Detection of Pathogenic Viruses, Pathogen Indicators, and Fecal-Source Markers within Tanker Water and Their Sources in the Kathmandu Valley, Nepal
Abstract
1. Introduction
2. Results
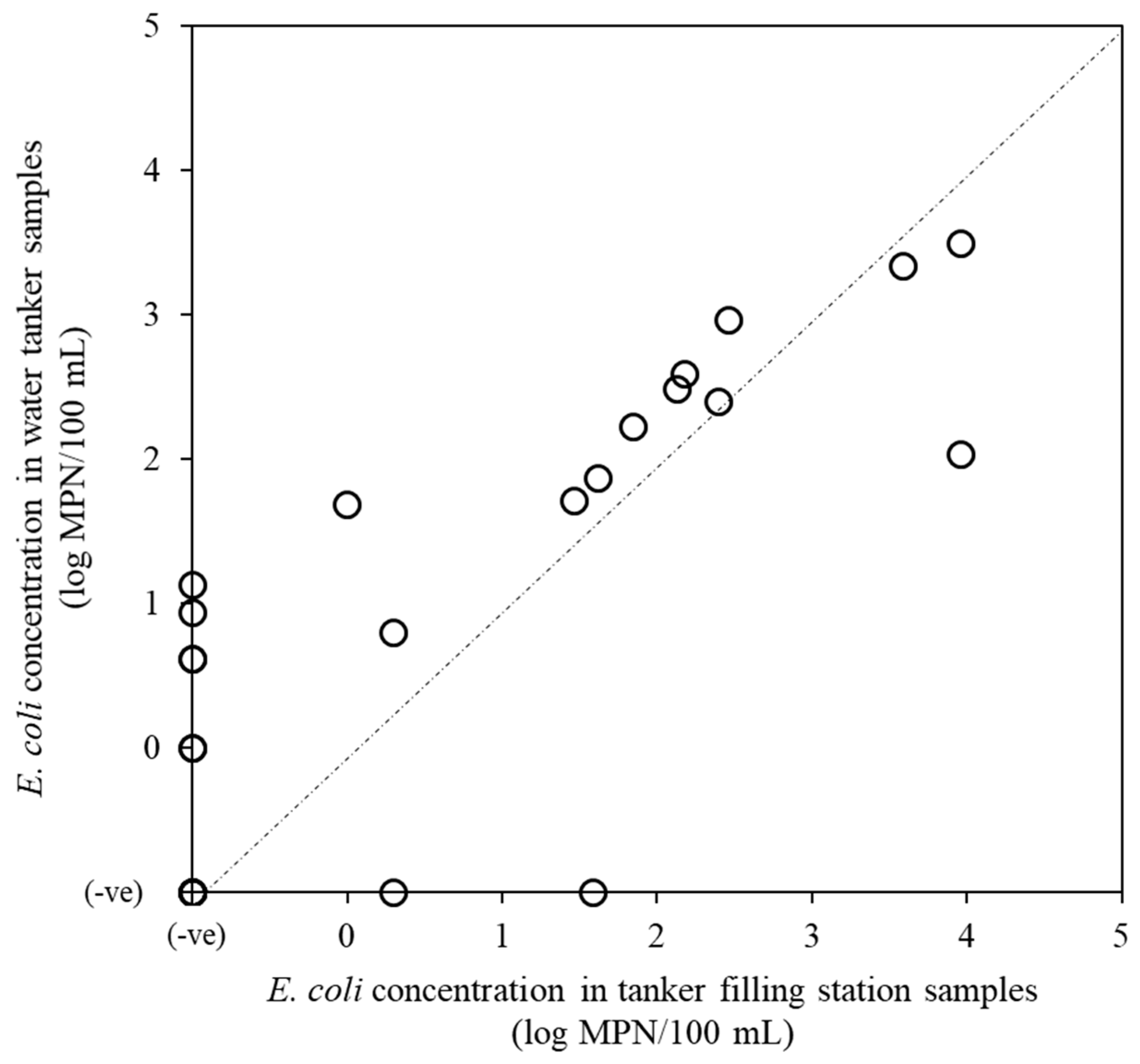
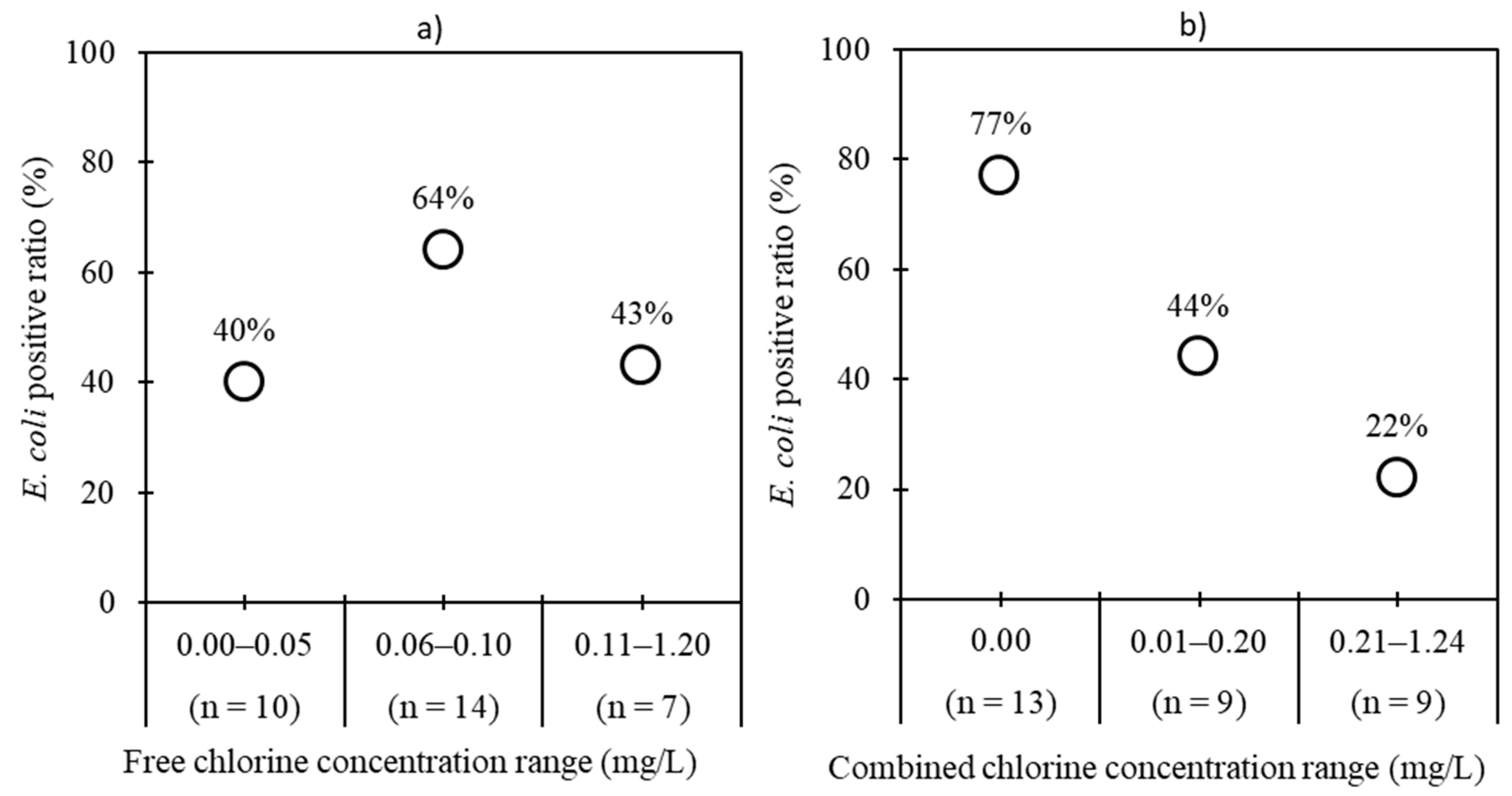
2.1. Detection of Fecal Indicator Bacteria and Index Viruses
2.2. Detection of Pathogenic Viruses
2.3. Detection of Host-Associated Fecal Markers
3. Discussion
4. Materials and Methods
4.1. Collection of Water Samples
4.2. Detection of Total Coliforms and E. coli
4.3. Concentration and Extraction of Bacterial, mtDNA, and Viral Markers and Viruses
4.4. Detection of Viruses and Fecal Markers
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Haramoto, E.; Yamada, K.; Nishida, K. Prevalence of protozoa, viruses, coliphages and indicator bacteria in groundwater and river water in the Kathmandu Valley, Nepal. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Malla, S.S.; Aihara, Y.; Kondo, N.; Nishida, K. Water Quality at supply source and point of use in Kathmandu Valley. JWET 2013, 11, 331–340. [Google Scholar] [CrossRef]
- Guragai, B.; Takizawa, S.; Hashimoto, T.; Oguma, K. Effects of inequality of supply hours on consumers’ coping strategies and perceptions of intermittent water supply in Kathmandu Valley, Nepal. Sci. Total Environ. 2017, 599–600, 431–441. [Google Scholar] [CrossRef] [PubMed]
- KUKL. KUKL 9th Annual Report; Kathmandu Upatyaka Khanepani Limited: Kathmandu, Nepal, 2017. [Google Scholar]
- Shrestha, S.; Aihara, Y.; Bhattarai, A.P.; Bista, N.; Rajbhandari, S.; Kondo, N.; Kazama, F.; Nishida, K.; Shindo, J. Dynamics of domestic water consumption in the urban area of the Kathmandu Valley: Situation analysis pre and post 2015 Gorkha Earthquake. Water 2017, 9, 222. [Google Scholar] [CrossRef]
- Dongol, R.; Kansakar, L.K.; Bajimaya, S.; Maharjan, S.; Shrestha, D. Overview of water markets in the Kathmandu Valley. In Kathmandu Valley Groundwater Outlook; Shrestha, S., Pradhananga, D., Pandey, V.P., Eds.; SEN: Kathmandu, Nepal; CREEW: Kathmandu, Nepal; ICRE-UY: Yamanashi, Japan; Asian Institute of Technology: Pathum Thani, Thailand, 2012; pp. 100–111. ISBN 978-9937-2-4442-8. [Google Scholar]
- Kejjlen, M.; Mcgranahan, G. Informal Water Vendors and the Urban Poor Human Settlements Discussion Paper Series; International Institute for Environment and Development: London, UK, 2006. [Google Scholar]
- World Health Organization (WHO); United Nations Children’s Fund (UNICEF). Core Questions on Drinking-water and Sanitation for Household Surveys; WHO: Geneva, Switzerland; UNICEF: Geneva, Switzerland, 2006; p. 125. [Google Scholar]
