Changes in Protein Composition in the Grain and Malt after Fusarium Infection Dependently of Wheat Resistance
Abstract
:1. Introduction
2. Results and Discussion
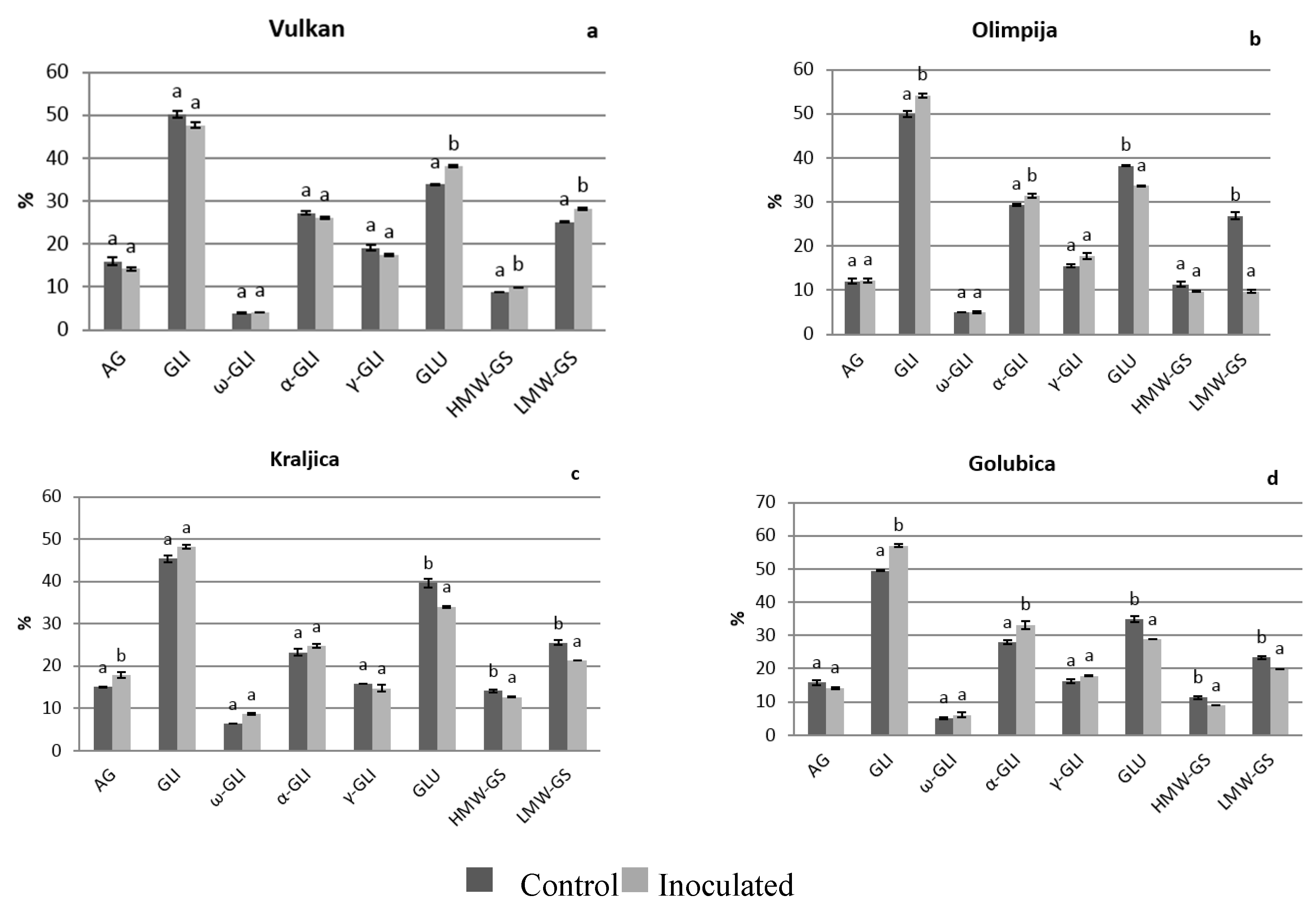
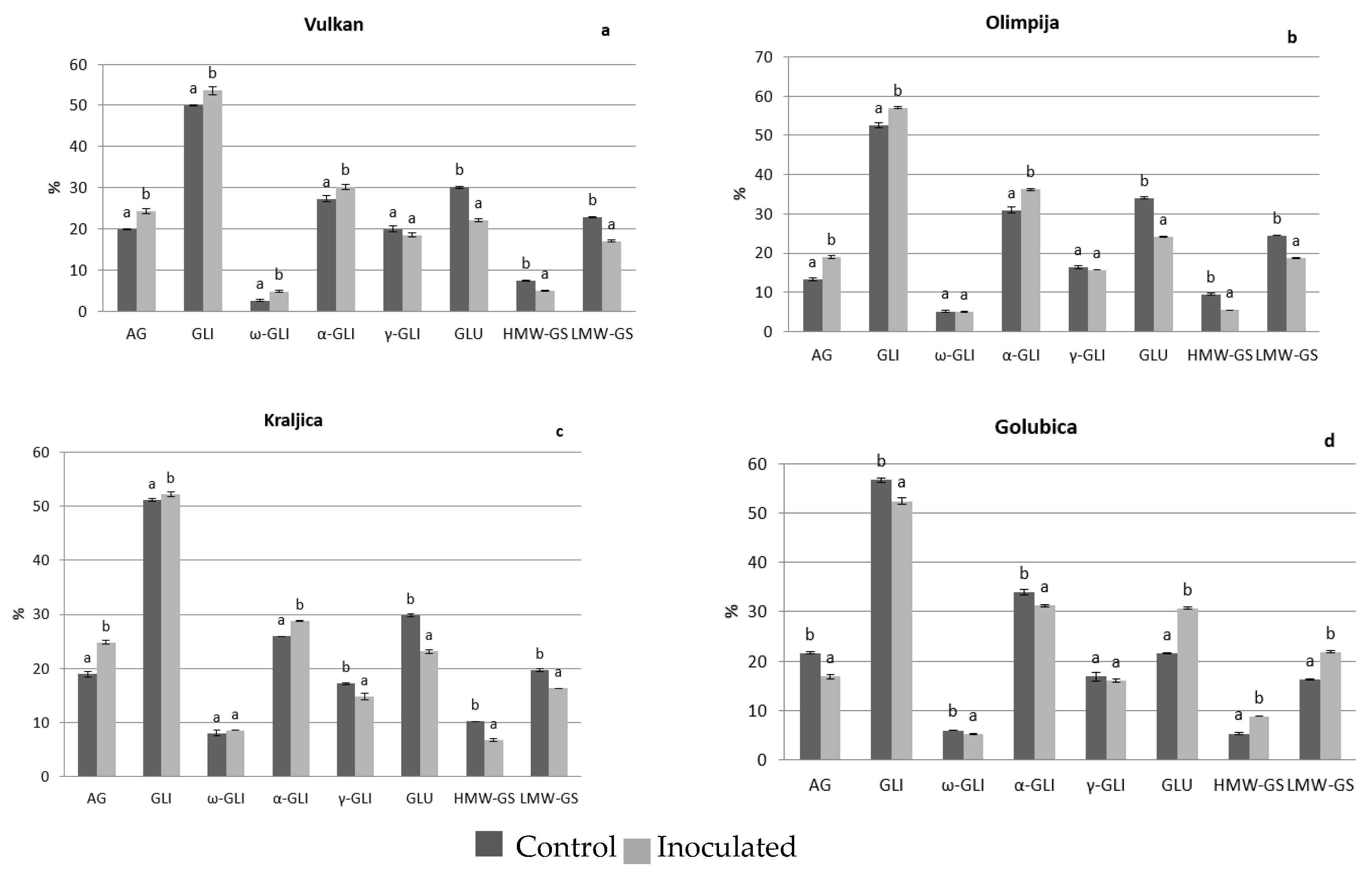
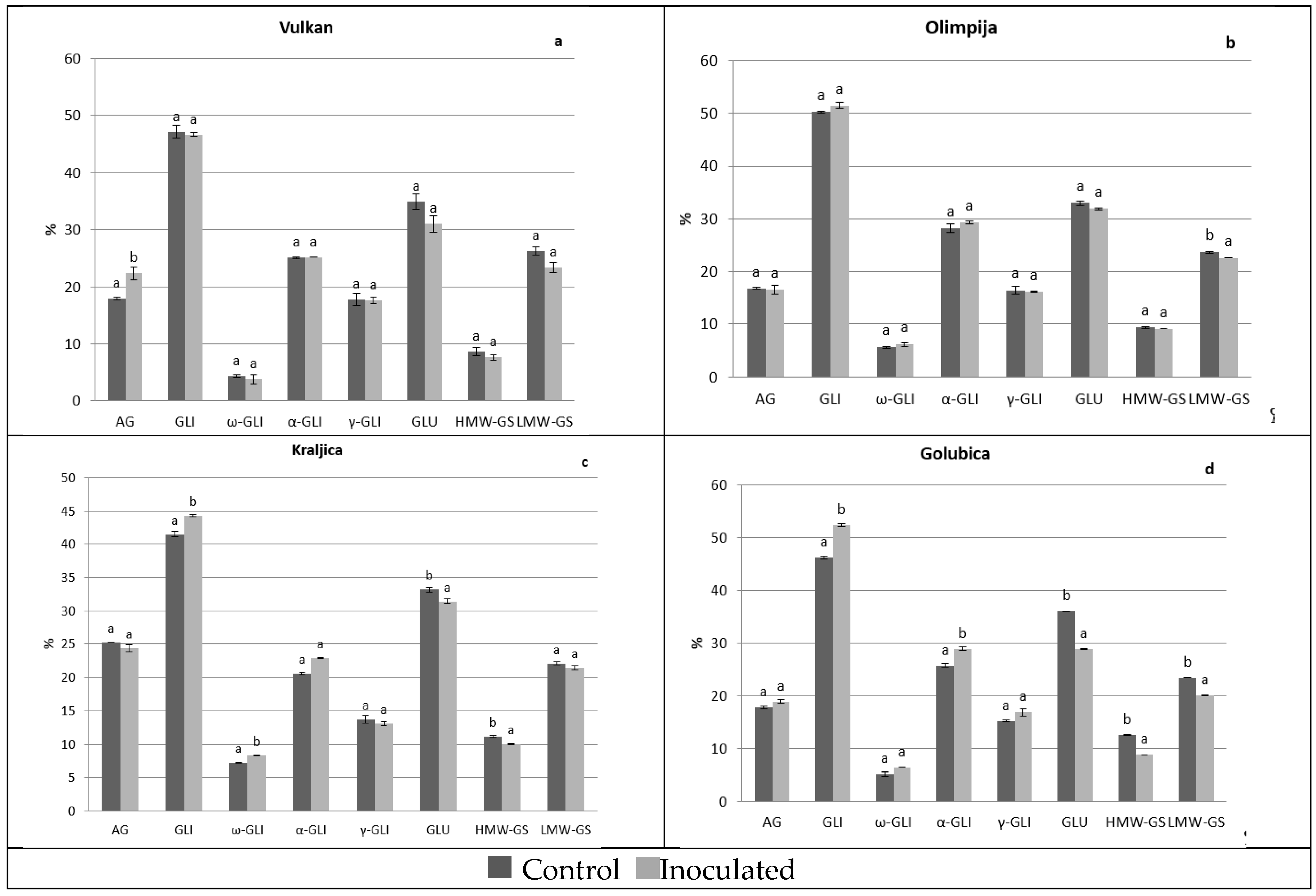
2.1. Protein Distribution in the Grain and Malt Dependently of Control and Inoculation
2.2. Grain Yield and Other Agronomical Traits
3. Materials and Methods
3.1. Field Trials
3.2. Inoculum Production, Inoculation and Disease Assessment
3.3. Agronomical and Physiological Traits
3.4. Malting
3.5. Protein Composition Analysis
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osman, A.M.; Coverdale, S.M.; Onley-Watson, K.; Bell, D.; Healy, P. The gel filtration chromatographic-profiles of proteins and peptides of wort and beer: Effects of processing-malting, mashing, kettle boiling, fermentation and filtering. J. Inst. Brew. 2003, 109, 41–50. [Google Scholar] [CrossRef]
- Faltermaier, A.; Waters, D.; Becker, T.; Arendt, E.; Gastl, M. Common wheat (Triticum aestivum L.) and its use as a brewing cereal—A review. J. Inst. Brew. 2014, 120, 1–15. [Google Scholar] [CrossRef]
- Horvat, D.; Spanic, V.; Dvojkovic, K.; Simic, G.; Magdic, D.; Nevistic, A. The Influence of Fusarium infection on wheat (Triticum. aestivum L.) proteins distribution and baking quality. Cereal Res. Commun. 2015, 43, 61–71. [Google Scholar] [CrossRef]
- Emebiri, L.C.; Moody, D.B.; Black, C.; van Ginkel, M.; Hernandez, E. Improvement in malting quality barley grain yield by manipulation of genes influencing grain protein content. Euphytica 2007, 156, 185–194. [Google Scholar] [CrossRef]
