Characterization and Protective Activity of Monoclonal Antibodies Directed against Streptococcus suis Serotype 2 Capsular Polysaccharide Obtained Using a Glycoconjugate
Abstract
1. Introduction
2. Results
2.1. Glycoconjugate Preparation
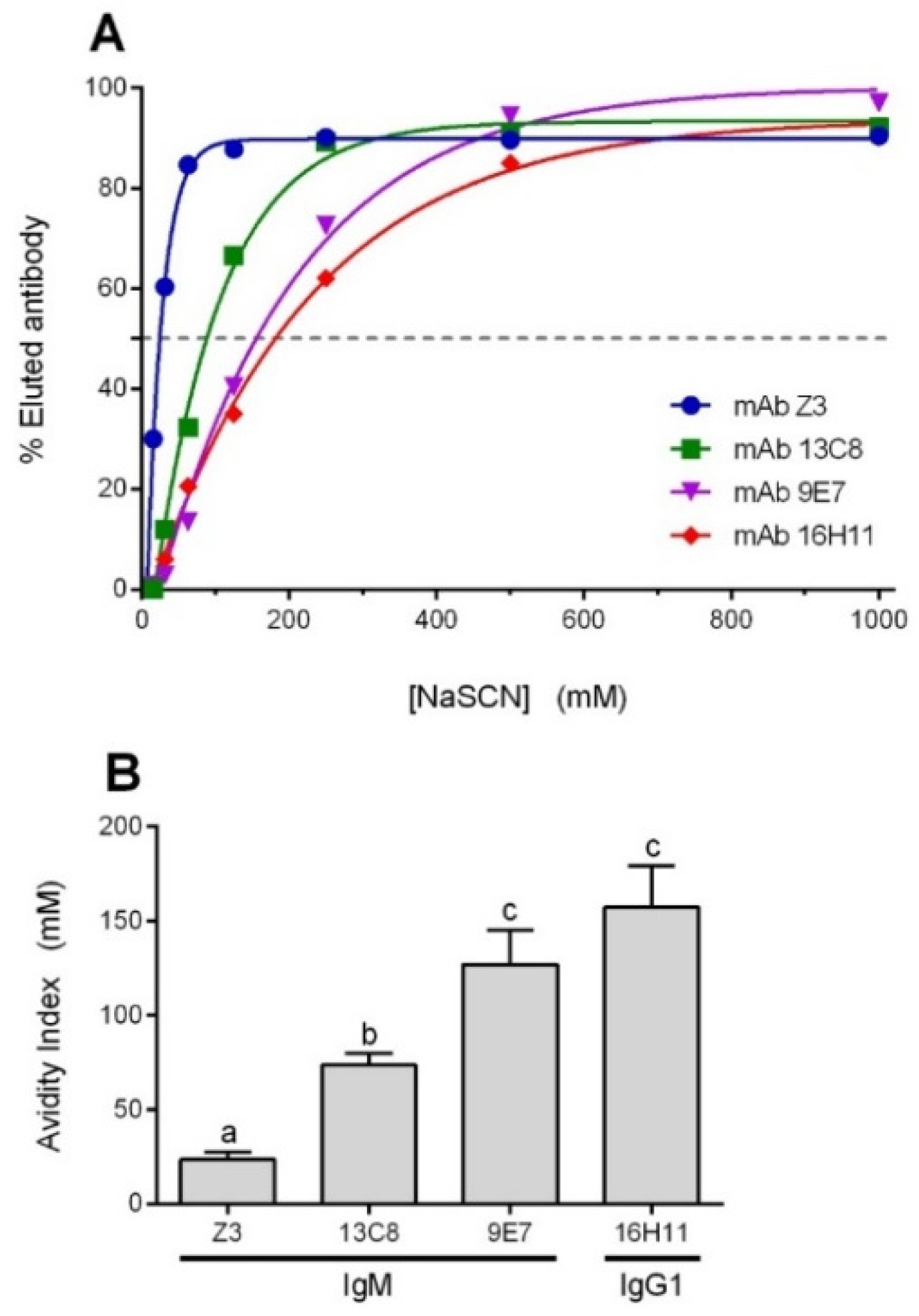
2.2. Characterization of mAbs
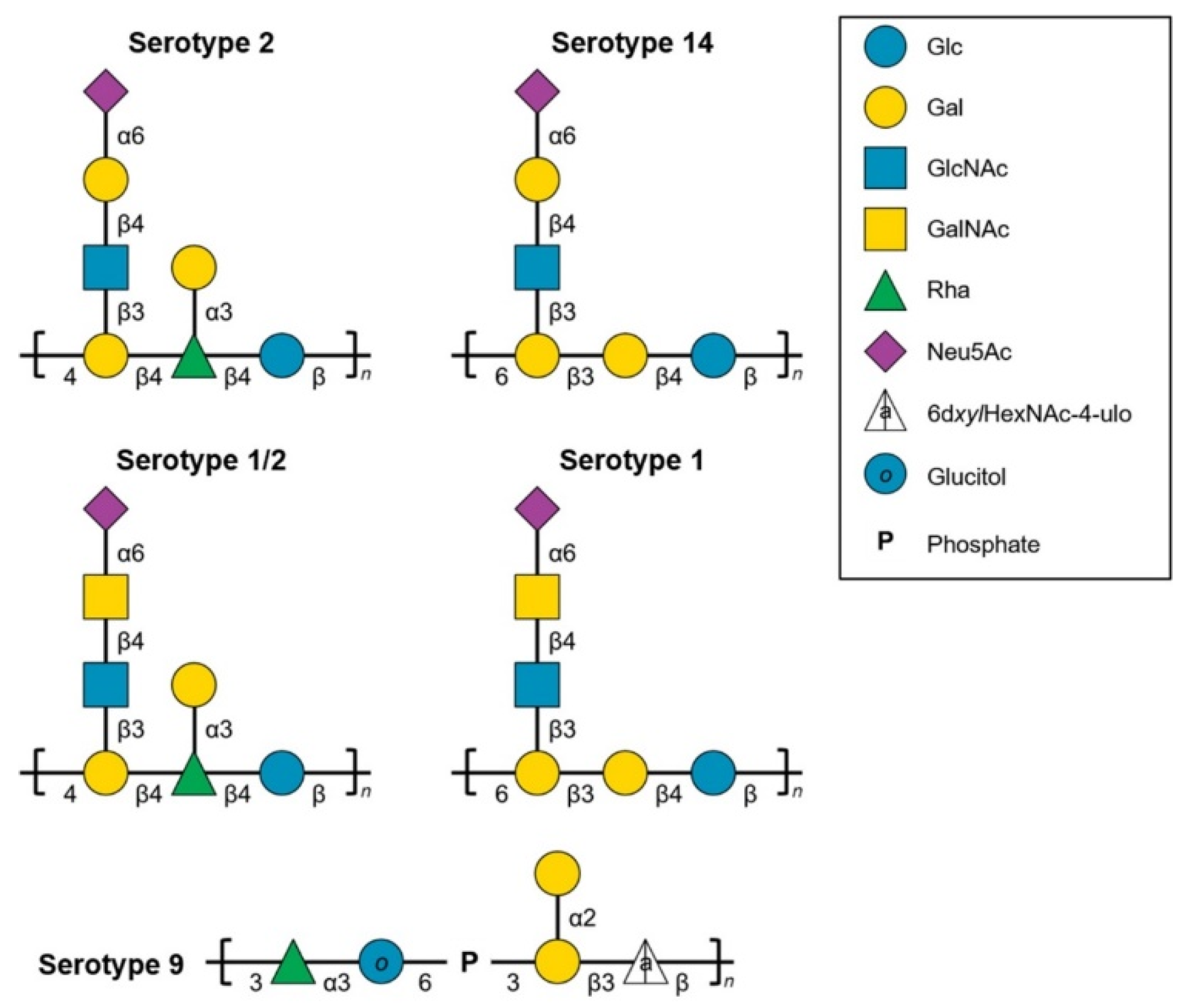
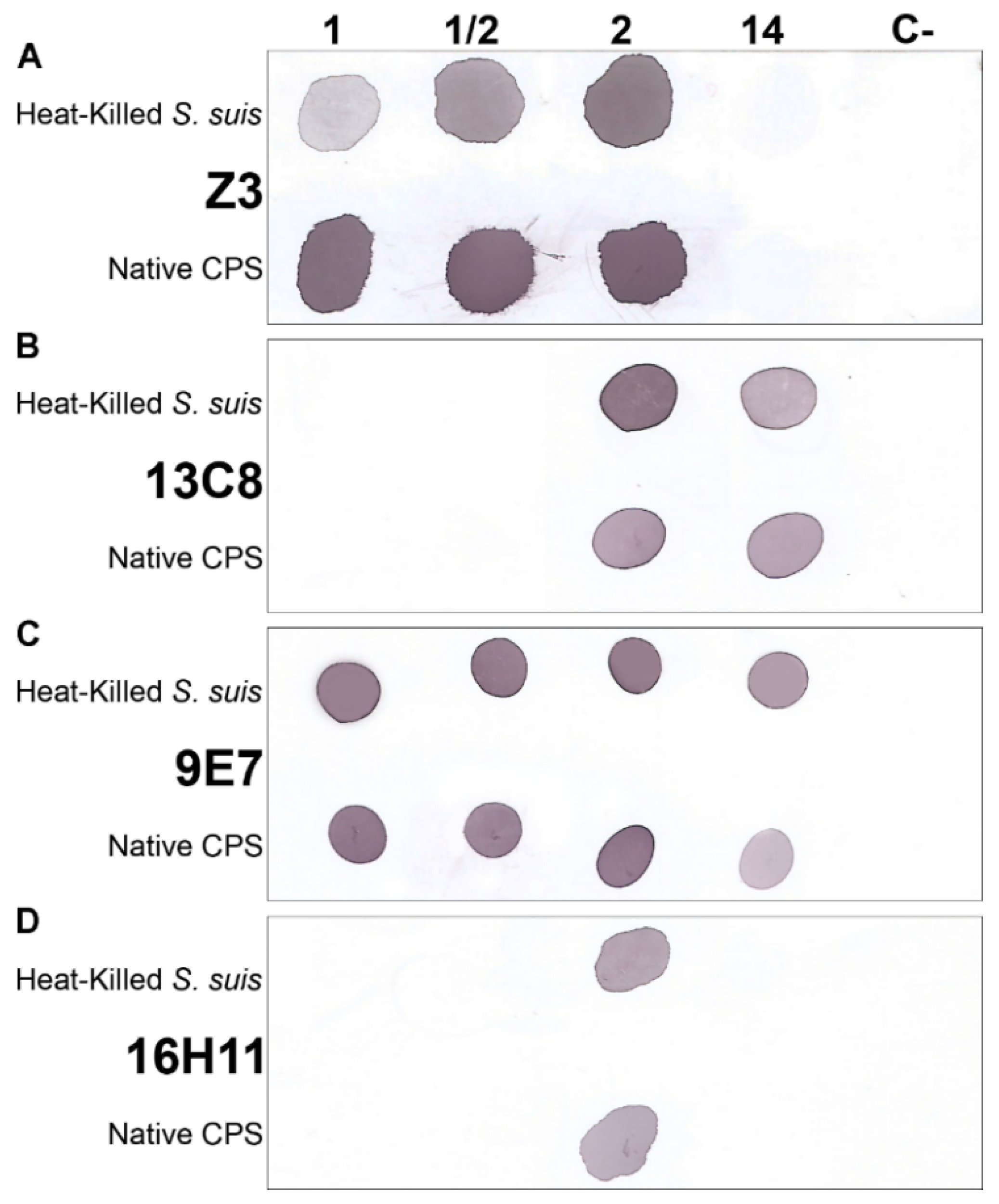
2.3. Characterization of the S. suis Capsular Epitopes Recognized by the mAbs
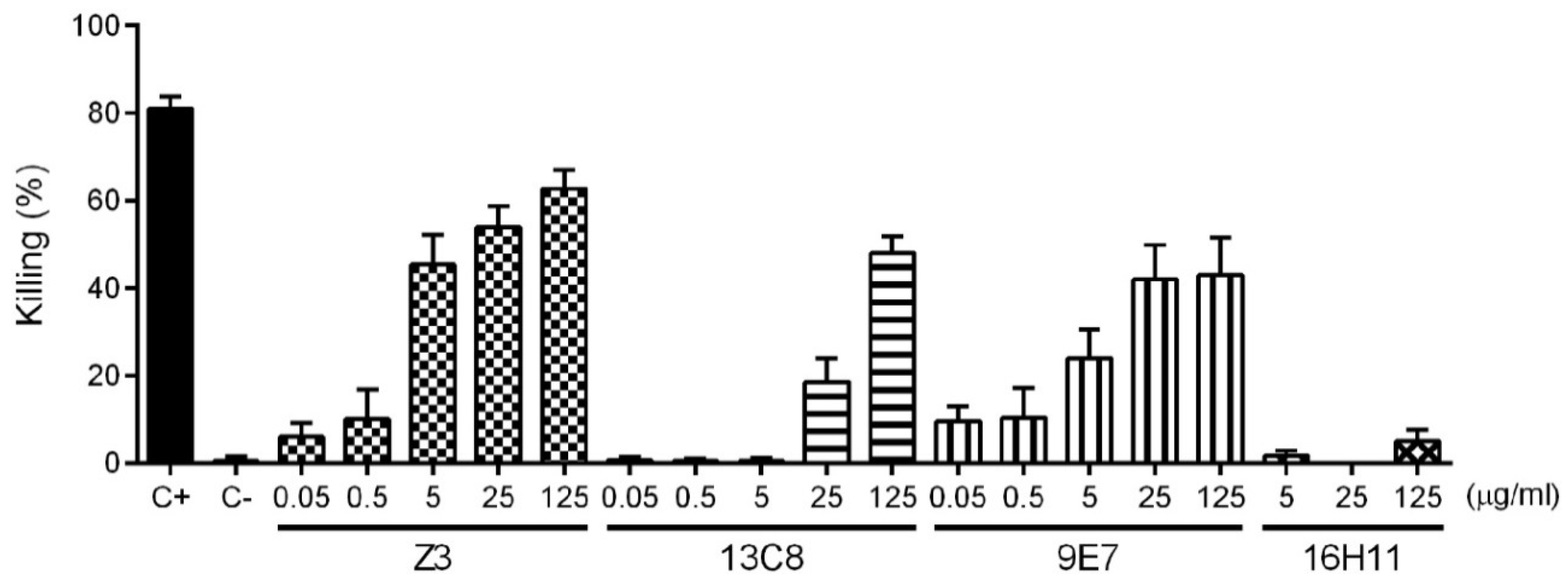
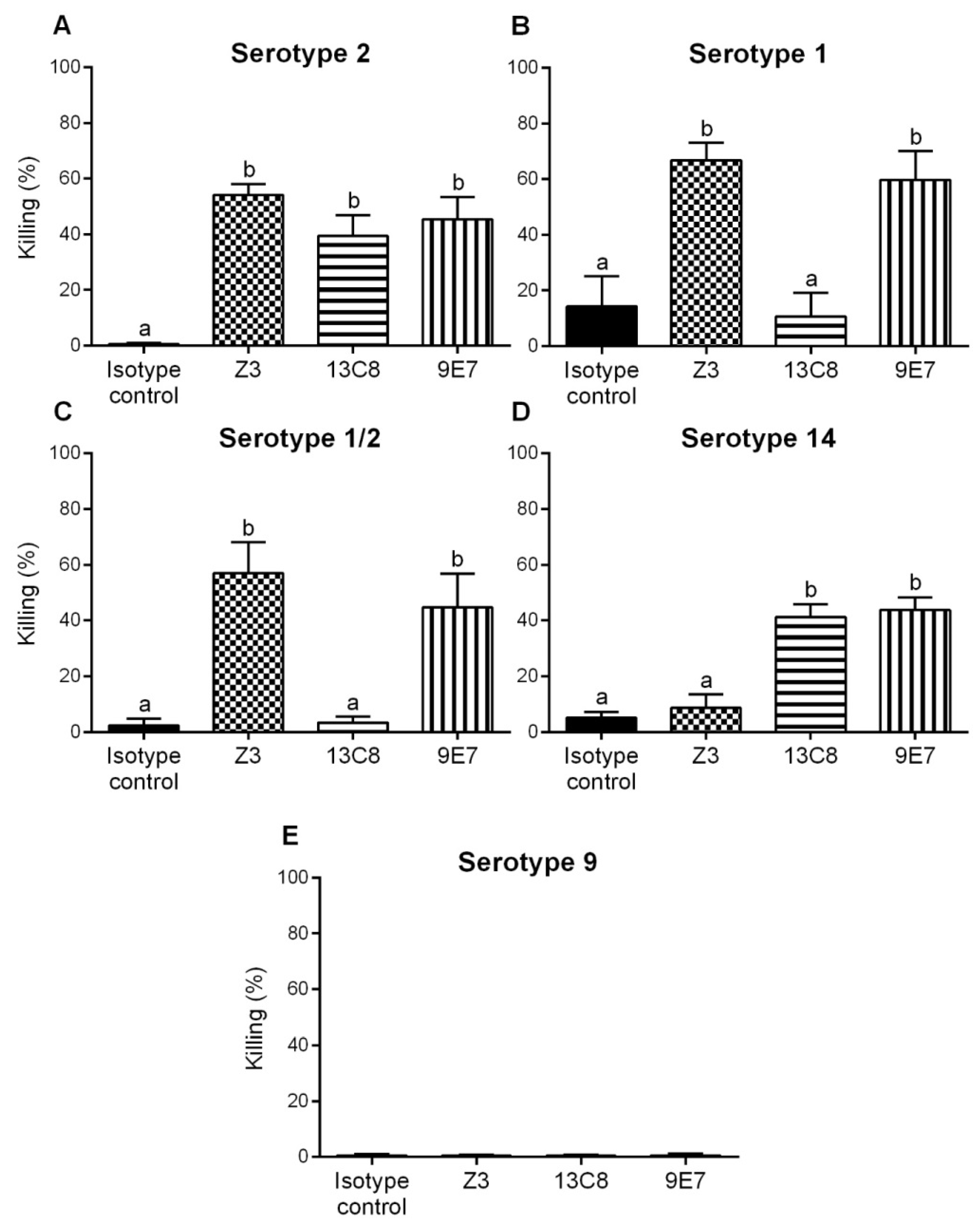
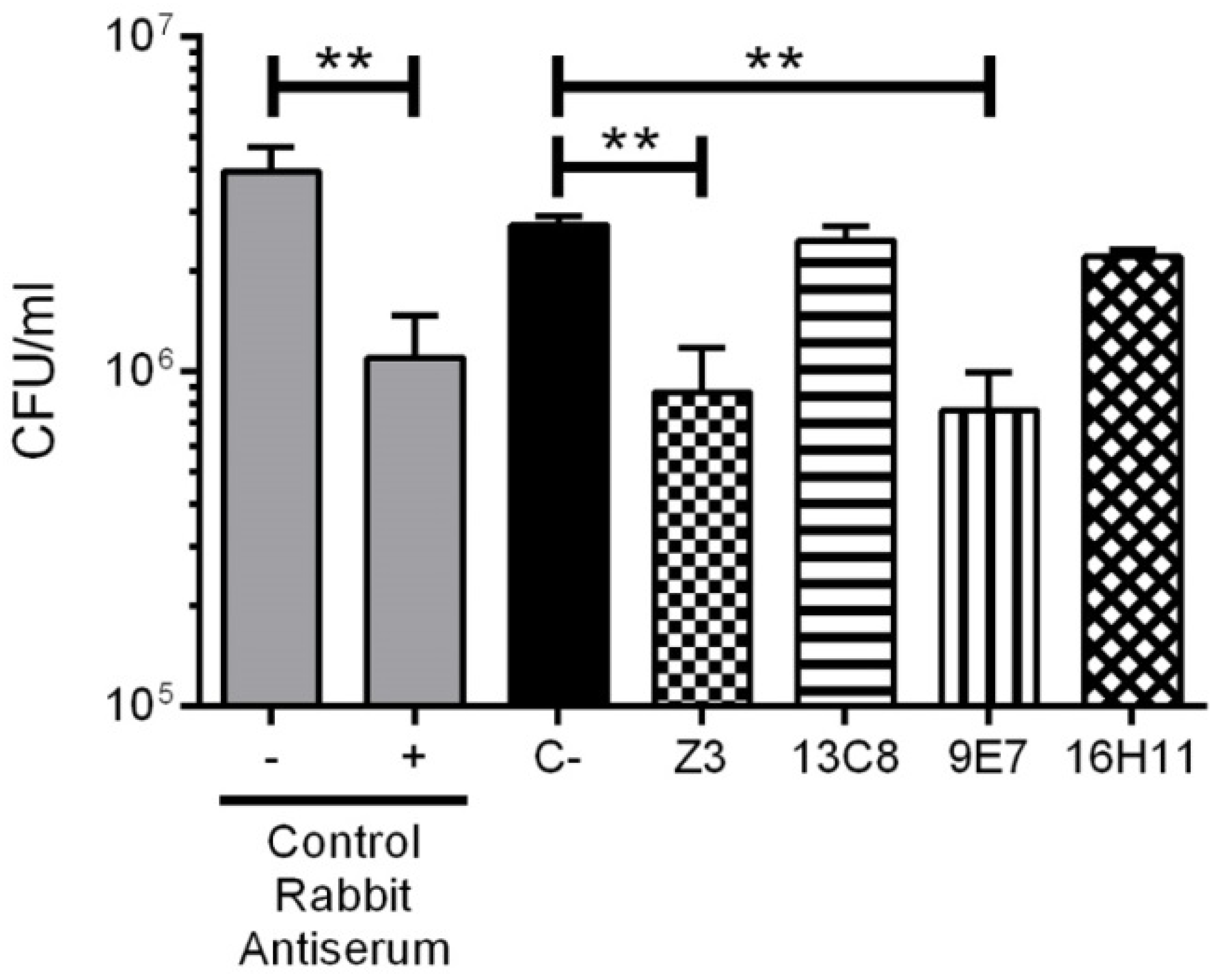
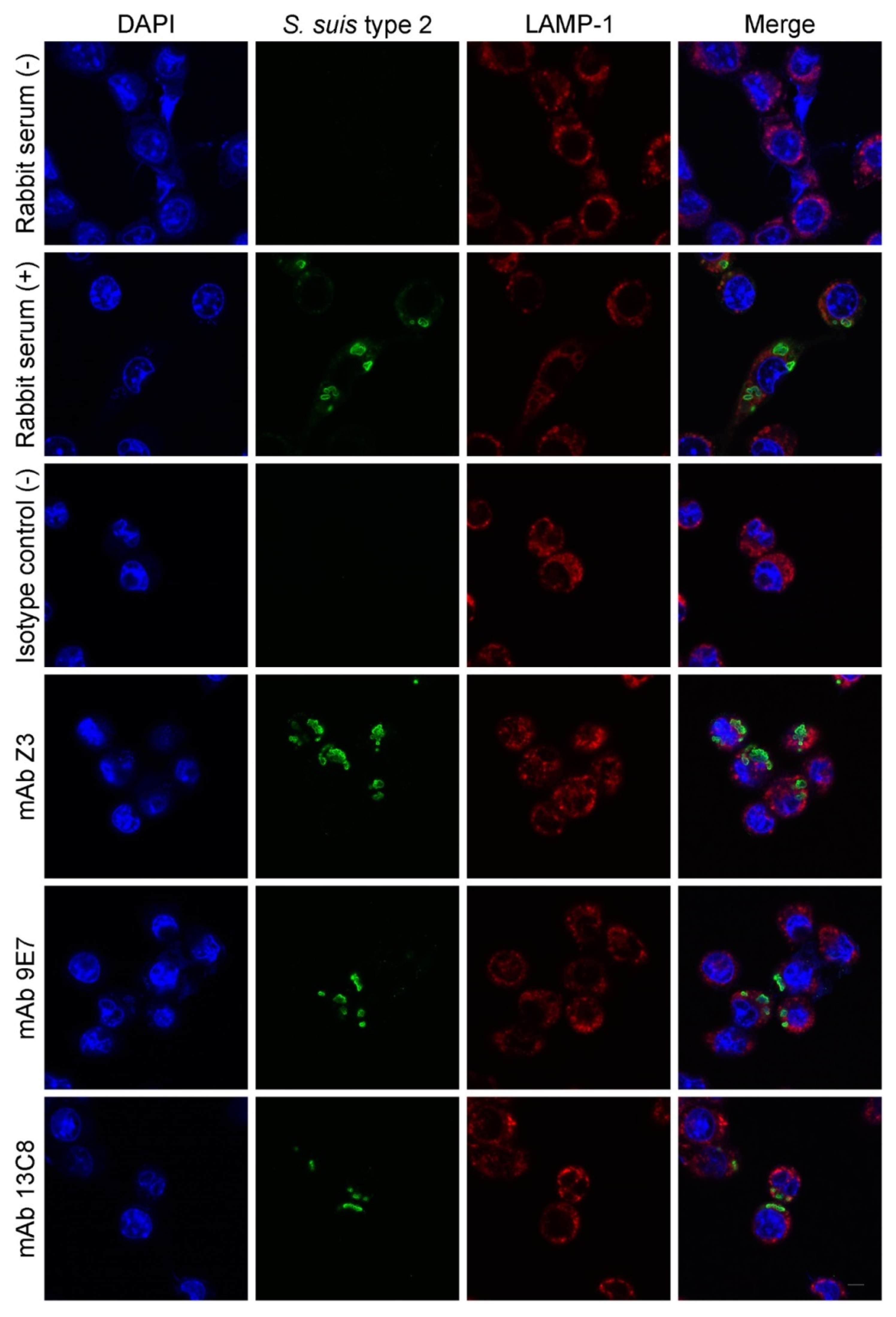
2.4. Functional Activity of mAbs
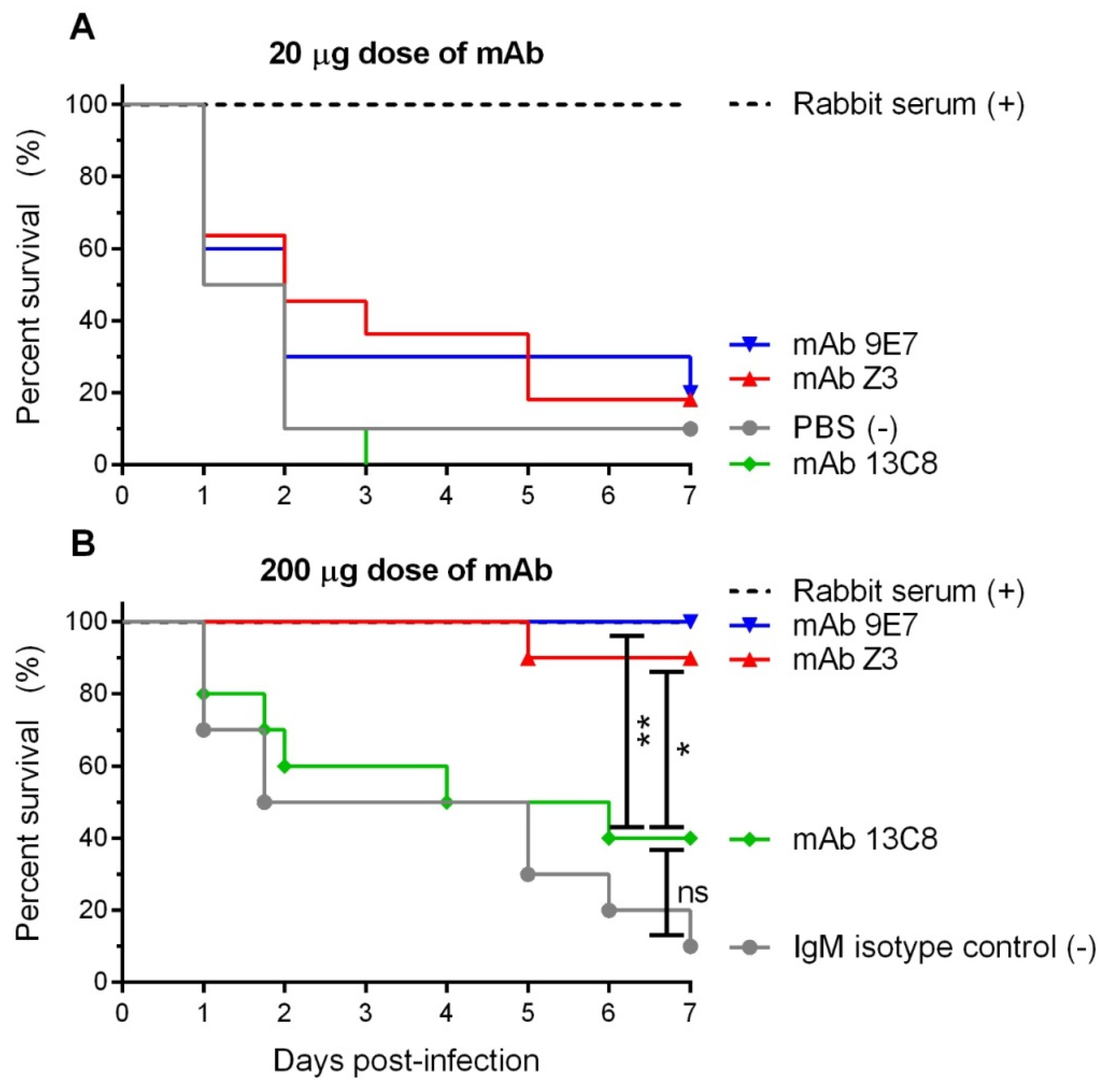
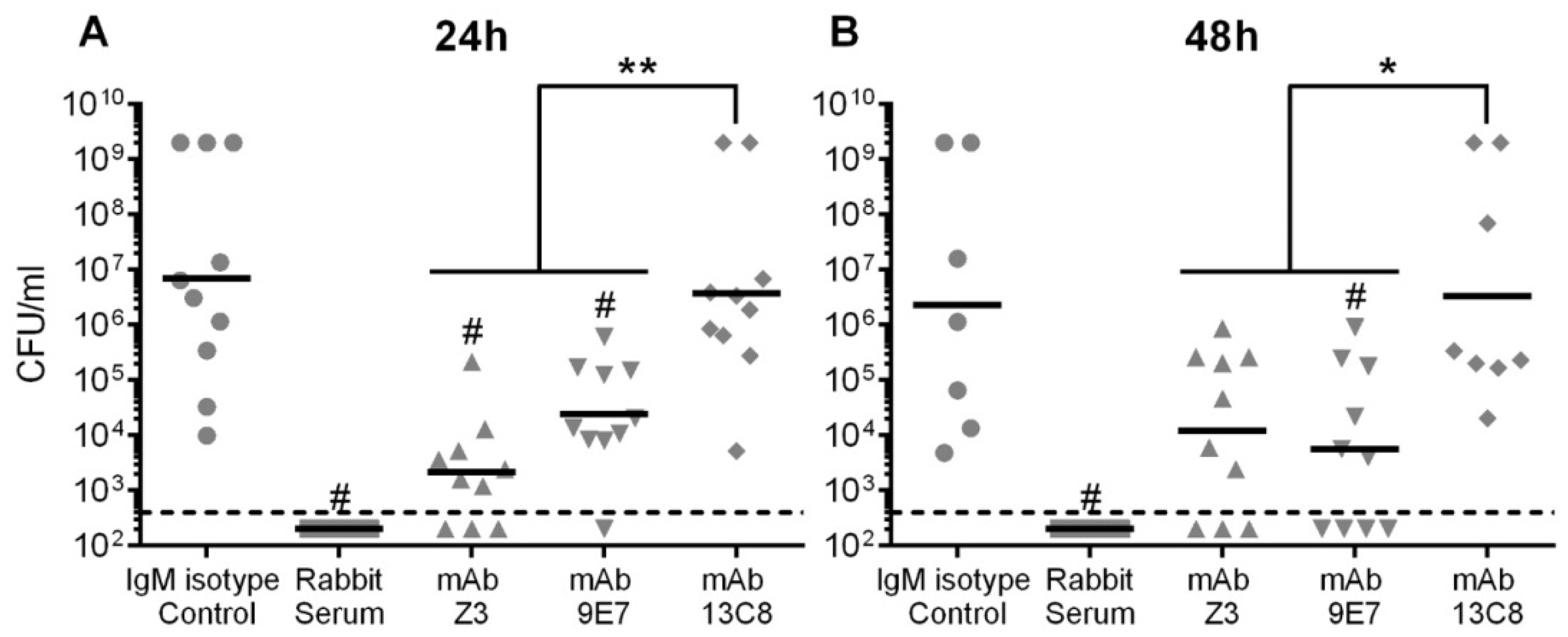
2.5. Protective Potential of mAbs
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Capsular Polysaccharide Purification and Mild Acid Hydrolysis
4.3. Monoclonal Antibody Production
4.4. Monoclonal Antibody Purification
4.5. Direct ELISA for Mouse Immunoglobulin (Ig) Quantification
4.6. Indirect ELISA for Antibodies against Type 2 S. suis CPS
4.7. Avidity Measurement
4.8. Dot-Blot
4.9. Opsonophagocytosis Assay (OPA)
4.10. Bacterial Agglutination Assay
4.11. Phagocytosis Assay by Confocal Microscopy
4.12. Passive Immunization and Protection Assay of Mice
4.13. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gottschalk, M.; Xu, J.; Calzas, C.; Segura, M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010, 5, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Segura, M. Streptococcus suis vaccines: Candidate antigens and progress. Expert Rev. Vaccines 2015, 14, 1587–1608. [Google Scholar] [CrossRef] [PubMed]
- Segura, M. Fisher scientific award lecture—The capsular polysaccharides of Group B Streptococcus and Streptococcus suis differently modulate bacterial interactions with dendritic cells. Can. J. Microbiol. 2012, 58, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Auger, J.P.; Segura, M.; Fittipaldi, N.; Takamatsu, D.; Okura, M.; Gottschalk, M. Role of the capsular polysaccharide as a virulence factor for Streptococcus suis serotype 14. Can. J. Vet. Res. 2015, 79, 141–146. [Google Scholar] [PubMed]
