Angiotensin-receptor blockers |
| CI | Confidence Intervals |
| CKD | Chronic kidney disease |
| DAAs | Direct-acting antiviral agents |
| DCV | Daclatasvir |
| 3D | Ritonavir-boosted paritaprevir/ombitasvir/dasabuvir |
| EBR | Elbasvir |
| eGFR | Estimated glomerular filtration rate |
| ESRD | End-stage renal disease |
| FDV | Faldaprevir |
| GRZ | Grazoprevir |
| GN | Glomerulonephritis |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HIV | Human immunodeficiency virus |
| HD | Haemodialysis |
| MCS | Mixed cryoglobulinemia syndrome |
| MPGN | Membranoproliferative glomerulonephritis |
| IFN | Interferon |
| LDV | Ledipasvir |
| pegIFN | Pegylated interferon |
| RBV | Ribavirin |
| RF | Rheumatoid factor |
| RT | Renal transplant |
| RTX | Rituximab |
| SIM | Simeprevir |
| SOF | Sofosbuvir |
| SVR | Sustained virological response |
References
- Fabrizi, F.; Plaisier, E.; Saadoun, D.; Martin, P.; Messa, P.; Cacoub, P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am. J. Kidney Dis. 2013, 61, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, S.; Chung, T.; Sise, M. Treatment of hepatitis C virus infection in patients with mixed cryoglobulinemic syndrome and cryoglobulinemic glomerulonephritis. Hemodial. Int. 2018, 22, S81–S96. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease Improving Global Outcomes (KDIGO) Hepatitis C Work Group. KDIGO 2018 Clinical Pratice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int. Suppl. 2018, 8, 91–165. [Google Scholar] [CrossRef] [PubMed]
- Kasuno, K.; Ono, T.; Matsumori, A.; Nogaki, F.; Kusano, H.; Watanabe, H.; Yodoi, J.; Muso, E. Hepatitis C virus-associated tubulo-interstitial injury. Am. J. Kidney Dis. 2003, 41, 767–775. [Google Scholar] [CrossRef]
- Fabrizi, F.; Donato, F.; Messa, P. Association between hepatitis C virus and chronic kidney disease: A systematic review and meta-analysis. Ann. Hepatol. 2018, 17, 364–391. [Google Scholar] [CrossRef]
- Fabrizi, F.; Donato, F.; Messa, P. Association between hepatitis B virus and chronic kidney disease: A systematic review and meta-analysis. Ann. Hepatol. 2017, 16, 21–47. [Google Scholar] [CrossRef]
- Rossi, C.; Raboud, J.; Walmsley, S.; Cooper, C.; Antoniou, T.; Burchell, A.N.; Hull, M.; Chia, J.; Hogg, R.S.; Moodie, E.E.; et al. The Canadian Observational Cohort (CANOC) Collaboration. The Hepatitis C co-infection is associated with an increased risk of incident chronic disease in HIV-infected patients initiating combination antiretroviral therapy. BMC Infect. Dis. 2017, 17, 246. [Google Scholar] [CrossRef]
- Johnson, R.J.; Gretch, D.R.; Yamabe, H.; Hart, J.; Bacchi, C.E.; Hartwell, P.; Couser, W.G.; Corey, L.; Wener, M.H.; Alpers, C.E.; et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N. Engl. J. Med. 1993, 328, 465–470. [Google Scholar] [CrossRef]
- Rossi, P.; Bertani, T.; Baio, P.; Caldara, R.; Luliri, P.; Tengattini, F.; Bellavita, P.; Mazzucco, G.; Misiani, R. Hepatitis C virus-related cryoglobulinemic glomerulonephritis: Long-term remission after antiviral therapy. Kidney Int. 2003, 63, 2236–2241. [Google Scholar] [CrossRef][Green Version]
- Alric, L.; Plaisier, E.; Thébault, S.; Péron, J.M.; Rostaing, L.; Pourrat, J.; Ronco, P.; Piette, J.C.; Cacoub, P. Influence of antiviral therapy in hepatitis C-associated cryoglobulinemic membranoproliferative glomerulonephritis. Am. J. Kidney Dis. 2004, 43, 617–623. [Google Scholar] [CrossRef]
- Fabrizi, F.; Dixit, V.; Messa, P. Interferon mono-therapy for symptomatic HCV associated mixed cryoglobulinemia: Meta-analysis of clinical studies. Acta Gastroenterol. Belg. 2013, 76, 363–371. [Google Scholar] [PubMed]
- Fabrizi, F.; Dixit, V.; Messa, P. Antiviral therapy of symptomatic HCV associated mixed cryoglobulinemia: Meta-analysis of clinical studies. J. Med. Virol. 2013, 85, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, A.; Cantarelli, C.; Cravedi, P. HCV-associated nephropathies in the era of direct acting antiviral agents. Front. Med. 2019, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- American Association for the Study of Liver Diseases (AASLD) and Infectious Disease Society of America (IDSA). HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Available online: http://hcv.guidelines.org (accessed on 21 September 2017).
