Infections with Carbapenem-Resistant Gram-Negative Bacteria are a Serious Problem Among Critically Ill Children: A Single-Centre Retrospective Study
Abstract
1. Introduction
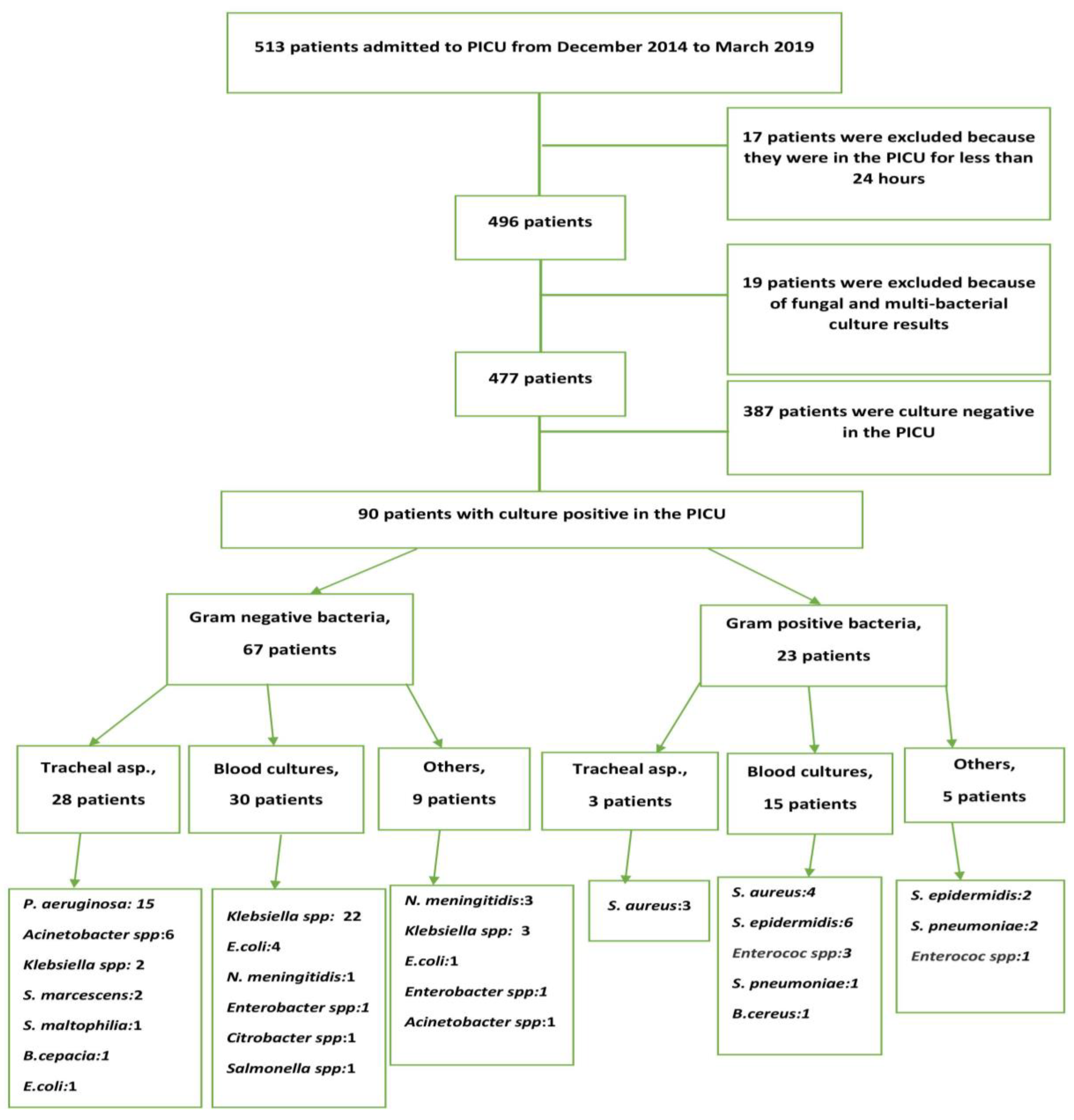
2. Results
2.1. Demographic Characteristics
2.2. Factors Associated with Culture-Positive Bacterial Infections in the PICU
2.3. Factors Associated with Gram-Positive and Gram-Negative Bacterial Infections in the PICU
2.4. Factors Associated with Gram-Negative CRIs
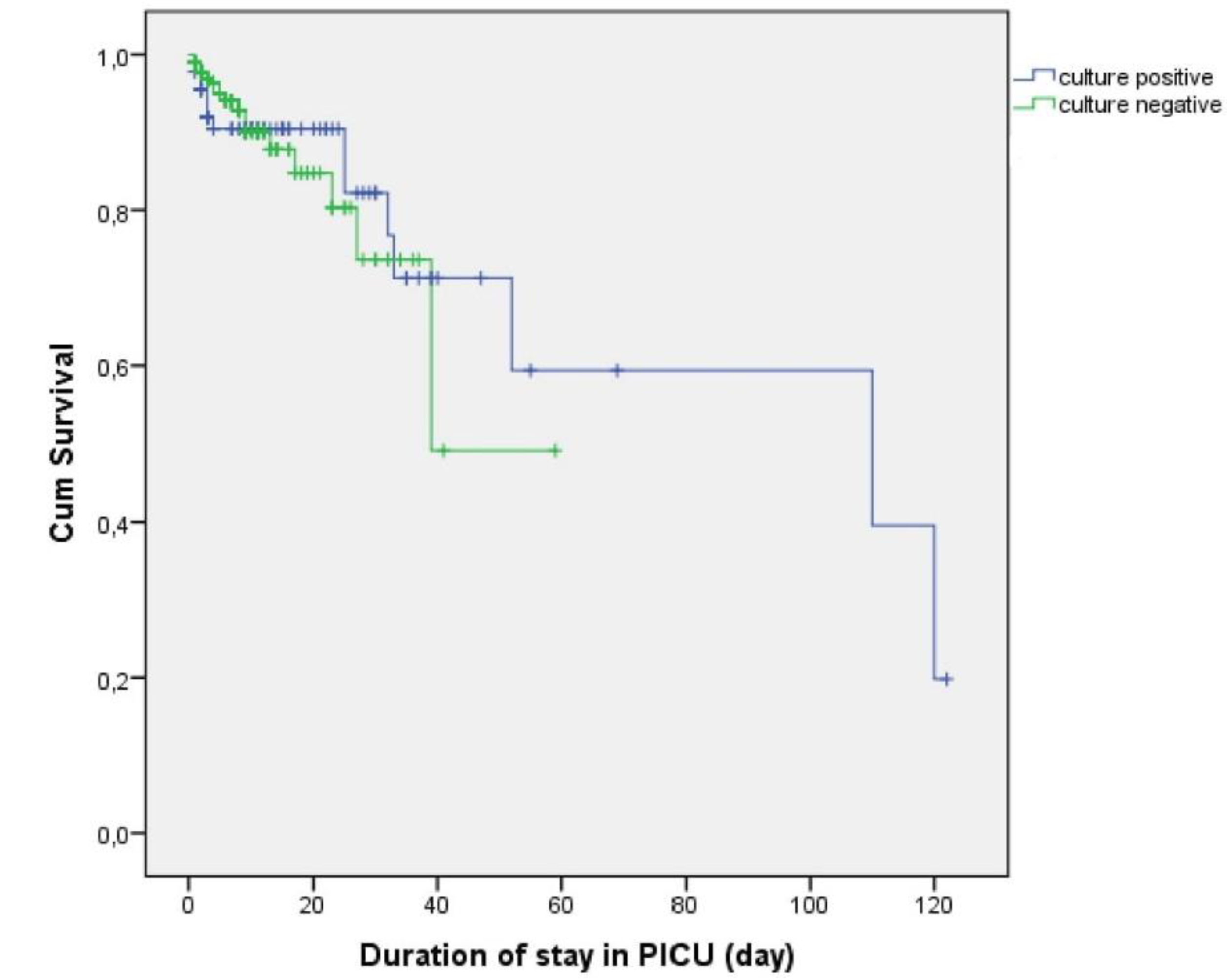
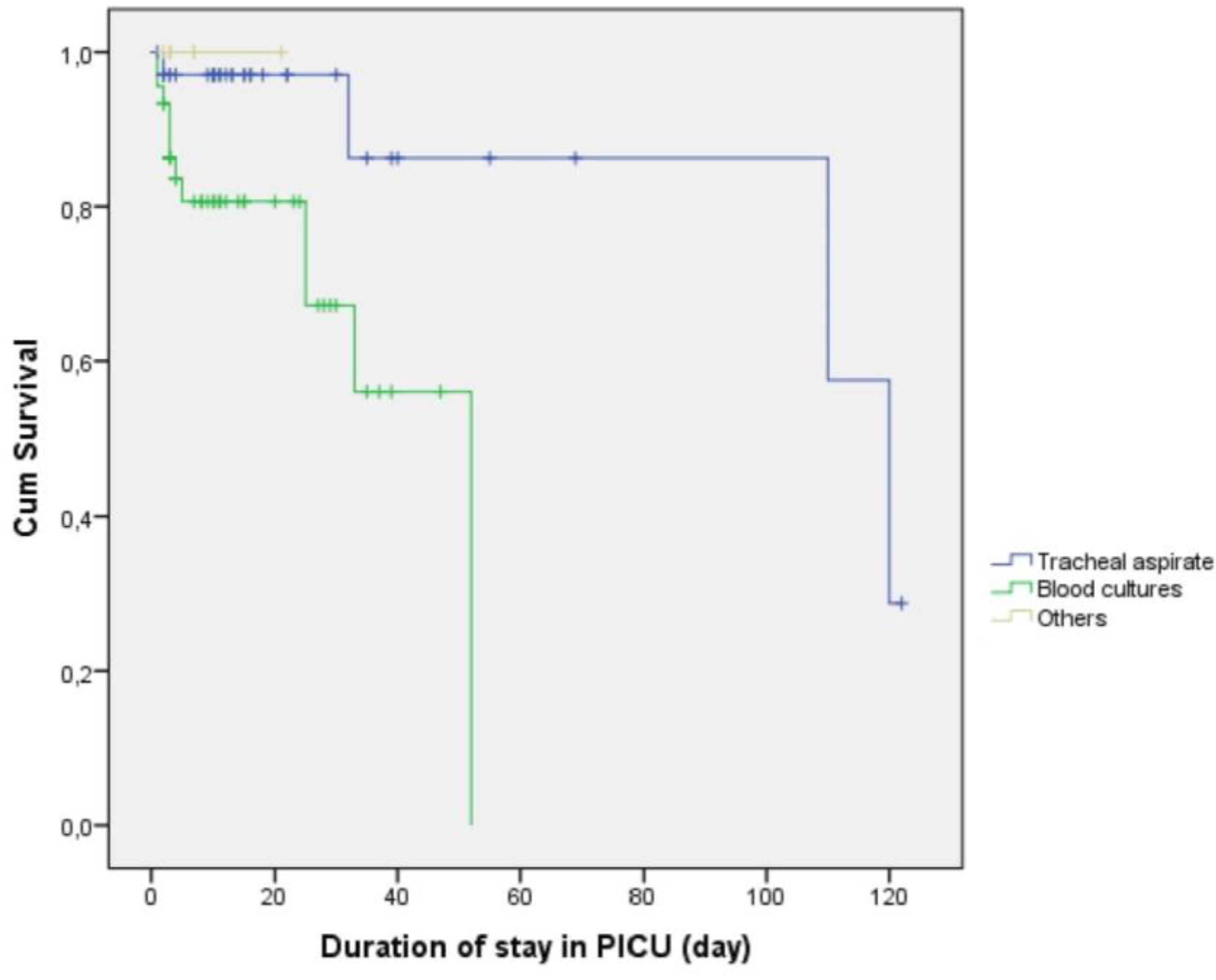
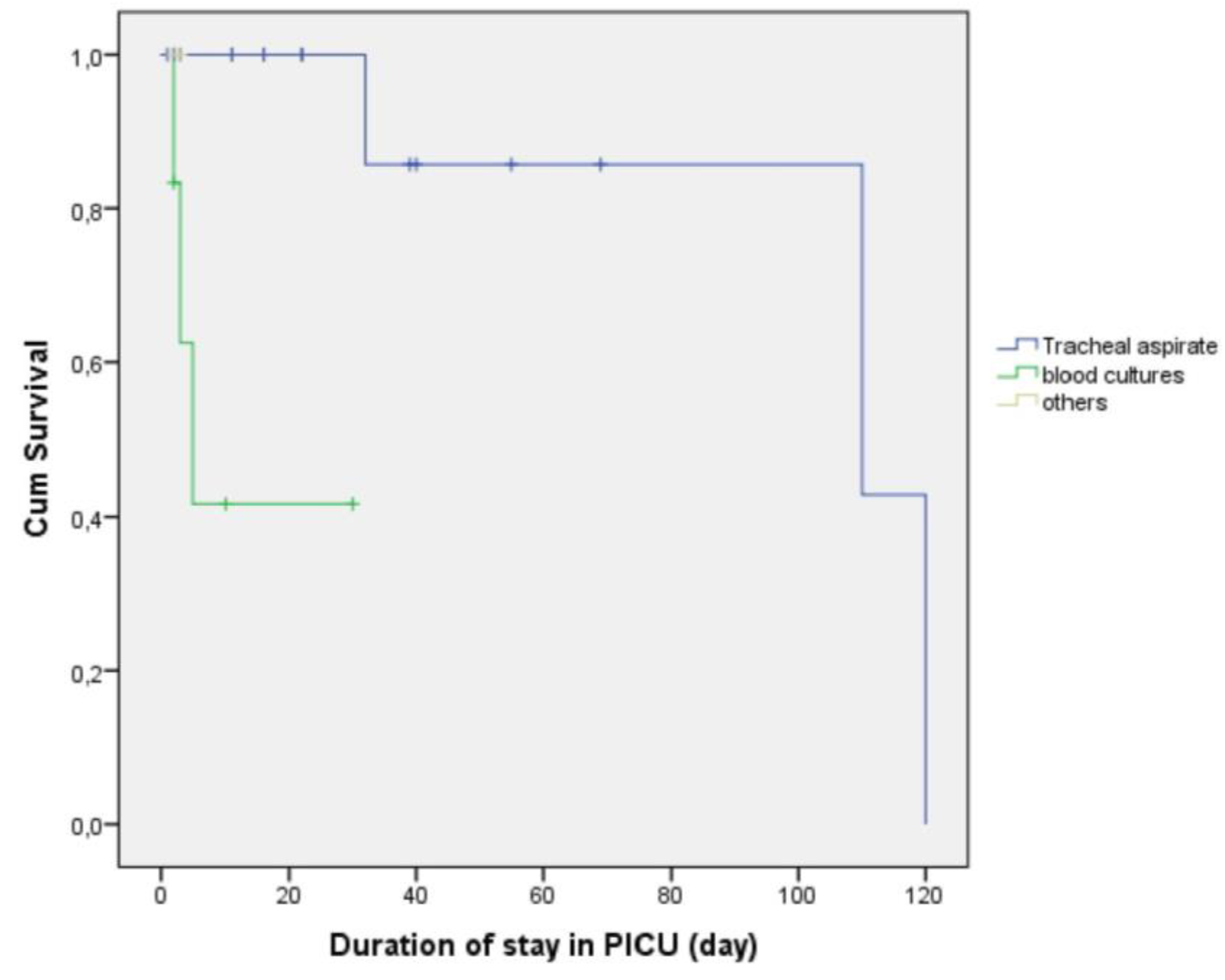
2.5. Survival Outcomes According to Culture Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Collection and Patient Comparisons
4.3. Evaluation of Culture Results
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dorofaeff, T.; Mohseni-Bod, H.; Cox, P.N. Infections in the PICU. In Textbook of Clinical Pediatrics; Elzouki, A.Y., Harfi, H.A., Nazer, H.M., Stapleton, B., Oh, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 2537–2563. [Google Scholar]
- Esteban, E.; Ferrer, R.; Urrea, M.; Suarez, D.; Rozas, L.; Balaguer, M. The impact of a quality improvement intervention to reduce nosocomial infections in a PICU. Pediatr. Crit. Care Med. 2013, 14, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Sinkowitz-Cochran, R.L.; Garrett, D.O.; Sohn, A.H.; Levine, G.L.; Siegel, J.D.; Stover, B.H.; Jarvis, W.R.; Pediatric Prevention Network. A national point-prevalence survey of pediatric intensive care unit-acquired infections in the United States. J. Pediatr. 2002, 140, 432–438. [Google Scholar] [CrossRef]
- Reymond, J.; Aujard, Y.; European Study Group. Nosocomial infections in pediatric patients: A European multicenter prospective study. Infect. Control Hosp. Epidemiol. 2000, 21, 260–263. [Google Scholar] [CrossRef]
- Dray, S.; Coiffard, B.; Persico, N.; Papazian, L.; Hraiech, S. Are tracheal surveillance cultures useful in the intensive care unit? Ann. Transl. Med. 2018, 6, 421. [Google Scholar] [CrossRef]
- Karageorgos, S.A.; Bassiri, H.; Siakallis, G.; Miligkos, M.; Tsioutis, C. Intravenous colistin use for infections due to MDR Gram-negative bacilli in critically ill paediatric patients: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2019. [Google Scholar] [CrossRef]