- Pandey, V.P.; Chapagain, S.K.; Shrestha, D.; Shrestha, S.; Kazama, F. Groundwater Markets for Domestic Water Use in Kathmandu Valley: An Analysis of Its Characteristics, Impacts and Regulations. Available online: https://www.academia.edu/11198401/Groundwater_markets_for_domestic_water_use_in_Kathmandu_Valley_an_analysis_of_its_characteristics_impacts_and_regulations (accessed on 13 May 2019).
- Shrestha, D. State and Services of Private Water Tanker Operation in Kathmandu. Unpublished M.Sc. Thesis, Nepal Engineering College, Pokhara University, Changunarayan, Nepal, 2011. [Google Scholar]
- Haramoto, E. Detection of waterborne protozoa, viruses, and bacteria in groundwater and other water samples in the Kathmandu Valley, Nepal. IOP Conf. Ser. Earth Environ. Sci. 2018, 120, 012004. [Google Scholar] [CrossRef]
- Maharjan, S.; Joshi, T.P.; Shrestha, S.J. Poor quality of treated water in Kathmandu: Comparison with Nepal drinking water quality standards. Tribhuvan Univ. J. Microbiol. 2018, 5, 83–88. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, S.; Shindo, J.; Sherchand, J.B.; Haramoto, E. Virological quality of irrigation water sources and pepper mild mottle virus and tobacco mosaic virus as index of pathogenic virus contamination level. Food Environ. Virol. 2018, 10, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Tandukar, S.; Sherchand, J.B.; Bhandari, D.; Sherchan, S.; Malla, B.; Ghaju Shrestha, R.; Haramoto, E. Presence of human enteric viruses, protozoa, and indicators of pathogens in the Bagmati River, Nepal. Pathogens 2018, 7, 38. [Google Scholar] [CrossRef]
- Harwood, V.J.; Staley, C.; Badgley, B.D.; Borges, K.; Korajkic, A. Microbial source tracking markers for detection of fecal contamination in environmental waters: Relationships between pathogens and human health outcomes. FEMS. Microbiol. Rev. 2014, 38, 1–40. [Google Scholar] [CrossRef]
- Haramoto, E.; Osada, R. Assessment and application of host-specific Bacteroidales genetic markers for microbial source tracking of river water in Japan. PLoS ONE 2018, 13, e0207727. [Google Scholar] [CrossRef]
- Kildare, B.J.; Leutenegger, C.M.; McSwain, B.S.; Bambic, D.G.; Rajal, V.B.; Wuertz, S. 16S rRNA-based assays for quantitative detection of universal, human-, cow-, and dog-specific fecal Bacteroidales: A Bayesian approach. Water Res. 2007, 41, 3701–3715. [Google Scholar] [CrossRef] [PubMed]
- Reischer, G.H.; Kasper, D.C.; Steinborn, R.; Mach, R.L.; Farnleitner, A.H. Quantitative PCR method for sensitive detection of ruminant fecal pollution in freshwater and evaluation of this method in alpine karstic regions. Appl. Environ. Microbiol. 2006, 72, 5610–5614. [Google Scholar] [CrossRef] [PubMed]