- Steiner, B.; Lemmens, M.; Griesser, M.; Scholz, U.; Schondelmaier, J.; Buerstmayr, H. Molecular mapping of resistance to Fusarium head blight in the spring wheat cultivar Frontana. Theor. Appl. Genet. 2004, 109, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Mergoum, M.; Frohberg, R.C.; Stack, R.W. Breeding Hard Red Spring Wheat for Fusarium Head Blight Resistance. In Wheat Production in Stressed Environments; Springer: Dordrecht, The Netherlands, 2007; Volume 12. [Google Scholar]
- Spanic, V.; Marcek, T.; Abicic, I.; Sarkanj, B. Effects of Fusarium head blight on wheat grain and malt infected by Fusarium culmorum. Toxins 2018, 10, 17. [Google Scholar] [CrossRef]
- Beccari, G.; Prodi, A.; Tini, F.; Bonciarelli, U.; Onofri, A.; Oueslati, S.; Limayma, M.; Covarelli, L. Changes in the fusarium head blight complex of malting barley in a three-year field experiment in Italy. Toxins 2017, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.M.; Liu, C.; Vander Fels-Klerx, H.J. Fusarium infection and trichothecenes in barley and its comparison with wheat. World Mycotoxin J. 2018, 11, 33–46. [Google Scholar] [CrossRef]
- Dodd, J.G.; Vegi, A.; Vashisht, A.; Tobias, D.; Schwarz, P.; Wolf-Hall, C.E. Effect of ozone treatment on the safety and quality of malting barley. J. Food Prot. 2011, 74, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Du, J.; Zhang, K.; Xie, L.; Li, P. Relationship between Kolbach index and other quality parameters of wheat malt. J. Inst. Brew. 2012, 118, 57–62. [Google Scholar] [CrossRef]
- De Vita, P.; Destri, L.; Nicosia, O.; Nigro, F.; Platani, C.; Riefolo, C.; Di Fonzo, N.; Cattivelli, L. Breeding progress in morpho-physiological, agronomical and qualitative traits of durum wheat cultivars released in Italy during the 20th century. Eur. J. Agron. 2007, 26, 39–53. [Google Scholar] [CrossRef]