- Stein, R.; Dogan, H.S.; Hoebeke, P.; Kočvara, R.; Nijman, R.J.M.; Radmayr, C.; Tekgül, S.; European Association of Urology; European Society for Pediatric Urology. Urinary tract infection in children: EAU/ESPU guidelines. Eur. Urol. 2015, 67, 546–558. [Google Scholar]
- El-Nawawy, A.; Ashraf, G.A.; Antonios, M.A.M.; Meheissen, M.A.; El-Alfy, M.M.R. Incidence of Multidrug-Resistant Organism Among Children Admitted to Pediatric Intensive Care Unit in a Developing Country. Microb. Drug Resist. 2018, 24, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Akturk, H.; Sutcu, M.; Somer, A.; Aydın, D.; Cihan, R.; Ozdemir, A. Carbapenem-resistant Klebsiella pneumoniae colonization in pediatric and neonatal intensive care units: Risk factors for progression to infection. Braz. J. Infect. Dis. 2016, 20, 134–140. [Google Scholar] [CrossRef]
- Aygun, F.; Aygun, F.D.; Varol, F.; Durak, C.; Cokugraş, H.; Camcioglu, Y.; Cam, H. Can Nebulised Colistin Therapy Improve Outcomes in Critically Ill Children with Multi-Drug Resistant Gram-Negative Bacterial Pneumonia? Antibiotics 2019, 8, 40. [Google Scholar] [CrossRef]
- Nikaido, H.; Pagès, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J.M.; Amaral, L. Mechanisms of drug efflux and strategies to combat them: Challenging the efflux pump of Gram-negative bacteria. Biochim. Biophys. Acta 2009, 1794, 826–833. [Google Scholar] [CrossRef]
- Pan, Y.P.; Xu, Y.H.; Wang, Z.X.; Fang, Y.P.; Shen, J.L. Overexpression of MexAB-OprM efflux pump in carbapenem-resistant Pseudomonas aeruginosa. Arch. Microbiol. 2016, 198, 565–571. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multiresistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Colistin: The revival of polymyxins for the management of multidrug resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef]
- Anthony, K.B.; Fishman, N.O.; Linkin, D.R.; Gasink, L.B.; Edelstein, P.H.; Lautenbach, E. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin. Infect. Dis. 2008, 46, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.J.; Lin, H.S.; Kuo, A.J.; Leu, H.S.; Chiang, P.C.; Huang, C.T.; Lee, M.H. The clinical implication and prognostic predictors of tigecycline treatment for pneumonia involving multidrug-resistant Acinetobacter baumannii. J. Infect. 2011, 63, 351–361. [Google Scholar] [CrossRef]