- Mieszkin, S.; Furet, J.P.; Corthier, G.; Gourmelon, M. Estimation of pig fecal contamination in a river catchment by real-time PCR using two Pig-Specific Bacteroidales 16S rRNA genetic markers. Appl. Environ. Microbiol. 2009, 75, 3045–3054. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.M.; Raley, M.E.; Levine, J.F. Mitochondrial multiplex real-time PCR as a source tracking method in fecal-contaminated effluents. Environ. Sci. Technol. 2007, 41, 3277–3283. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.M.; Levine, J.F. Domestic wastewater influent profiling using mitochondrial real-time PCR for source tracking animal contamination. J. Microbiol. Methods 2009, 77, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.T.; Lipp, E.K. Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiol. Mol. Biol. Rev. 2005, 69, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Albinana-Gimenez, N.; Clemente-Casares, P.; Bofill-Mas, S.; Hundesa, A.; Ribas, F.; Girones, R. Distribution of human polyomaviruses, adenoviruses, and hepatitis E virus in the environment and in a drinking-water treatment plant. Environ. Sci. Technol. 2006, 40, 7416–7422. [Google Scholar] [CrossRef] [PubMed]
- Carratalà, A.; Rusinol, M.; Hundesa, A.; Biarnes, M.; Rodriguez-Manzano, J.; Vantarakis, A.; Kern, A.; Suñen, E.; Girones, R.; Bofill-Mas, S. A novel tool for specific detection and quantification of chicken/turkey parvoviruses to trace poultry fecal contamination in the environment. App. Environ. Microbiol. 2012, 78, 7496–7499. [Google Scholar] [CrossRef] [PubMed]
- Hundesa, A.; Maluquer de Motes, C.; Albinana-Gimenez, N.; Rodriguez-Manzano, J.; Bofill-Mas, S.; Suñen, E.; Rosina Girones, R. Development of a qPCR assay for the quantification of porcine adenoviruses as an MST tool for swine fecal contamination in the environment. J. Virol. Methods 2009, 158, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Bhandari, D.; Inoue, D.; Sei, K.; Tanaka, Y.; Sherchand, J.B.; Haramoto, E. Validation of host-specific Bacteroidales quantitative PCR assays and their application to microbial source tracking of drinking water sources in the Kathmandu Valley, Nepal. J. Appl. Microbiol. 2018, 125, 609–619. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Constantine, K.; Massoud, M.; Alameddine, I.; El-Fadel, M. The role of water tankers market in water stressed semi-arid urban areas: Implications on water quality and economic burden. J. Environ. Manag. 2017, 188, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Ono, K.; Nukina, M.; Itoh, M.; Thapa, U.; Rai, S.K. Detection of diarrheagenic viruses from diarrheal fecal samples collected from children in Kathmandu, Nepal. Nepal Med. Coll. J. 2004, 6, 17–23. [Google Scholar] [PubMed]