- Bottomley, M.J.; Serruto, D.; Sáfadi, M.A.P.; Klugman, K.P. Future challenges in the elimination of bacterial meningitis. Vaccine 2012, 30, B78–B86. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Moseley, M.A.; Burton, R.L.; Nahm, M.H. Pneumococcal vaccine and opsonic pneumococcal antibody. J. Infect. Chemother. 2013, 19, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Mond, J.J.; Kokai-Kun, J.F. The multifunctional role of antibodies in the protective response to bacterial T cell-independent antigens. In Specialization and Complementation of Humoral Immune Responses to Infection; Manser, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 319, pp. 17–40. [Google Scholar]
- Zimmermann, S.; Lepenies, B. Glycans as vaccine antigens and adjuvants: Immunological considerations. Methods Mol. Biol. 2015, 1331, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Shiao, T.C. Organic chemistry and immunochemical strategies in the design of potent carbohydrate-based vaccines. Chimia (Aarau) 2011, 65, 24–29. [Google Scholar] [CrossRef]
- Goyette-Desjardins, G.; Calzas, C.; Shiao, T.C.; Neubauer, A.; Kempker, J.; Roy, R.; Gottschalk, M.; Segura, M. Protection against Streptococcus suis serotype 2 infection using a capsular polysaccharide glycoconjugate vaccine. Infect. Immun. 2016, 84, 2059–2075. [Google Scholar] [CrossRef]
- Van Calsteren, M.R.; Gagnon, F.; Lacouture, S.; Fittipaldi, N.; Gottschalk, M. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem. Cell Biol. 2010, 88, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Van Calsteren, M.R.; Gagnon, F.; Calzas, C.; Goyette-Desjardins, G.; Okura, M.; Takamatsu, D.; Gottschalk, M.; Segura, M. Structure determination of Streptococcus suis serotype 14 capsular polysaccharide. Biochem. Cell Biol. 2013, 91, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Van Calsteren, M.R.; Goyette-Desjardins, G.; Gagnon, F.; Okura, M.; Takamatsu, D.; Roy, R.; Gottschalk, M.; Segura, M. Explaining the serological characteristics of Streptococcus suis serotypes 1 and 1/2 from their capsular polysaccharide structure and biosynthesis. J. Biol. Chem. 2016, 291, 8387–8398. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, E.; Goyette-Desjardins, G.; Okura, M.; Takamatsu, D.; Gottschalk, M.; Segura, M. Structure determination of Streptococcus suis serotype 9 capsular polysaccharide and assignment of functions of the cps locus genes involved in its biosynthesis. Carbohydr. Res. 2016, 433, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Vinogradov, E.; Okura, M.; Takamatsu, D.; Gottschalk, M.; Segura, M. Streptococcus suis serotype 3 and serotype 18 capsular polysaccharides contain di-N-acetyl-bacillosamine. Carbohydr. Res. 2018, 466, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Vinogradov, E.; Okura, M.; Takamatsu, D.; Gottschalk, M.; Segura, M. Structure determination of Streptococcus suis serotypes 7 and 8 capsular polysaccharides and assignment of functions of the cps locus genes involved in their biosynthesis. Carbohydr. Res. 2019, 473, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Takamatsu, D.; Maruyama, F.; Nozawa, T.; Nakagawa, I.; Osaki, M.; Sekizaki, T.; Gottschalk, M.; Kumagai, Y.; Hamada, S. Genetic analysis of capsular polysaccharide synthesis gene clusters from all serotypes of Streptococcus suis: Potential mechanisms for generation of capsular variation. Appl. Environ. Microbiol. 2013, 79, 2796–2806. [Google Scholar] [CrossRef]
- Athey, T.B.; Teatero, S.; Lacouture, S.; Takamatsu, D.; Gottschalk, M.; Fittipaldi, N. Determining Streptococcus suis serotype from short-read whole-genome sequencing data. BMC Microbiol. 2016, 16, 162. [Google Scholar] [CrossRef]
- Roy, D.; Athey, T.B.T.; Auger, J.-P.; Goyette-Desjardins, G.; Van Calsteren, M.-R.; Takamatsu, D.; Okura, M.; Teatero, S.; Alcorlo, M.; Hermoso, J.A.; et al. A single amino acid polymorphism in the glycosyltransferase CpsK defines four Streptococcus suis serotypes. Sci. Rep. 2017, 7, 4066. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Charland, N.; Jacques, M.; Lacouture, S.; Gottschalk, M. Characterization and protective activity of a monoclonal antibody against a capsular epitope shared by Streptococcus suis serotypes 1, 2 and 1/2. Microbiology 1997, 143, 3607–3614. [Google Scholar] [CrossRef] [PubMed]
- del Campo Sepulveda, E.M.; Altman, E.; Kobisch, M.; D’Allaire, S.; Gottschalk, M. Detection of antibodies against Streptococcus suis capsular type 2 using a purified capsular polysaccharide antigen-based indirect ELISA. Vet. Microbiol. 1996, 52, 113–125. [Google Scholar] [CrossRef]