- Tsala, M.; Vourli, S.; Georgiou, P.C.; Pournaras, S.; Daikos, G.R.L.; Mouton, J.W.; Meletiadis, J. Triple combination of meropenem, colistin and tigecycline was bactericidal in a dynamic model despite mere additive interactions in chequerboard assays against carbapenemase-producing Klebsiella pneumoniae isolates. J. Antimicrob. Chemother. 2019, 74, 387–394. [Google Scholar] [CrossRef]
- Ni, W.; Wei, C.; Zhou, C.; Zhao, J.; Liang, B.; Cui, J.; Wang, R.; Liu, Y. Tigecycline-Amikacin Combination Effectively Suppresses the Selection of Resistance in Clinical Isolates of KPC-Producing Klebsiella pneumoniae. Front. Microbiol. 2016, 7, 1304. [Google Scholar] [CrossRef]
- Elemam, A.; Rahimian, J.; Mandell, W. Infection with panresistant Klebsiella pneumoniae: A report of 2 cases and a brief review of the literature. Clin. Infect. Dis. 2009, 49, 271–274. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kang, C.I.; Chung, D.R.; Peck, K.R.; Song, J.H.; Jang, J.H. Breakthrough bacteremia due to extended-spectrum-beta lactamase-producing Klebsiella pneumoniae during combination therapy with colistin and tigecycline. Antimicrob. Agents Chemother. 2012, 56, 4994–4995. [Google Scholar] [CrossRef]
- Song, J.Y.; Lee, J.; Heo, J.Y.; Noh, J.Y.; Kim, W.J.; Cheong, H.J.; Hwang, I.S. Colistin and rifampicin combination in the treatment of ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 2008, 32, 281–284. [Google Scholar] [CrossRef]
- Majumdar, S.S.; Padiglione, A.A. Nosocomial infections in the intensive care unit. Anaesthasia Intensiv. Care Med. 2012, 13, 204–208. [Google Scholar] [CrossRef]
- Gupta, A.; Kapil, A.; Lodha, R.; Kabra, S.K.; Sood, S.; Dhawan, B.; Das, B.K.; Sreenivas, V. Burden of healthcare-associated infections in a paediatric intensive care unit of a developing country: A single centre experience using active surveillance. J. Hosp. Infect. 2011, 78, 323–326. [Google Scholar] [CrossRef]
- Venier, A.G.; Gruson, D.; Lavigne, T.; Jarno, P.; L’hériteau, F.; Coignard, B.; Savey, A.; Rogues, A.M.; REA-RAISIN Group. Identifying new risk factors for Pseudomonas aeruginosa pneumonia in intensive care units: Experience of the French national surveillance, REA-RAISIN. J. Hosp. Infect. 2011, 79, 44–48. [Google Scholar] [CrossRef]