- Sherchand, J.B.; Schluter, W.W.; Sherchan, J.B.; Tandukar, S.; Dhakwa, J.R.; Choudhary, G.R.; Mahaseth, C. Prevalence of group A genotype human rotavirus among children with diarrhoea in Nepal, 2009–2011. WHO South-East Asia J. Public Health 2012, 1, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Sherchand, J.B.; Rijal, B.P.; Parajuli, K.; Mishra, S.K.; Dahal, R.K.; Shrestha, S.; Tandukar, S.; Chaudhary, R.; Kattel, H.P.; et al. Characterization of rotavirus causing acute diarrhoea in children in Kathmandu, Nepal, showing the dominance of serotype G12. J. Med. Microbiol. 2013, 62, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Kitajima, M. Quantification and genotyping of aichi virus 1 in water samples in the Kathmandu Valley, Nepal. Food Environ. Virol. 2017, 9, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Tandukar, S.; Sherchand, J.B.; Karki, S.; Malla, B.; Ghaju Shrestha, R.; Bhandari, D.; Thakali, O.; Haramoto, E. Co-Infection by Waterborne Enteric Viruses in Children with Gastroenteritis in Nepal. Healthcare 2019, 7, 9. [Google Scholar] [CrossRef]
- Shrestha, S.; Haramoto, E.; Shindo, J. Assessing the infection risk of enteropathogens from consumption of raw vegetables washed with contaminated water in Kathmandu Valley, Nepal. J. Appl. Microbiol. 2017, 123, 1321–1334. [Google Scholar] [CrossRef]
- Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Bhandari, D.; Inoue, D.; Sei, K.; Tanaka, Y.; Sherchand, J.B.; Haramoto, E. Identification of human and animal fecal contamination in drinking water sources in the Kathmandu Valley, Nepal, using host-associated Bacteroidales quantitative PCR assays. Water 2018, 10, 1796. [Google Scholar] [CrossRef]
- Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Sherchand, J.B.; Haramoto, E. Performance evaluation of human-specific viral markers and application of pepper mild mottle virus and crAssphage to environmental water samples as fecal pollution markers in the Kathmandu Valley, Nepal. Food Environ. Virol. 2019. [Google Scholar] [CrossRef]
- Uy, D.; Haka, S.; Huya, C.; Srey, M.; Chunhieng, T.; Phoeurng, S.; Nasir, H.M.; Fredricks, D. Comparison of tube-well and dug-well groundwater in the arsenic polluted areas in Cambodia. In Southeast Asian Water Environment 4; Fukusi, K., Kurisu, F., Oguma, K., Furumai, H., Fontanos, P., Eds.; International Water Association Publishing: London, UK, 2010. [Google Scholar]
- Ferguson, A.S.; Mailloux, B.J.; Ahmed, K.M.; Van Geen, A.; McKay, L.D.; Culligan, P.J. Hand-pumps as reservoirs for microbial contamination of well water. J. Water Health 2011, 9, 708–717. [Google Scholar] [CrossRef]