- Calzas, C.; Lemire, P.; Auray, G.; Gerdts, V.; Gottschalk, M.; Segura, M. Antibody response specific to the capsular polysaccharide is impaired in Streptococcus suis serotype 2-infected animals. Infect. Immun. 2015, 83, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Calzas, C.; Taillardet, M.; Fourati, I.S.; Roy, D.; Gottschalk, M.; Soudeyns, H.; Defrance, T.; Segura, M. Evaluation of the Immunomodulatory Properties of Streptococcus suis and Group B Streptococcus Capsular Polysaccharides on the Humoral Response. Pathogens 2017, 6, 16. [Google Scholar] [CrossRef]
- Gottschalk, M.; Higgins, R.; Jacques, M.; Mittal, K.R.; Henrichsen, J. Description of 14 new capsular types of Streptococcus suis. J. Clin. Microbiol. 1989, 27, 2633–2636. [Google Scholar] [PubMed]
- Wibroe, P.P.; Helvig, S.Y.; Moein Moghimi, S. The role of complement in antibody therapy for infectious diseases. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Underhill, D.M.; Ozinsky, A. Phagocytosis of microbes: Complexity in action. Annu. Rev. Immunol. 2002, 20, 825–852. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcγ receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef]
- Goyette-Desjardins, G.; Roy, R.; Segura, M. Murine whole-blood opsonophagocytosis assay to evaluate protection by antibodies raised against encapsulated extracellular bacteria. In Carbohydrate-Based Vaccines—Methods and Protocols; Lepenies, B., Ed.; Springer: New York, NY, USA, 2015; Volume 1331, pp. 81–92. [Google Scholar]
- Huynh, K.K.; Eskelinen, E.L.; Scott, C.C.; Malevanets, A.; Saftig, P.; Grinstein, S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007, 26, 313–324. [Google Scholar] [CrossRef]
- Racine, R.; Winslow, G.M. IgM in microbial infections: Taken for granted? Immunol. Lett. 2009, 125, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, K.; Manix, C.; Guimaraes, A.J.; Nosanchuk, J.D.; Pirofski, L.-A. Aggregation of Streptococcus pneumoniae by a pneumococcal capsular polysaccharide-specific human monoclonal IgM correlates with antibody efficacy in vivo. Clin. Vaccine Immunol. 2010, 17, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hwang, Y.-I.; Nahm, M.H. Avidity, potency, and cross-reactivity of monoclonal antibodies to pneumococcal capsular polysaccharide serotype 6B. Infect. Immun. 2001, 69, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Weber, S.; Thorkildson, P.; Kozel, T.R.; Pirofski, L.A. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect. Immun. 