- Saxena, V.; Koraishy, F.M.; Sise, M.E.; Lim, J.K.; Schmidt, M.; Chung, R.T.; Liapakis, A.; Nelson, D.R.; Fried, M.W.; Terrault, N.A.; et al. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016, 36, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.; Nelson, D.R.; Bruchfeld, A.; Liapakis, A.; Silva, M.; Monsour, H., Jr.; Martin, P.; Pol, S.; Londoño, M.C.; Hassanein, T.; et al. Grazoprevir plus elbasvir in treatment naïve and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study). Lancet 2015, 386, 1537–1545. [Google Scholar] [CrossRef]
- Gane, E.; Lawitz, E.; Pugatch, D.; Papatheodoridis, G.; Bräu, N.; Brown, A.; Pol, S.; Leroy, V.; Persico, M.; Moreno, C.; et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N. Engl. J. Med. 2017, 377, 1448–1455. [Google Scholar] [CrossRef]
- Sise, M.E.; Wisocky, J.; Rosales, I.A.; Chute, D.; Holmes, J.A.; Corapi, K.M.; Babitt, J.L.; Tangren, J.S.; Hashemi, N.; Lundquist, A.L.; et al. Lupus-like immune complex-mediated glomerulonephritis in patients with hepatitis C virus infection treated with oral, interferon-free, direct-acting antiviral therapy. Kidney Int. Rep. 2016, 1, 135–143. [Google Scholar] [CrossRef]
- Ghosn, M.; Palmer, M.; Najem, C.; Haddad, D.; Merkel, P.; Hogan, J. New-onset hepatitis C virus-associated glomerulonephritis following sustained virologic response with direct acting antiviral therapy. Clin. Nephrol. 2017, 87, 261–266. [Google Scholar] [CrossRef]
- Barbieri, D.; García-Prieto, A.; Torres, E.; Verde, E.; Goicoechea, M.; Luño, J. Mixed cryoglobulinaemia vasculitis after sustained hepatitis C virological response with direct-acting antivirals. Clin. Kidney J. 2018, 12, 362–364. [Google Scholar] [CrossRef]
- Gragnani, L.; Visentini, M.; Fognani, E.; Urraro, T.; De Santis, A.; Petraccia, L.; Perez, M.; Ceccotti, G.; Colantuono, S.; Mitrevski, M.; et al. Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology 2016, 64, 1473–1482. [Google Scholar] [CrossRef]
- Sise, M.E.; Bloom, A.K.; Wisocky, J.; Lin, M.V.; Gustafson, J.L.; Lundquist, A.L.; Steele, D.; Thiim, M.; Williams, W.W.; Hashemi, N.; et al. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with sofosbuvir-based direct-acting antiviral agents. Hepatology 2016, 63, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, D.; Thibault, V.; Ahmed, S.N.S.; Alric, L.; Mallet, M.; Guillaud, C.; Izzedine, H.; Plaisier, A.; Fontaine, H.; Costopoulos, M.; et al. Sofosbuvir plus ribavirin for hepatitis C-associated cryoglobulinaemia vasculitis: Vascuvaldic study. Ann. Rheum. Dis. 2016, 75, 1777–1782. [Google Scholar] [CrossRef]