- Bajracharya, R.; Nakamura, T.; Shakya, B.M.; Kei, N.; Shrestha, S.D.; Tamrakar, N.K. Identification of river water and groundwater interaction at central part of the Kathmandu valley, Nepal using stable isotope tracers. Int. J. Adv. Sci. Tech. Res. 2018, 8, 29–41. [Google Scholar] [CrossRef]
- Ghaju Shrestha, R.; Tanaka, Y.; Malla, B.; Bhandari, D.; Tandukar, S.; Inoue, D.; Sei, K.; Sherchand, J.B.; Haramoto, E. Next-generation sequencing identification of pathogenic bacterial genes and their relationship with fecal indicator bacteria in different water sources in the Kathmandu Valley, Nepal. Sci. Total Environ 2017, 601–602, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Katayama, H.; Asami, M.; Akiba, M. Development of a novel method for simultaneous concentration of viruses and protozoa from a single water sample. J. Virol. Methods 2012, 182, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Kitajima, M.; Hata, A.; Torrey, J.R.; Masago, Y.; Sano, D.; Katayama, H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018, 135, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Hata, A.; Yamashita, T.; Haramoto, E.; Minagawa, H.; Katayama, H. Development of a reverse transcription-quantitative PCR system for detection and genotyping of aichi viruses in clinical and environmental samples. Appl. Environ. Microbiol. 2013, 79, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Sirota, L.; Maudru, T.; Peden, K.; Lewis, A.M. Realtime, quantitative PCR assays for the detection of virus-specific DNA in samples with mixed populations of polyomaviruses. J. Virol. Methods 2006, 135, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.S.; Wait, D.; Tai, L.; Sobsey, M.D. Methods to remove inhibitors in sewage and other fecal wastes for enterovirus detection by the polymerase chain reaction. J. Virol. Methods 1995, 54, 51–66. [Google Scholar] [CrossRef]
- Katayama, H.; Shimasaki, A.; Ohgaki, S. Development of a virus concentration method and its application to detection of enterovirus and norwalk virus from coastal seawater. Appl. Environ. Microbiol. 2002, 68, 1033–1039. [Google Scholar] [CrossRef]
- Zhang, T.; Breitbart, M.; Lee, W.H.; Run, J.-Q.; Wei, C.L.; Soh, S.W.L.; Hibberd, M.L.; Liu, E.T.; Rohwer, F.; Ruan, Y. RNA viral community in human feces: Prevalence of plant pathogenic viruses. PLoS Biol. 2005, 4, 108–118. [Google Scholar] [CrossRef]
- Haramoto, E.; Kitajima, M.; Kishida, N.; Konno, Y.; Katayama, H.; Asami, M.; Akiba, M. Occurrence of pepper mild mottle virus in drinking water sources in Japan. Appl. Environ. Microbiol. 2013, 79, 7413–7418. [Google Scholar] [CrossRef]