- Torre, F.P.F.; Baldanzi, G.; Troster, E.J. Risk factors for vascular catheter-related bloodstream infections in pediatric intensive care units. Revista Brasileira de terapia intensiva 2018, 30, 436–442. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Guzman, S.; Migone, O.; Crnich, C.J. The attributable cost, length of hospital stay, and mortality of central line-associated bloodstream infection in intensive care departments in Argentina A prospective, matched analysis. Am. J. Infect. Control 2003, 31, 475–480. [Google Scholar] [CrossRef]
- Wylie, M.C.; Graham, D.A.; Potter-Bynoe, G.; Kleinman, M.E.; Randolph, A.G.; Costello, J.M.; Sandora, T.J. Risk factors for central line-associated bloodstream infection in pediatric intensive care units. Infect. Control Hosp. Epidemiol. 2010, 31, 1049–1056. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef]
- Dellinger, E.P.; Anaya, D.A. Infectious and immunologic consequences of blood transfusion. Crit. Care 2004, 8, S18. [Google Scholar] [CrossRef]
- Muszynski, J.A.; Spinella, P.C.; Cholette, J.M.; Acker, J.P.; Hall, M.W.; Juffermans, N.P.; Kelly, D.P.; Blumberg, N.; Nicol, K.; Liedel, J.; et al. Transfusion-related immunomodulation: Review of the literature and implications for pediatric critical illness. Transfusion 2017, 57, 195–206. [Google Scholar] [CrossRef]
- Sanchez-Pinto, L.N.; Goldstein, S.L.; Schneider, J.B.; Khemani, R.G. Association between Progression and Improvement of Acute Kidney Injury and Mortality in Critically Ill Children. Pediatr. Crit. Care Med. 2015, 16, 703–710. [Google Scholar] [CrossRef]
- Bajwa, A.; Kinsey, G.R.; Okusa, M.D. Immune mechanisms and novel pharmacological therapies of acute kidney injury. Curr. Drug Targets 2009, 10, 1196–1204. [Google Scholar] [CrossRef]
- Thakar, C.V.; Yared, J.P.; Worley, S.; Cotman, K.; Paganini, E.P. Renal dysfunction and serious infections after open-heart surgery. Kidney Int. 2003, 64, 239–246. [Google Scholar] [CrossRef]
