A Simple Genotyping Method for Rapid Differentiation of Blastocystis Subtypes and Subtype Distribution of Blastocystis spp. in Thailand
Abstract
:1. Introduction
2. Results
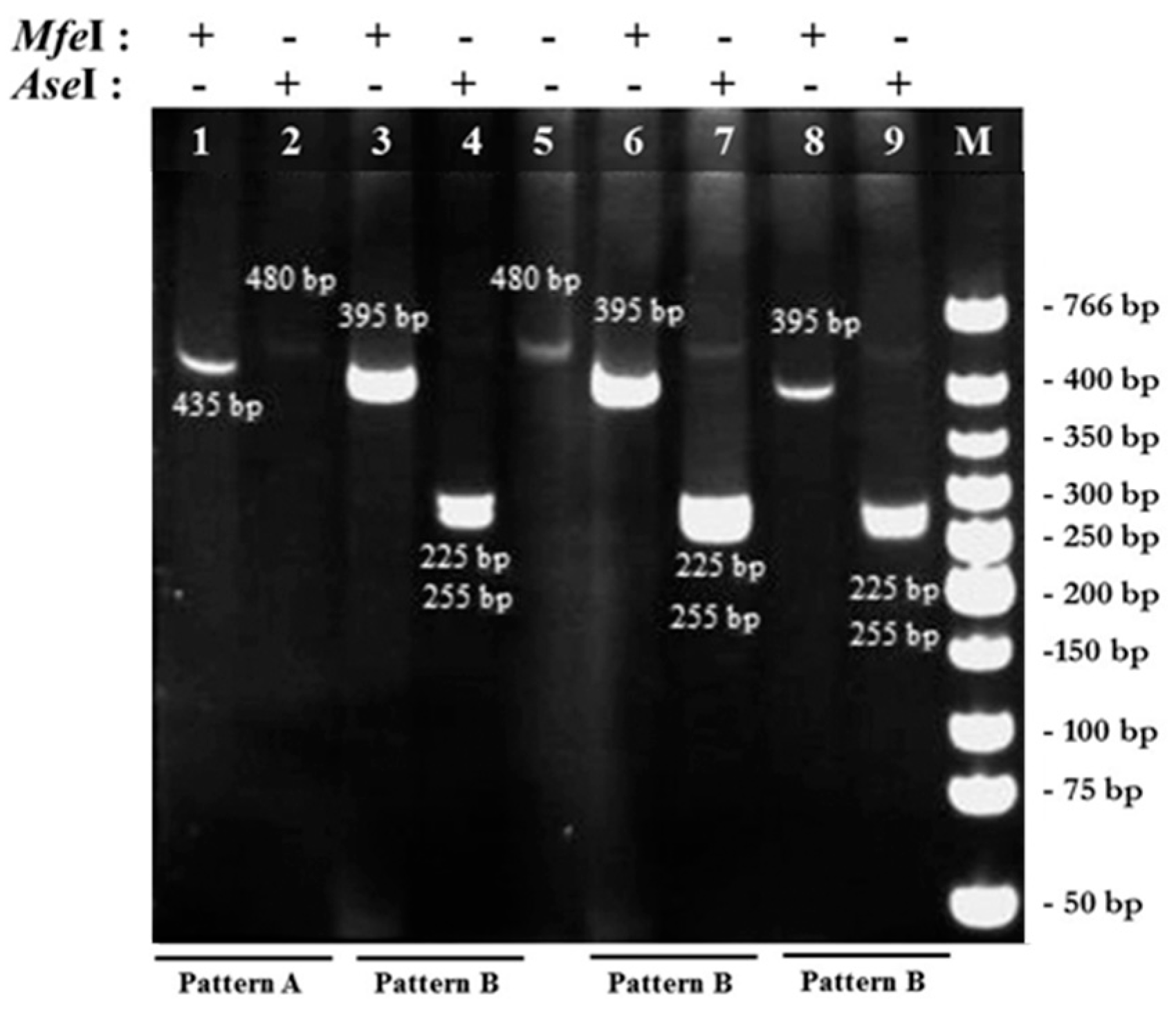