2009, 77, 1502–1513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elliott, S.D.; Clifton-Hadley, F.; Tai, J. Streptococcal infection in young pigs. V. An immunogenic polysaccharide from Streptococcus suis type 2 with particular reference to vaccination against streptococcal meningitis in pigs. J. Hyg. (Lond.) 1980, 85, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Wisselink, H.J.; Stockhofe-Zurwieden, N.; Hilgers, L.A.T.; Smith, H.E. Assessment of protective efficacy of live and killed vaccines based on a non-encapsulated mutant of Streptococcus suis serotype 2. Vet. Microbiol. 2002, 84, 155–168. [Google Scholar] [CrossRef]
- Fabbrini, M.; Rigat, F.; Rinaudo, C.D.; Passalaqua, I.; Khacheh, S.; Creti, R.; Baldassarri, L.; Carboni, F.; Anderloni, G.; Rosini, R.; et al. The protective value of maternal Group B Streptococcus antibodies: Quantitative and functional analysis of naturally acquired responses to capsular polysaccharides and pilus proteins in european maternal sera. Clin. Infect. Dis. 2016, 63, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Hughes, R.; Parker, A.; Quinti, I.; Thon, V.; Cavaliere, M.; Würfel, M.; Herzog, W.; Gessner, J.E.; Baumann, U. Kinetics of IgM and IgA antibody response to 23-valent pneumococcal polysaccharide vaccination in healthy subjects. J. Clin. Immunol. 2013, 33, 288–296. [Google Scholar] [CrossRef]
- Weeratna, R.D.; McCluskie, M.J.; Xu, Y.; Davis, H.L. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 2000, 18, 1755–1762. [Google Scholar] [CrossRef]
- Berhanu, A.; Wilson, R.L.; Kirkwood-Watts, D.L.; King, D.S.; Warren, T.K.; Lund, S.A.; Brown, L.L.; Krupkin, A.K.; VanderMay, E.; Weimers, W.; et al. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J. Virol. 2008, 82, 3517–3529. [Google Scholar] [CrossRef]
- Martelet, L.; Lacouture, S.; Goyette-Desjardins, G.; Beauchamp, G.; Surprenant, C.; Gottschalk, M.; Segura, M. Porcine dendritic cells as an in vitro model to assess the immunological behaviour of Streptococcus suis subunit vaccine formulations and the polarizing effect of adjuvants. Pathogens 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M. IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol. Cell Biol. 2016, 94, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Sarvas, H.O.; Seppala, I.J.; Tahtinen, T.; Peterfy, F.; Makela, O. Mouse IgG antibodies have subclass associated affinity differences. Mol. Immunol. 1983, 20, 239–246. [Google Scholar] [CrossRef]