- Jothikumar, N.; Kang, G.; Hill, V.R. Broadly reactive TaqMan assay for real-time RT-PCR detection of rotavirus in clinical and environmental samples. J. Virol. Methods 2009, 155, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Balique, F.; Colson, P.; Barry, A.O.; Nappez, C.; Ferretti, A.; Al Moussawi, K.; Ngounga, T.; Lepidi, H.; Ghigo, E.; Mege, J.-L.; et al. Tobacco mosaic virus in the lungs of mice following intra-tracheal inoculation. PLoS ONE 2013, 8, e54993. [Google Scholar] [CrossRef] [PubMed]
- Heim, A.; Ebnet, C.; Harste, G.; Pring-Åkerblom, P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 2003, 70, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Clavero, M.A.; Fernadez, C.; Ortiz, J.A.; Pro, J.; Carbonell, G.; Tarazona, J.V.; Roblas, N.; Ley, V. Teschoviruses as indicators of porcine fecal contamination of surface water. App. Environ. Microbiol. 2003, 69, 6311–6315. [Google Scholar] [CrossRef] [PubMed]
| Water Sample | No. of Tested Samples | Fecal Indicator Bacteria | Index Viruses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | Total Coliforms | PMMoV | TMV | ||||||
| No. of Positive Samples (%) | Concentration a (log MPN b/100 mL) | No. of Positive Samples (%) | Concentration a (log MPN b/100 mL) | No. of Positive Samples (%) | Concentration a (log copies/L) | No. of Positive Samples (%) | Concentration a (log copies/L) | ||
| Tanker filling station | 31 | 16 (52) | 0.0–4.0 | 27 (87) | 0.0–5.4 | 22 (71) | 1.7–4.7 | 28 (90) | 2.7–6.0 |
| Water tanker | 30 | 21 (70) | 0.0–3.5 | 27 (90) | 1.0–4.8 | 22 (73) | 2.1–4.9 | 29 (97) | 2.8–6.3 |
| Total | 61 | 37 (61) | 54 (89) | 44 (72) | 57 (93) | ||||
| Water Sample | No. of Tested Samples | AiV-1 | EVs | HAdVs | NoVs-GI | NoVs-GII | RVAs | At Least One Pathogen Detected | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Positive Samples (%) | Conc. a (log copies/L) | No. of Positive Samples (%) | Conc. a (log copies/L) | No. of Positive Samples (%) | Conc. a (log copies/L) | No. of Positive Samples (%) | Conc. a (log copies/L) | No. of Positive Samples (%) | Conc. a (log copies/L) | No. of Positive Samples (%) | Conc. a (log copies/L) | No. of Positive Samples (%) | ||
| Tanker filling station | 31 | 0 (0) | NA | 11 (35) | 2.7–4.6 | 4 (13) | 3.6–4.9 | 0 (0) | NA | 7 (23) | 2.0–3.9 | 2 (6) | 3.3–3.7 | 14 (45) |
| Water tanker | 30 | 0 (0) | NA | 4 (13) | 3.1–4.6 | 2 (7) | 4.3–5.0 | 0 (0) | NA | 6 (20) | 1.8–4.5 | 3 (10) | 2.8–3.4 | 8 (27) |
| Total | 61 | 0 (0) | 15 (25) | 6 (10) | 0 (0) | 13 (21) | 5 (8) | 22 (36) | ||||||
| Fecal Markers | Detection % (No. of Positive Samples/No. of Tested Samples) | Concentration d (log copies/L) | |