- Altenbach, S.; Tanaka, C.; Pineau, F.; Lupi, R.; Drouet, M.; Beaudouin, E.; Morisset, M.; Denery-Papini, S. Assessment of the allergenic potential of transgenic wheat (Triticum aestivum) with reduced levels of ω5-gliadins, the major sensitizing allergen in wheat-dependent exercise-induced anaphylaxis. J. Agric. Food Chem. 2015, 63, 9323–9332. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.M.; Palermo, C.; De Santis, M.A.; Mentana, A.; Pompa, M.; Giuzio, L.; Masci, S.; Centonze, D.; Flagella, Z. Differential expression of durum wheat gluten proteome under water stress during grain filling. J. Agric. Food Chem. 2015, 63, 6501–6512. [Google Scholar] [CrossRef] [PubMed]
- Spanic, V.; Viljevac Vuletic, M.; Drezner, G.; Zdunic, Z.; Horvat, D. Performance indices in wheat chlorophyll a fluorescence and protein quality influenced by FHB. Pathogens 2017, 6, 59. [Google Scholar] [CrossRef]
- Eggert, K.; Wieser, H.; Pawelzik, E. The influence of Fusarium infection and growing location on the quantitative protein composition of (part I) emmer (Triticum dicoccum). Eur. Food Res. Technol. 2010, 230, 837–847. [Google Scholar] [CrossRef]
- Eggert, K.; Rawel, H.M.; Pawelzik, E. In vitro degradation of wheat gluten fractions by Fusarium graminearum proteases. Eur. Food Res. Technol. 2011, 233, 697–705. [Google Scholar] [CrossRef]
- Huebner, F.R.; Kaczkowski, J.; Bietz, J.A. Quantitative variation in wheat proteins from grain at different stages of maturity from different spike locations. Cereal Chem. 1990, 67, 464. [Google Scholar]
- Galano, T.; Fininsa, C.; Bultosa, G. Effects of net blotch (Pyrenophora teres) on malt barley yield and grain quality at Holeta. Central Ethiopia. East Afr. J. Sci. 2008, 2, 150–158. [Google Scholar] [CrossRef]