- Michaelsen, T.E.; Kolberg, J.; Aase, A.; Herstad, T.K.; Høiby, E.A. The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. Scand. J. Immunol. 2004, 59, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.H.; Moran, E.; Maresh, G.; Jennings, H.J.; Pritchard, D.G.; Egan, M.L.; Blixt, O. Fine specificity and cross-reactions of monoclonal antibodies to Group B streptococcal capsular polysaccharide type III. Vaccine 2012, 30, 4849–4858. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weber, S.; Tian, H.; van Rooijen, N.; Pirofski, L.A. A serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibody requires Fcgamma receptor III and macrophages to mediate protection against pneumococcal pneumonia in mice. Infect. Immun. 2012, 80, 1314–1322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doyle, C.R.; Moon, J.Y.; Daily, J.P.; Wang, T.; Pirofski, L.A. A capsular polysaccharide-specific antibody alters Streptococcus pneumoniae gene expression during nasopharyngeal colonization of mice. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Goldblatt, D.; Pinto Vaz, A.R.J.P.M.; Miller, E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 1998, 177, 1112–1115. [Google Scholar] [CrossRef]
- Usinger, W.R.; Lucas, A.H. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 1999, 67, 2366–2370. [Google Scholar]
- Romero-Steiner, S.; Musher, D.M.; Cetron, M.S.; Pais, L.B.; Groover, J.E.; Fiore, A.E.; Plikaytis, B.D.; Carlone, G.M. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 1999, 29, 281–288. [Google Scholar] [CrossRef]
- Roche, A.M.; Richard, A.L.; Rahkola, J.T.; Janoff, E.N.; Weiser, J.N. Antibody blocks acquisition of bacterial colonization through agglutination. Mucosal Immunol. 2014, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Dalia, A.B.; Weiser, J.N. Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell Host Microbe 2011, 10, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zhong, Z.; Lees, A.; Pekna, M.; Pirofski, L. Structure-Function Relationships for Human Antibodies to Pneumococcal Capsular Polysaccharide from Transgenic Mice with Human Immunoglobulin Loci. Infect. Immun. 2002, 70, 4977–4986. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, K.; Manix, C.; Tian, H.; van Rooijen, N.; Pirofski, L.