- Santiago, M.J.; López-Herce, J.; Urbano, J.; Solana, M.J.; del Castillo, J.; Ballestero, Y.; Botrán, M.; Bellón, J.M. Clinical course and mortality risk factors in critically ill children requiring continuous renal replacement therapy. Intensiv. Care Med. 2010, 36, 843–849. [Google Scholar] [CrossRef]
- Belletti, A.; Castro, M.L.; Silvetti, S.; Greco, T.; Biondi-Zoccai, G.; Pasin, L.; Zangrillo, A.; Landoni, G. The Effect of inotropes and vasopressors on mortality: A meta-analysis of randomized clinical trials. Br. J. Anaesth. 2015, 115, 656–675. [Google Scholar] [CrossRef]
- Benbenishty, J.; Weissman, C.; Sprung, C.L.; Brodsky-Israeli, M.; Weiss, Y. Characteristics of patients receiving vasopressors. Heart Lung 2011, 40, 247–252. [Google Scholar] [CrossRef]
- Dominguez, T.E.; Chalom, R.; Costarino, A.T., Jr. The impact of adverse patient occurrences on hospital costs in the pediatric intensive care unit. Crit. Care Med. 2001, 29, 169–174. [Google Scholar] [CrossRef]
- Hattori, H.; Maeda, M.; Nagatomo, Y.; Takuma, T.; Niki, Y.; Naito, Y.; Sasaki, T.; Ishino, K. Epidemiology and risk factors for mortality in bloodstream infections: A single-center retrospective study in Japan. Am. J. Infect. Control 2018, 46, e75–e79. [Google Scholar] [CrossRef]
| Sex | |
|---|---|
| Male | 265 (55.6%) |
| Female | 212 (44.4%) |
| Reason for Hospitalisation | |
| Respiratory disease | 118 (24.7%) |
| Metabolic disease | 85 (17.8%) |
| Sepsis | 61 (12.8%) |
| Surgery | 57 (11.9%) |
| Neurologic disease | 50 (10.5%) |
| Haematology-oncology | 30 (6.3%) |
| Nephrology | 28 (5.9%) |
| Cardiovascular disease | 16 (3.4%) |
| Other | 32 (7.2%) |
| Age | 2.0 years (1 day to 18 years) |
| Acute kidney injury | 137 (28.7%) |
| Inotropic medication | 103 (21.6%) |
| Continuous renal replacement therapy | 100 (21.0%) |
| Invasive mechanical ventilation | 194 (40.7%) |
| Duration of PICU stay (days) | 3.00 (2–122) |
| Central venous catheter | 251 (52.6%) |
| Red blood cell transfusion | 216 (45.3%) |
| Non-invasive mechanical ventilation | 161 (33.8%) |
| Mortality | 37 (7.8%) |
| Intravenous immunoglobulin use | 55 (11.5%) |
| Infections detected at PICU admission | 71 (14.9%) |
| Nosocomial infection in the PICU | 19 (4.0%) |
| Total parenteral nutrition use | 126 (26.4%) |
| Previous hospitalization | 250 (52.4%) |
| Culture Positive (n = 90) | Culture Negative (n = 387) | P | |