|---|---|---|---|
| Human- | BacHum a | 5 (1/22) | 6.3 |
| HAdVs b | 13 (4/31) | 3.6–4.9 | |
| BKPyVs b | 29 (9/31) | 4.9–5.7 | |
| JCPyVs b | 10 (3/31) | 5.0–5.9 | |
| At least one human marker | 39 (12/31) | 3.6–6.3 | |
| Ruminant- | BacR a | 14 (3/22) | 5.4–5.9 |
| Bovine mtDNAc | 0 (0/22) | NAe | |
| Pig- | Pig2Bac a | 5 (1/22) | 6.1 |
| PoAdVs b | 0 (0/31) | NA | |
| Swine mtDNA c | 0 (0/22) | NA | |
| Dog- | Dog mtDNA c | 0 (0/22) | NA |
| Chicken- | ChkPVs b | 3 (1/31) | 3.4 |
| Assay | Primer/Probe | Sequence (5′–3′) | Product Length (bp) | Reference |
|---|---|---|---|---|
| AiV-1 | Forward primer | GTCTCCACHGACACYAAYTGGAC | 108–111 | [43] |
| Reverse primer | GTTGTACATRGCAGCCCAGG | |||
| TaqMan MGB probe | FAM-TTYTCCTTYGTGCGTGC-MGB-NFQ | |||
| BacHum | Forward primer | TGAGTTCACATGTCCGCATGA | 82 | [17] |
| Reverse primer | CGTTACCCCGCCTACTATCTAATG | |||
| TaqMan probe | FAM-TCCGGTAGACGATGGGGATGCGTT-TAMRA | |||
| BacR | Forward primer | GCGTATCCAACCTTCCCG | 118 | [18] |
| Reverse primer | CATCCCCATCCGTTACCG | |||
| TaqMan MGB probe | FAM-CTTCCGAAAGGGAGATT-MGB-NFQ | |||
| BKPyVs | Forward primer | GGCTGAAGTATCTGAGACTTGGG | 78 | [44] |
| Reverse primer | GAAACTGAAGACTCTGGACATGGA | |||
| TaqMan probe | FAM-CAAGCACTGAATCCCAATCACAATGCTC-TAMRA | |||
| Bovine-mtDNA | Forward primer | CAGCAGCCCTACAAGCAATGT | 191 | [20] |
| Reverse primer | GAGGCCAAATTGGGCGGATTAT | |||
| TaqMan probe | FAM-CATCGGCGACATTGGTTTCATTTTAG-TAMRA | |||
| ChkPVs | Forward primer | AGTCCACGAGATTGGCAACA | 82 | [24] |
| Reverse primer | GCAGGTTAAAGATTTTCACG | |||
| TaqMan probe | FAM-AATTATTCGAGATGGCGCCCACG-TAMRA | |||
| Dog-mtDNA | Forward primer | GGCATGCCTTTCCTTACAGGATTC | 109 | [21] |
| Reverse primer | GGGATGTGGCAACGAGTGTAATTATG | |||
| TaqMan probe | FAM-TCATCGAGTCCGCTAACACGTCGAAT-TAMRA | |||
| EVs | Forward primer | CCTCCGGCCCCTGAATG | 195 | [45] |
| Reverse primer | ACCGGATGGCCAATCCAA | |||
| TaqMan probe | FAM-CCGACTACTTTGGGTGTCCGTGTTTC-TAMRA | [46] | ||
| HAdVs | Forward primer | GCCACGGTGGGGTTTCTAAACTT | 132 | [51] |
| Reverse primer | GCCCCAGTGGTCTTACATGCACATC | |||
| TaqMan probe | FAM-TGCACCAGACCCGGGCTCAGGTACTCCGA-TAMRA | |||
| JCPyVs | Forward primer | GGAAAGTCTTTAGGGTCTTCTACCTTT | 89 | [44] |
| Reverse primer | ATGTTTGCCAGTGATGATGAAAA | |||
| TaqMan probe | FAM-GATCCCAACACTCTACCCCACCTAAAAAGA-TAMRA | |||
| NoVs-GI | Forward primer | CGYTGGATGCGNTTYCATGA | 85 | [52] |
| Reverse primer | CTTAGACGCCATCATCATTYAC | |||
| TaqMan probe | FAM-AGATYGCGATCYCCTGTCCA-TAMRA | |||
| NoVs-GII | Forward primer | CARGARBCNATGTTYAGRTGGATGAG | 98 | [52] |
| Reverse primer | TCGACGCCATCTTCATTCACA | |||
| TaqMan probe | FAM-TGGGAGGGCGATCGCAATCT-TAMRA | |||