- Sollima, S.; Milazzo, L.; Peri, A.M.; Torre, A.; Antinori, S.; Galli, M. Persistent mixed cryoglobulinemia vasculitis despite hepatitis C virus eradication after interferon-free antiviral therapy. Rheumatology 2016, 55, 2084–2085. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.S.; Kuczynski, M.; La, D.; Almarzooqi, S.; Kowgier, M.; Shah, H.; Wong, D.; Janssen, H.L.; Feld, J.J. Efficacy and safety of direct acting antivirals for the treatment of mixed cryoglobulinemia. Am. J. Gastroenterol. 2017, 112, 1298–1308. [Google Scholar] [CrossRef]
- Saadoun, D.; Pol, S.; Ferfar, Y.; Alric, L.; Hezode, C.; Ahmed, S.N.S.; de Saint Martin, L.; Comarmond, C.; Bouyer, A.S.; Musset, L.; et al. Efficacy and safety of sofosbuvir plus daclatasvir for treatment of HCV-associated cryoglobulinemia vasculitis. Gastroenterology 2017, 153, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, M.; Lens, S.; Mariño, Z.; Londoño, M.C.; Rodriguez-Tajes, S.; Sánchez-Tapias, J.M.; Ramos-Casals, M.; Hernández-Rodríguez, J.; Forns, X. Long-term outcomes of patients with HCV-associated cryoglobulinemic vasculitis after virologic cure. Gastroenterology 2018, 155, 311–315. [Google Scholar] [CrossRef]
- Fabrizi, F.; Aghemo, A.; Lampertico, P.; Fraquelli, M.; Cresseri, D.; Moroni, G.; Passerini, P.; Donato, F.M.; Messa, P. Immunosuppressive and antiviral treatment of hepatitis C virus-associated glomerular disease: A long-term follow-up. Int. J. Artif. Organs 2018, 41, 306–318. [Google Scholar] [CrossRef]
- Obrisca, B.; Juribita, R.; Sorohan, B.; Iliescu, L.; Baston, C.; Bobeică, R.; Andronesi, A.; Leca, N.; Ismail, G. Clinical outcomes of HCV-associated cryoglobulinemic glomerulonephritis following treatment with direct acting antiviral agents: A case-based review. Clin. Rheumatol. 2019. [Google Scholar] [CrossRef]
- Fabrizi, F.; Paolucci, A.; Antonelli, B.; Cerutti, R.; Donato, F.M.; Lampertico, P.; Messa, P. Hepatitis C virus induced glomerular disease and posterior reversible encephalopathy syndrome after liver transplant: Case report and literature review. Saudi J. Kidney Dis. Transpl. 2019, 30, 239–249. [Google Scholar] [CrossRef]
- Zarębska-Michaluk, D.; Stępień, P.M.; Paluch, K.; Pabjan, P. Retreatment of symptomatic HCV genotype 3 associated mixed cryoglobulinemia with sofosbuvir plus ribavirin: A case report. Clin. Exp. Hepatol. 2018, 4, 100–103. [Google Scholar]
- Elmowafy, A.Y.; El Maghrabi, H.M.; Zahab, M.A.; Elwasif, S.M.; Bakr, M.A. Sofosbuvir and daclatasvir in treatment of HCV-related membranoproliferative glomerulonephritis with cryoglobulinemia in a patient with HCV genotype 4. Iran J. Kidney Dis. 2018, 12, 372–384. [Google Scholar]