|---|---|---|---|
| Sex | |||
| Male | 49 (54.4%) | 216 (55.8%) | 0.814 |
| Female | 41 (45.6%) | 171 (44.2%) | |
| Age (years) | 1.0 (1 month–18 years) | 2.25 (1 day–18 years) | 0.013 |
| IMV support | 58 (64.4%) | 136 (35.1%) | <0.001 |
| Duration of IMV support (days) | 10 (1–110) | 3 (1–50) | <0.001 |
| Inotropic drug use | 39 (43.3%) | 64 (16.5%) | <0.001 |
| Acute kidney injury | 50 (55.6%) | 87 (22.5%) | <0.001 |
| Continuous renal replacement therapy | 31 (34.4%) | 69 (17.8%) | 0.077 |
| NIV | 51 (56.7%) | 110 (28.4%) | <0.001 |
| Duration of NIV support (h) | 8 (1–144) | 3 (1–120) | 0.004 |
| Mortality | 13 (14.4%) | 24 (6.2%) | 0.008 |
| Duration of PICU stay (days) | 10 (1–122) | 3 (1–59) | <0.001 |
| Surgery | 14 (15.6%) | 49 (12.7%) | 0.436 |
| Body temperature at admission (°C) | 37.0 (34.5–39.3) | 36.7 (33.9–39.4) | 0.002 |
| Intravenous immunoglobulin use | 30 (33.3%) | 25 (6.5%) | <0.001 |
| Red blood cell transfusion | 60 (66.7%) | 156 (40.3%) | <0.001 |
| C-reactive protein (mg/dL) | 2.0 (0.3–32.2) | 1.0 (0.1–279.3) | 0.244 |
| Leucocyte count (/mm3) | 11,800 (200–111,300) | 10,800 (100–192,000) | 0.206 |
| Neutrophil count (/mm3) | 6400 (0–105,200) | 6150 (0–54,400) | 0.089 |
| Lactate (mmol/L) | 1.75 (0.5–17.0) | 1.70 (0.3–20.0) | 0.018 |
| Total parenteral nutrition use | 40 (44.4%) | 86 (22.2%) | <0.001 |
| P | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|
| Invasive mechanical ventilation | 0.009 | 2.254 | 1.229–4.131 |
| Catheter use | 0.328 | 1.419 | 0.703–2.866 |
| Surgery | 0.497 | 1.270 | 0.637–2.530 |
| Red blood cell transfusion | 0.005 | 2.624 | 1.346–5.115 |
| Acute kidney injury | 0.478 | 1.312 | 0.620–2.777 |
| Previous hospitalization | 0.180 | 1.475 | 0.836–2.602 |
| Inotropic drug use | 0.017 | 2.262 | 1.154–4.433 |
| Mortality | 0.561 | 1.339 | 0.500–3.581 |
| Gram-Negative (n = 67) | Gram-Positive (n = 23) | P | |
|---|---|---|---|
| Sex | |||
| Male | 34 (54.4%) | 14 (55.8%) | 0.401 |
| Female | 33 (45.6%) | 9 (44.2%) | |
| Age (years) | 1.0 (1 month–18 years) | 1.80 (1 month–17 years) | 0.139 |
| Invasive mechanical ventilation support | 50 (64.4%) | 12 (35.1%) | 0.045 |
| Inotropic drug use | 35 (43.3%) | 10 (16.5%) | 0.468 |