-A. The efficacy of pneumococcal capsular polysaccharide-specific antibodies to serotype 3 Streptococcus pneumoniae requires macrophages. Vaccine 2010, 28, 7542–7550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weinstein, J.R.; Quan, Y.; Hanson, J.F.; Colonna, L.; Iorga, M.; Honda, S.-I.; Shibuya, K.; Shibuya, A.; Elkon, K.B.; Möller, T. IgM-dependent phagocytosis in microglia is mediated by complement receptor 3, not Fcα/μ receptor. J. Immunol. 2015, 195, 5309–5317. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, M.; Saito, T.; Kataoka, Y.; Itoh, T.; Kikuchi, N.; Hiramune, T. Comparative preparation methods of sialylated capsule antigen from Streptococcus suis type 2 with type specific antigenicity. J. Vet. Med. Sci. 1996, 58, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Stankova, J.; Gottschalk, M. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect. Immun. 1999, 67, 4646–4654. [Google Scholar]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Hale, G. Isolation and purification of monoclonal antibodies from tissue culture supernatant. In Monoclonal Antibodies: A Practical Approach; Shepherd, P., Dean, C., Eds.; Oxford University Press: Oxford, UK, 2000; pp. 149–180. [Google Scholar]
- Colino, J.; Duke, L.; Arjunaraja, S.; Chen, Q.; Liu, L.; Lucas, A.H.; Snapper, C.M. Differential idiotype utilization for the in vivo type 14 capsular polysaccharide-specific Ig responses to intact Streptococcus pneumoniae versus a pneumococcal conjugate vaccine. J. Immunol. 2012, 189, 575–586. [Google Scholar] [CrossRef]
- Pullen, G.R.; Fitzgerald, M.G.; Hosking, C.S. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 1986, 86, 83–87. [Google Scholar] [CrossRef]
- Goldblatt, D. Simple solid phase assays of avidity. In Immunochemistry 2; Johnstone, A., Turner, M.W., Eds.; IRL Press at Oxford University Press: New York, NY, USA, 1997; pp. 31–51. [Google Scholar]
- Higgins, R.; Gottschalk, M. An update on Streptococcus suis identification. J. Vet. Diagn. Invest. 1990, 2, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Gottschalk, M. Streptococcus suis interactions with the murine macrophage cell line J774: adhesion and cytotoxicity. Infect. Immun. 2002, 70, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Punaro, M.C.; Segura, M.; Plante, M.M.; Lacouture, S.; Rivest, S.; Gottschalk, M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J. Immunol. 2007, 179, 1842–1854. [Google Scholar] [CrossRef] [PubMed]