- Nayak, S.; Kataria, A.; Sharma, M.; Rastogi, A.; Gupta, E.; Singh, A.; Tiwari, S. HCV associated membranoproliferative glomerulonepritis treated with direct-acting antivirals. Indian J. Nephrol. 2018, 28, 462–464. [Google Scholar] [PubMed]
- Chia, X.X.; Cherepanoff, S.; Danta, M.; Furlong, T. Successful treatment of HCV-related glomerulonephritis with sofosbuvir and daclatasvir. Nephrology 2018, 33, 37–380. [Google Scholar] [CrossRef] [PubMed]
- De Vita, S.; Quartuccio, L.; Isola, M.; Mazzaro, C.; Scaini, P.; Lenzi, M.; Campanini, M.; Naclerio, C.; Tavoni, A.; Pietrogrande, M.; et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012, 64, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Sneller, M.; Hu, Z.; Langford, C. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus associated cryoglobulinemic vasculitis. Arthritis Rheum. 2012, 64, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Roccatello, D.; Sciascia, S.; Rossi, D.; Solfietti, L.; Fenoglio, R.; Menegatti, E.; Baldovino, S. The challenge of treating hepatitis C virus-associated cryoglobulinemic vasculitis in the era of anti-CD20 monoclonal antibodies and direct antiviral agents. Oncotarget 2017, 8, 41764–41777. [Google Scholar] [CrossRef][Green Version]
- Roccatello, D.; Sciascia, S.; Baldovino, S.; Rossi, D.; Alpa, M.; Naretto, C.; Di Simone, D.; Menegatti, E. Improved (4 plus 2) rituximab protocol for severe cases of mixed cryoglobulinaemia: A 6-year observational study. Am. J. Nephrol. 2016, 43, 251–260. [Google Scholar] [CrossRef]
- Terrier, B.; Launay, D.; Kaplanski, G.; Hot, A.; Larroche, C.; Cathébras, P.; Combe, B.; De Jaureguiberry, J.P.; Meyer, O.; Schaeverbeke, T.; et al. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis, data from the French Autoimmunity and Rituximab registry. Arthritis Care Res. 2010, 62, 1787–1795. [Google Scholar] [CrossRef]
- Fabrizi, F.; Cresseri, D.; Fogazzi, G.B.; Moroni, G.; Passerini, P.; Martin, P.; Messa, P. Rituximab therapy for primary glomerulonepritis: Reports on two cases. World J. Clin. Cases 2015, 3, 736–742. [Google Scholar] [CrossRef]
- Fabrizi, F.; Martin, P.; Elli, A.; Montagnino, G.; Banfi, G.; Passerini, P.; Campise, M.R.; Tarantino, A.; Ponticelli, C. Hepatitis C virus infection and rituximab therapy after renal transplantation. Int. J. Artif. Organs 2007, 30, 445–449. [Google Scholar] [CrossRef]