| Acute kidney injury | 21 (55.6%) | 8 (22.5%) | 0.833 |
| Continuous renal replacement therapy | 14 (34.4%) | 5 (17.8%) | 0.932 |
| Non-invasive mechanical ventilation | 31 (56.7%) | 16 (28.4%) | 0.054 |
| Mortality | 14 (14.4%) | 2 (6.2%) | 0.187 |
| Duration of PICU stay (days) | 9 (1–120) | 12 (3–122) | 0.665 |
| Body temperature at admission (°C) | 37.0 (34.5–39.3) | 37.2 (35.2–38.7) | 0.588 |
| Intravenous immunoglobulin use | 23 (33.3%) | 10 (6.5%) | 0.598 |
| Catheter-related sepsis | 3 (4.5%) | 5 (17.8%) | 0.006 |
| Red blood cell transfusion | 48 (66.7%) | 15 (40.3%) | 0.261 |
| C-reactive protein (mg/dL) | 2.0 (0.3–32.2) | 2.0 (0.4–30.6) | 0.433 |
| Leucocyte count (/mm3) | 13,600 (200–111,800) | 10,300 (200–40,500) | 0.194 |
| Neutrophil count (/mm3) | 7200 (0–105,200) | 5800 (100–39,500) | 0.333 |
| Carbapenem-Resistant Gram-Negative | P | |||
|---|---|---|---|---|
| Yes (n = 25) | No (n = 42) | |||
| Sex | Female | 10 (40.0%) | 25 (59.5%) | 0.122 |
| Male | 15 (60.0%) | 17 (40.5%) | ||
| Age (years) | 1.0 (2 month–18 years) | 1.0 (1 month–17 years) | 0.289 | |
| Duration of IMV support (days) | 23 (92.0%) | 28 (66.7%) | 0.019 | |
| Inotropic drug use | 9.0 (1–110) | 9.5 (1–52) | 0.559 | |
| Inotropic drug use | 10 (40.0%) | 25 (59.5%) | 0.122 | |
| Acute kidney injury | 6 (24.0%) | 15 (35.7%) | 0.320 | |
| Continuous renal replacement therapy | 3 (12.0%) | 10 (23.8%) | 0.237 | |
| NIV | 11 (44.0%) | 20 (47.6%) | 0.774 | |
| Duration of NIV support (h) | 48.0 (2–144) | 6.0 (1–96) | 0.083 | |
| Mortality | 6 (24.0%) | 7 (16.7%) | 0.463 | |
| Duration of PICU stay (days) | 11.0 (1–120) | 7.5 (1–52) | 0.031 | |
| Intravenous immunoglobulin use | 6 (24.0%) | 17 (40.5%) | 0.275 | |
| Previous hospitalization | 23 (92.0%) | 30 (71.4%) | 0.045 | |
| Surgery | 3 (12.0%) | 3 (7.1%) | 0.501 | |
| Red blood cell transfusion | 12 (48.0%) | 35 (83.3%) | 0.007 | |
| Central venous catheter use | 19 (76.0%) | 33 (78.6%) | 0.807 | |
| Total parenteral nutrition usage before infection | 19 (76.0%) | 15 (35.7%) | <0.001 | |
| Positive culture samples taken at admission | 20 (80.0%) | 36 (85.7%) | 0.541 | |
| Culture results | Klebsiella spp. | 9 (36.0%) | 18 (42.9%) | <0.001 |
| P. aeruginosa | 8 (32.0%) | 7 (16.7%) | ||