- Guo, M.; Jin, Y.; Du, J.; Zhang, K.; Zhao, D. Effects of wheat protein compositions on malt quality. Qual. Assur. Saf. Crop. 2013, 6, 73–80. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Waters, D.M.; Arendt, E.K. The impact of Fusarium culmorum infection on the protein fractions of raw barley and malted grains. Appl. Microbiol. Biotechnol. 2013, 97, 2053–2065. [Google Scholar] [CrossRef]
- Sarlin, T.; Laitila, A.; Pekkarinen, A.; Haikara, A. Effects of three Fusarium Species on the quality of barley and malt. J. Am. Soc. Brew. Chem. 2005, 63, 43–49. [Google Scholar] [CrossRef]
- Bechtel, D.B.; Kaleikau, L.A.; Gaines, R.L.; Seitz, L.M. The effects of Fusarium graminearum infection on wheat kernels. Cereal Chem. 1985, 62, 191–197. [Google Scholar]
- Spanic, V.; Drezner, G. Influence of Fusarium artificial infection on agronomic traits of wheat. Glasnik zaštite bilja 2011, 34, 60–64. [Google Scholar] [CrossRef]
- Salgado, J.D.; Madden, L.V.; Paul, P.P. Quantifying the Effects of Fusarium head blight on grain yield and test weight in soft red winter wheat. Phytopathology 2014, 105, 295–306. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Bartok, T.; Mirocha, C.G.; Komoroczy, R. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant. Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- Martin, C.; Schöneberg, T.; Vogelgsang, S.; Vincenti, J.; Bertossa, M.; Mauch–Mani, B.; Mascher, F. Factors of wheat grain resistance to Fusarium head blight. Phytopathol. Mediterr. 2017, 56, 154–166. [Google Scholar] [CrossRef]
- Kilic, H.; Sanal, T.; Erdemci, I.; Karaca, K. Screening Bread Wheat Genotypes for High Molecular Weight Glutenin Subunits and Some Quality Parameters. J. Agric. Sci. Technol. 2017, 19, 1393–1404. [Google Scholar] [CrossRef]