- Hamzeh, M.; Hosseinimehr, S.J.; Khalatbary, A.R.; Mohammadi, H.R.; Dashti, A.; Amiri, F.T. Atorvastatin mitigates cyclophosphamide–induced hepatotoxicity via suppression of oxidative stress and apoptosis in rat model. Res. Pharm. Sci. 2018, 13, 440–449. [Google Scholar] [PubMed]
- Reed, M.J.; Alexander, G.J.M.; Thiru, S.; Smith, K.G.C. Hepatitis C-associated glomerulonephritis—A novel therapeutic approach. Nephrol. Dial. Transpl. 2001, 16, 869–871. [Google Scholar] [CrossRef] [PubMed]
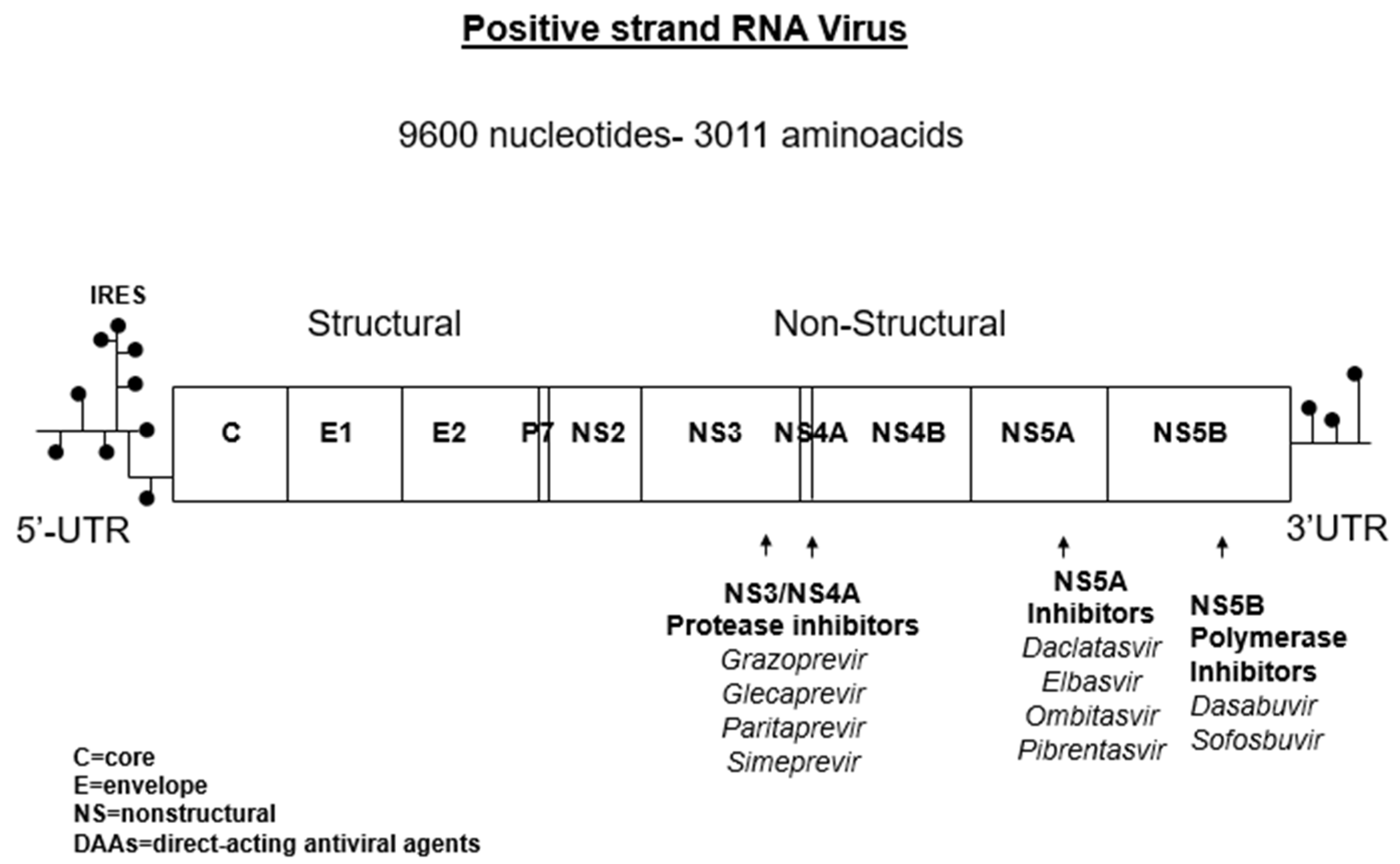
| Kidney Disease | Pathogenesis | Clinical Presentation |
|---|---|---|
| Cryoglobulinemic membranoproliferative GNs | Subendothelial and mesangial cryoglobulin deposits; mesangial deposits of immune complexes (HCV viral antigens, Ig and complement) | Nephritic or nephrotic syndrome |
| Noncryoglobulinemic membranoproliferative GN | Mesangial deposits of immune complexes (HCV viral antigens, Ig and complement) | Nephritic or nephrotic syndrome |
| Mesangial proliferative GN | Direct activity of HCV on mesangium | Proteinuria and/or haematuria |
| Membranous nephropathy | Subepithelial deposits of immune complexes | Nephrotic syndrome |
| Berger’s disease (IgA nephropathy) | Mesangial deposits of immune complexes | Nephritic syndrome, isolated proteinuria and/or haematuria |
| Tubulo-interstitial nephritis | HCV deposition in tubular epithelial (perinuclear areas) and infiltrating cells | Proteinuria |
| Focal and segmental glomerulosclerosis | Direct injury by HCV on podocytes of epithelial cells | Nephrotic syndrome, isolated proteinuria |
| Polyarteritis nodosa | Immune complexes in medium-sized muscular arteries | Haematuria and/or proteinuria |
| Immunotactoid glomerulopathy | Deposits (glomerular capillary wall and mesangium) containing microtubular structures | Nephrotic syndrome, isolated proteinuria and/or haematuria |
| Fibrillary GN | Mesangial deposits (containing randomly oriented fibrillar material) (fibrils composed of antigen-antibody immune complexes) | Nephrotic syndrome, isolated proteinuria and/or haematuria |
| Daclatasvir (60 mg) | CKD stage 1,2,3 |
| Elbasvir/Grazoprevir (50 mg/100 mg) | |
| Glecaprevir/Pibrentasvir (300 mg/120 mg) | |
| Ledipasvir/Sofosbuvir (90 mg/400 mg) | |
| Sofosbuvir/Velpatasvir (400 mg/100 mg) | |
| Simeprevir (150 mg) | |
| Sofosbuvir (400 mg) | |
| Sofosbuvir/Velpatasvir/Voxilaprevir (400 mg/100 mg/100 mg) | |
| Ritonavir-boosted Paritaprevir/Ombitasvir/Dasabuvir±Ribavirin (PrOD or 3D regimen) (50 mg/75 mg/12.5 mg/250 mg/200 mg) | CKD stage 4,5 |
| Elbasvir/Grazoprevir (50 mg/100 mg) | |