| Acinetobacter spp. | 6 (24.0%) | 1 (2.4%) | ||
| Others | 2 (8.0%) | 16 (38.1%) | ||
| Culture sample source: Antibiotics used for salvage treatment in addition to IV colistin treatment | Tracheal aspirate | 17 (68.0%) | 12 (28.6%) | 0.007 |
| Blood | 6 (24.0%) | 22 (52.4%) | ||
| Others | 2 (8.0%) | 8 (19.0%) | ||
| Tigecycline | 9 (36.0%) | - | ||
| Rifampin | 11 (44.0) | - | ||
| Nebulised colistin | 23 (92.0%) | - | ||
| P | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|
| Invasive mechanical ventilation | 0.005 | 7.626 | 1.010–57.580 |
| Surgery | 0.896 | 1.112 | 0.226–5.468 |
| Red blood cell transfusion | 0.007 | 0.094 | 0.017–0.525 |
| Acute kidney injury | 0.318 | 0.415 | 0.074–2.330 |
| Previous hospitalization | 0.560 | 1.965 | 0.203–19.046 |
| Inotropic drug use | 0.669 | 1.322 | 0.923–4.421 |
| Mortality | 0.706 | 1.341 | 0.292–6.156 |
| Total parenteral nutrition use | 0.016 | 5.870 | 1.386–24.870 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aygun, F.; Aygun, F.D.; Varol, F.; Durak, C.; Çokuğraş, H.; Camcıoğlu, Y.; Çam, H. Infections with Carbapenem-Resistant Gram-Negative Bacteria are a Serious Problem Among Critically Ill Children: A Single-Centre Retrospective Study. Pathogens 2019, 8, 69. https://doi.org/10.3390/pathogens8020069
Aygun F, Aygun FD, Varol F, Durak C, Çokuğraş H, Camcıoğlu Y, Çam H. Infections with Carbapenem-Resistant Gram-Negative Bacteria are a Serious Problem Among Critically Ill Children: A Single-Centre Retrospective Study. Pathogens. 2019; 8(2):69. https://doi.org/10.3390/pathogens8020069
Chicago/Turabian StyleAygun, Fatih, Fatma Deniz Aygun, Fatih Varol, Cansu Durak, Haluk Çokuğraş, Yıldız Camcıoğlu, and Halit Çam. 2019. "Infections with Carbapenem-Resistant Gram-Negative Bacteria are a Serious Problem Among Critically Ill Children: A Single-Centre Retrospective Study" Pathogens 8, no. 2: 69. https://doi.org/10.3390/pathogens8020069
APA StyleAygun, F., Aygun, F. D., Varol, F., Durak, C., Çokuğraş, H., Camcıoğlu, Y., & Çam, H. (2019). Infections with Carbapenem-Resistant Gram-Negative Bacteria are a Serious Problem Among Critically Ill Children: A Single-Centre Retrospective Study. Pathogens, 8(2), 69. https://doi.org/10.3390/pathogens8020069