- Spanic, V.; Viljevac Vuletic, M.; Abicic, I.; Marcek, T. Early response of wheat antioxidant system with special reference to Fusarium head blight stress. Plant Physiol. Biochem. 2017, 115, 34–43. [Google Scholar] [CrossRef]
- Spanic, V.; Lemmens, M.; Drezner, G. Morphological and molecular identification of Fusarium species associated with head blight on wheat in East Croatia. Eur. J. Plant Pathol. 2010, 128, 511–516. [Google Scholar] [CrossRef]
- Spanic, V.; Lemmens, M.; Drezner, G. Variability of components of fusarium head blight resistance among wheat genotypes. Cereal Res. Commun. 2013, 41, 420–430. [Google Scholar] [CrossRef]
- Lemmens, M.; Haim, K.; Lew, H.; Ruckenbauer, P. The effect of nitrogen fertilization on Fusarium head blight development and deoxynivalenol contamination in wheat. J. Phytopathol. 2004, 152, 1–8. [Google Scholar] [CrossRef]
- Snijders, C.H.A.; Van Eeuwijk, F.A. Genotype X strain interactions for resistance to Fusarium head blight caused by Fusarium culmorum in winter wheat. Theor. Appl. Genet. 1991, 81, 239–244. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzac, F.C.A. Decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Wieser, H.; Antes, S.; Selmeier, W. Quantitative determination of gluten protein types in wheat flour by reverse-phase high preformance liquid chromatography. Cereal Chem. 1998, 75, 644–650. [Google Scholar] [CrossRef]

| 2016 | ||||||
| 10 | 14 | 18 | 22 | 26 | AUDPC | |
| Vulkan | 0.00 | 0.0 | 1.0 | 4.0 | 7.5 | 18.3 |
| Olimpija | 0.00 | 0.0 | 1.5 | 7.5 | 17.5 | 38.3 |
| Kraljica | 0.00 | 0.0 | 2.5 | 6.5 | 20.0 | 44.0 |
| Golubica | 0.00 | 0.0 | 4.0 | 20.0 | 55.0 | 114.5 |
| 2017 | ||||||
| Vulkan | 1.75 | 4.0 | 7.5 | 17.5 | 20.0 | 90.8 |