| Glecaprevir/Pibrentasvir (300 mg/120 mg) |
| DAAs | SVR12 | Complete Clinical Response | Partial Clinical Response | Concomitant IS | |
|---|---|---|---|---|---|
| Gragnani L, et al. (2016) (n = 4) | SOF-based regimen | 4(100%) | 3(75%) | 1(25%) | 1(25%) |
| Sise M, et al. (2016) (n = 7) | SOF+SIM (n = 6) SOF+RBV (n = 1) | 6(86%) | 3(43%) | 4(57%) | 2(29%) |
| Saadoun D, et al. (2016) (n = 5). | SOF+RBV | 4(80%) | 0 | 4(80%) | 2(40%) |
| Sollima S, et al. (2016) (n = 5) | SOF+RBV SOF+DCV SOF+SIM 3D | 5(100%) | 0 | 1(20%) | 0 |
| Emery J, et al. (2017) (n = 10) | SOF+RBV SOF+SIM 3D±RBV SOF+LDV±RBV | 7(70%) | 2(20%) | 2(20%) | 4(40%) |
| Saadoun D, et al. (2017) (n = 5) | SOF+DCV | 5(100%) | 3(60%) | 1(20%) | NA |
| Bonacci M, et al. (2018) (n = 9) | SOF-based regimen±RBV 3D±RBV SIM+DCV GZR+EBR pegIFN+DAAs FDV+DLR | 9(100%) | 6(67%) | 3(33%) | 5(55%) |
| Fabrizi F, et al. (2018) (n = 13) | SOF+RBV (n = 6) 3D+RBV (n = 4) SOF+LDV (n = 1) SOF+DCV+RBV (n = 2) | 13(100%) | 3(23%) | 7(54%) | 9(69.2%) |
| Obrisca B, et al. (2019) (n = 9) | 3D | 9(100%) | 2(23%) | 1(10%) | 6(67%) |
| Presentation | Treatment |
|---|---|
| Non-nephrotic proteinuria | DAA-based regimen (Table 1) ACEIs and/or ARBs Diuretics, anti-hypertensive agents |
| Stable and mild kidney dysfunction (GFR > 30 mL/min/1.72 m2) | |
| Nephrotic syndrome | Rituximab, Plasma-exchange, IV steroids, mycophenolate mofetil DAA based regimen (Table 1) ACEIs and/or ARBs Diuretics, anti-hypertensive agents |
| Cryoglobulinemic flare | |
| Rapidly progressive glomerulonephritis |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabrizi, F.; Cerutti, R.; Porata, G.; Messa, P.; Ridruejo, E. Direct-Acting Antiviral Agents for HCV-Associated Glomerular Disease and the Current Evidence. Pathogens 2019, 8, 176. https://doi.org/10.3390/pathogens8040176
Fabrizi F, Cerutti R, Porata G, Messa P, Ridruejo E. Direct-Acting Antiviral Agents for HCV-Associated Glomerular Disease and the Current Evidence. Pathogens. 2019; 8(4):176. https://doi.org/10.3390/pathogens8040176
Chicago/Turabian StyleFabrizi, Fabrizio, Roberta Cerutti, Giulia Porata, Piergiorgio Messa, and Ezequiel Ridruejo. 2019. "Direct-Acting Antiviral Agents for HCV-Associated Glomerular Disease and the Current Evidence" Pathogens 8, no. 4: 176. https://doi.org/10.3390/pathogens8040176
APA StyleFabrizi, F., Cerutti, R., Porata, G., Messa, P., & Ridruejo, E. (2019). Direct-Acting Antiviral Agents for HCV-Associated Glomerular Disease and the Current Evidence. Pathogens, 8(4), 176. https://doi.org/10.3390/pathogens8040176