- Lecours, M.P.; Fittipaldi, N.; Takamatsu, D.; Okura, M.; Segura, M.; Goyette-Desjardins, G.; Van Calsteren, M.R.; Gottschalk, M. Sialylation of Streptococcus suis serotype 2 is essential for capsule expression but is not responsible for the main capsular epitope. Microbes Infect. 2012, 14, 941–950. [Google Scholar] [CrossRef] [PubMed]
| Z3 | 13C8 | 9E7 | 16H11 | |
|---|---|---|---|---|
| Isotype/Subclass | IgM | IgM | IgM | IgG1 |
| Avidity index (mM) | 24 | 74 | 127 | 157 |
| Serotype specificity | 2, 1, and 1/2 | 2 and 14 | 2, 1, 1/2, and 14 | 2 |
| Opsonic index (µg/mL) | 2.35 | 27.8 | 4.35 | Non-opsonic |
| Agglutination | + | − | + | − |
| Phagocytosis | + | + | + | ND 1 |
| Passive protection | + | +/− | + | − |
| Origin | [22] | This study | This study | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyette-Desjardins, G.; Lacouture, S.; Auger, J.-P.; Roy, R.; Gottschalk, M.; Segura, M. Characterization and Protective Activity of Monoclonal Antibodies Directed against Streptococcus suis Serotype 2 Capsular Polysaccharide Obtained Using a Glycoconjugate. Pathogens 2019, 8, 139. https://doi.org/10.3390/pathogens8030139
Goyette-Desjardins G, Lacouture S, Auger J-P, Roy R, Gottschalk M, Segura M. Characterization and Protective Activity of Monoclonal Antibodies Directed against Streptococcus suis Serotype 2 Capsular Polysaccharide Obtained Using a Glycoconjugate. Pathogens. 2019; 8(3):139. https://doi.org/10.3390/pathogens8030139
Chicago/Turabian StyleGoyette-Desjardins, Guillaume, Sonia Lacouture, Jean-Philippe Auger, René Roy, Marcelo Gottschalk, and Mariela Segura. 2019. "Characterization and Protective Activity of Monoclonal Antibodies Directed against Streptococcus suis Serotype 2 Capsular Polysaccharide Obtained Using a Glycoconjugate" Pathogens 8, no. 3: 139. https://doi.org/10.3390/pathogens8030139
APA StyleGoyette-Desjardins, G., Lacouture, S., Auger, J.-P., Roy, R., Gottschalk, M., & Segura, M. (2019). Characterization and Protective Activity of Monoclonal Antibodies Directed against Streptococcus suis Serotype 2 Capsular Polysaccharide Obtained Using a Glycoconjugate. Pathogens, 8(3), 139. https://doi.org/10.3390/pathogens8030139