| Olimpija | 1.25 | 5.0 | 5.0 | 10.0 | 17.5 | 72.5 |
| Kraljica | 2.00 | 3.5 | 5.0 | 10.0 | 20.0 | 75.5 |
| Golubica | 4.25 | 15.0 | 52.5 | 75.0 | 87.5 | 430.0 |
| Relative Losses (%) | ||||||
|---|---|---|---|---|---|---|
| 2016 | ||||||
| Varieties | GY* | TW | TKW | SL | PRO | ST |
| Vulkan | 10.3 | 0.8 | 5.0 | −21.4 | −0.4 | 2.2 |
| Olimpija | 38.5 | 3.0 | 8.7 | 0.0 | −4.6 | 3.2 |
| Kraljica | −13.0 | −7.9 | −20.4 | 16.7 | −11.9 | 4.8 |
| Golubica | 37.0 | 15.4 | −2.2 | 17.4 | −1.5 | 4.1 |
| 2017 | ||||||
| Vulkan | 15.6 | 2.7 | 6.6 | 5.9 | −0.9 | 1.6 |
| Olimpija | 8.1 | 3.5 | 5.4 | 11.1 | −1.0 | 7.1 |
| Kraljica | 25.6 | 4.0 | 16.6 | 0.0 | 0.6 | 2.2 |
| Golubica | 56.2 | 15.4 | 30.6 | 13.6 | 2.2 | −2.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanic, V.; Horvat, D.; Drezner, G.; Zdunic, Z. Changes in Protein Composition in the Grain and Malt after Fusarium Infection Dependently of Wheat Resistance. Pathogens 2019, 8, 112. https://doi.org/10.3390/pathogens8030112
Spanic V, Horvat D, Drezner G, Zdunic Z. Changes in Protein Composition in the Grain and Malt after Fusarium Infection Dependently of Wheat Resistance. Pathogens. 2019; 8(3):112. https://doi.org/10.3390/pathogens8030112
Chicago/Turabian StyleSpanic, Valentina, Daniela Horvat, Georg Drezner, and Zvonimir Zdunic. 2019. "Changes in Protein Composition in the Grain and Malt after Fusarium Infection Dependently of Wheat Resistance" Pathogens 8, no. 3: 112. https://doi.org/10.3390/pathogens8030112
APA StyleSpanic, V., Horvat, D., Drezner, G., & Zdunic, Z. (2019). Changes in Protein Composition in the Grain and Malt after Fusarium Infection Dependently of Wheat Resistance. Pathogens, 8(3), 112. https://doi.org/10.3390/pathogens8030112