| Pig2Bac | Forward primer | GCATGAATTTAGCTTGCTAAATTTGAT | 117 | [19] |
| Reverse primer | ACCTCATACGGTATTAATCCGC | |||
| TaqMan MGB probe | FAM-TCCACGGGATAGCC-MGB-NFQ | |||
| PMMoV | Forward primer | GAGTGGTTTGACCTTAACGTTTGA | 68 | [47] |
| Reverse primer | TTGTCGGTTGCAATGCAAGT | [48] | ||
| TaqMan MGB probe | FAM-CCTACCGAAGCAAATG-MGB-NFQ | [47] | ||
| PoAdVs | Forward primer | AACGGCCGCTACTGCAAG | 68 | [25] |
| Reverse primer | AGCAGCAGGCTCTTGAGG | |||
| TaqMan MGB probe | FAM-CACATCCAGGTGCCGC-MGB-NFQ | |||
| PoTeVs | Forward primer | CACCAGCGTGGAGTTCCTGTA | 66 | [53] |
| Reverse primer | AGCCGCGACCCTGTCA | |||
| TaqMan probe | FAM-TGCAGGACTGGACTTG-TAMRA | |||
| RVAs | Forward primer | CAGTGGTTGATGCTCAAGATGGA | 131 | [49] |
| Reverse primer | TCATTGTAATCATATTGAATACCA | |||
| TaqMan probe | FAM-ACAACTGCAGCTTCAAAAGAAGWGT-TAMRA | |||
| Swine-mtDNA | Forward primer | ACAGCTGCACTACAAGCAATGC | 197 | [20] |
| Reverse primer | GGATGTAGTCCGAATTGAGCTGATTAT | |||
| TaqMan probe | FAM-CATCGGAGACATTGGATTTGTCCTAT-TAMRA | |||
| TMV | Forward primer | CAAGCTGGAACTGTCGTTCA | 120 | [50] |
| Reverse primer | CGGGTCTAAYACCGCATTGT | |||
| TaqMan probe | FAM-CAGTGAGGTGTGGAAACCTTCACCACA-TAMRA | |||
| FAM, 6-carboxyfluorescein; MGB, minor groove binder; NFQ, nonfluorescent quencher; TAMRA, 5-carboxytetramethylrhodamine. | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Bhandari, D.; Thakali, O.; Sherchand, J.B.; Haramoto, E. Detection of Pathogenic Viruses, Pathogen Indicators, and Fecal-Source Markers within Tanker Water and Their Sources in the Kathmandu Valley, Nepal. Pathogens 2019, 8, 81. https://doi.org/10.3390/pathogens8020081
Malla B, Ghaju Shrestha R, Tandukar S, Bhandari D, Thakali O, Sherchand JB, Haramoto E. Detection of Pathogenic Viruses, Pathogen Indicators, and Fecal-Source Markers within Tanker Water and Their Sources in the Kathmandu Valley, Nepal. Pathogens. 2019; 8(2):81. https://doi.org/10.3390/pathogens8020081
Chicago/Turabian StyleMalla, Bikash, Rajani Ghaju Shrestha, Sarmila Tandukar, Dinesh Bhandari, Ocean Thakali, Jeevan B. Sherchand, and Eiji Haramoto. 2019. "Detection of Pathogenic Viruses, Pathogen Indicators, and Fecal-Source Markers within Tanker Water and Their Sources in the Kathmandu Valley, Nepal" Pathogens 8, no. 2: 81. https://doi.org/10.3390/pathogens8020081
APA StyleMalla, B., Ghaju Shrestha, R., Tandukar, S., Bhandari, D., Thakali, O., Sherchand, J. B., & Haramoto, E. (2019). Detection of Pathogenic Viruses, Pathogen Indicators, and Fecal-Source Markers within Tanker Water and Their Sources in the Kathmandu Valley, Nepal. Pathogens, 8(2), 81. https://doi.org/10.3390/pathogens8020081