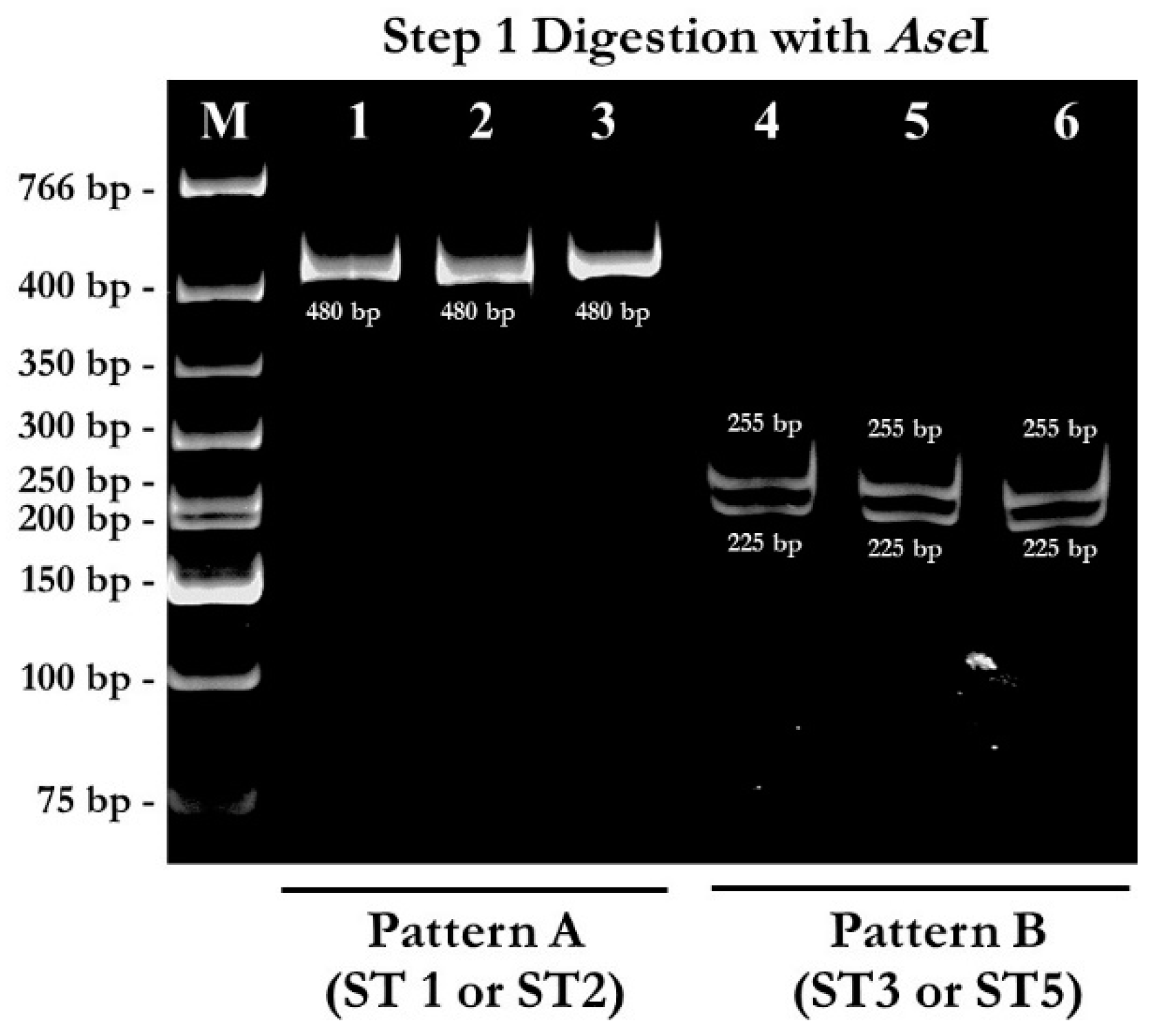
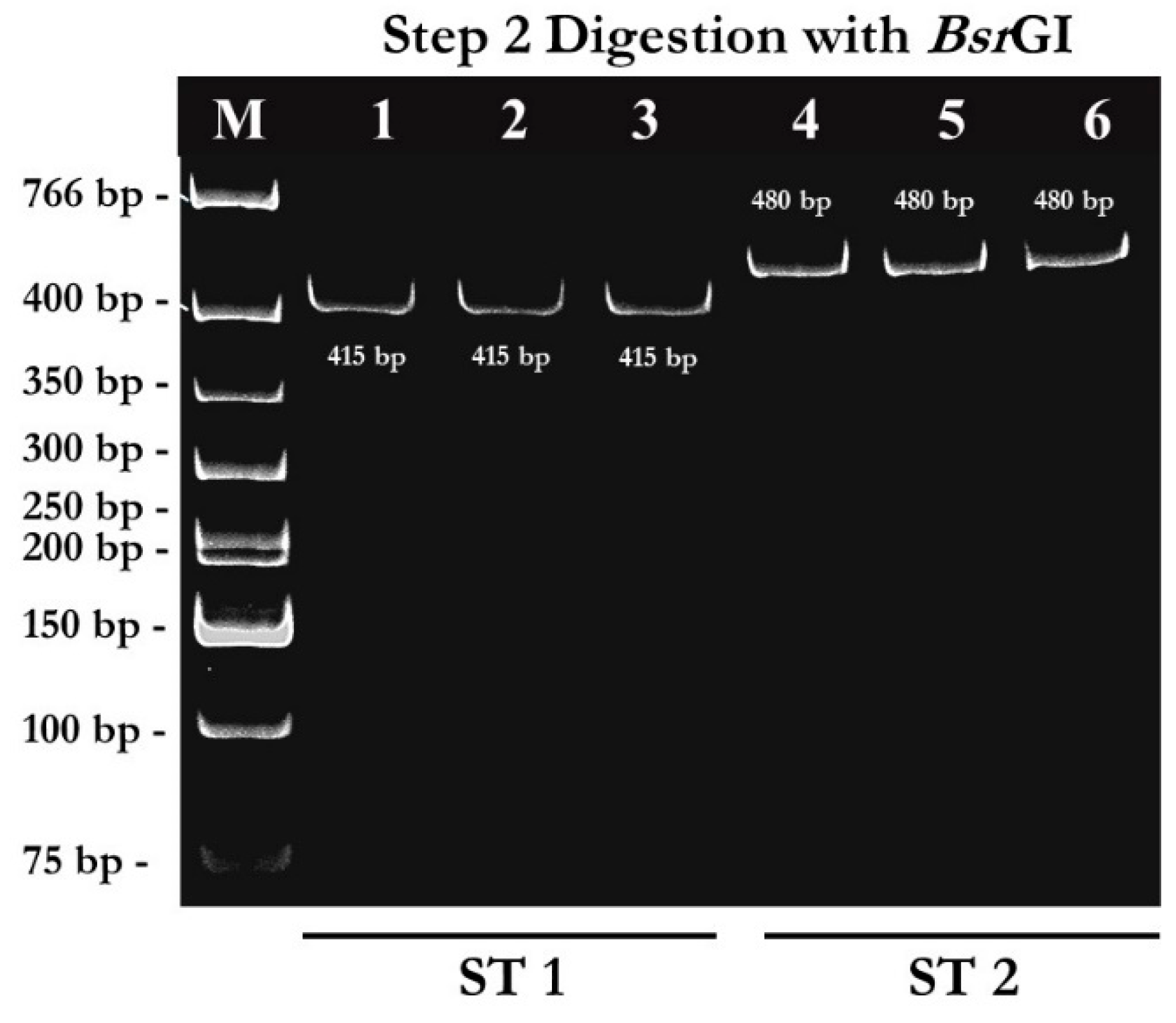
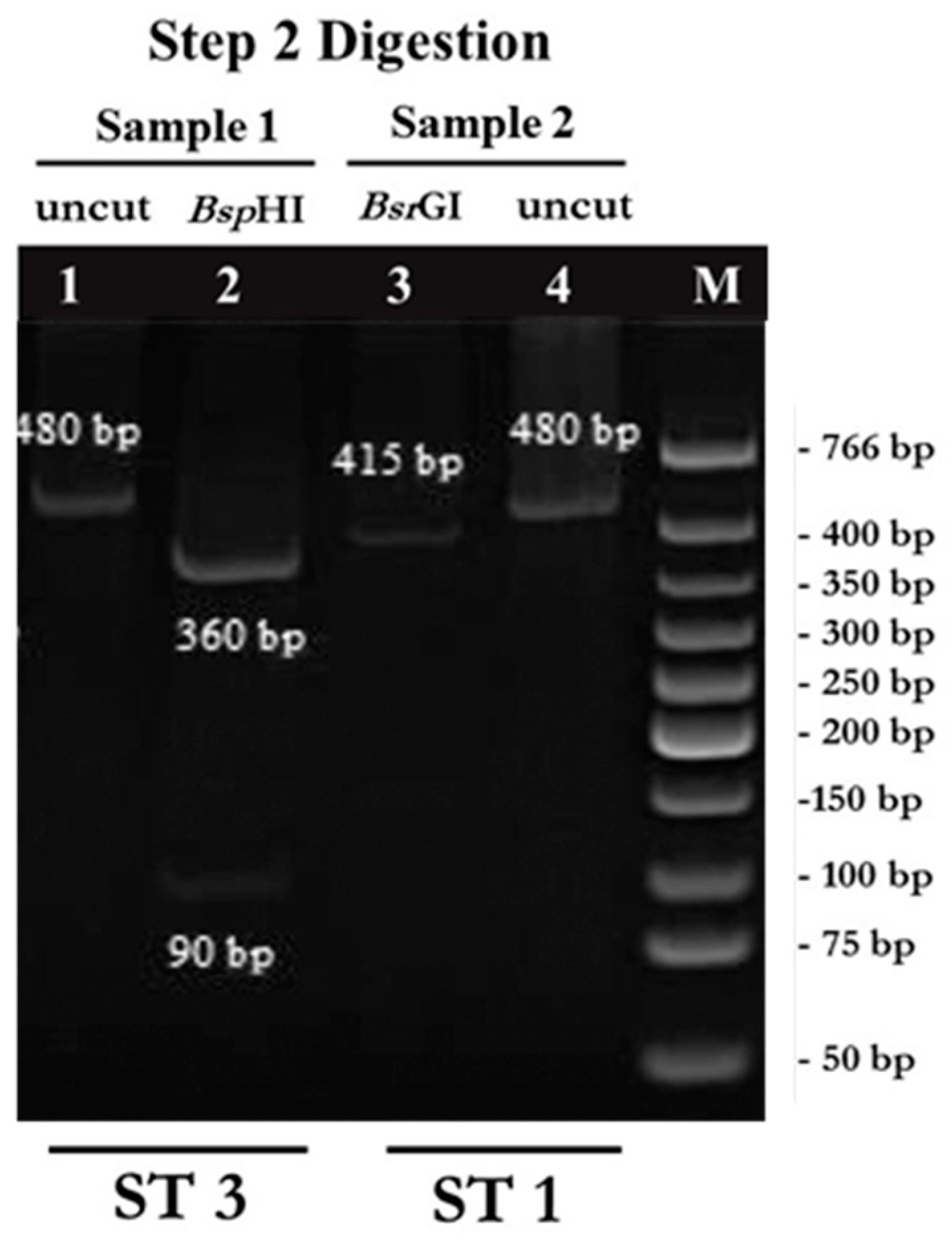
2.1. PCR-RFPL Analysis for Differentiation of Blastocystis Subtypes
2.2. Phylogenetic Analysis
2.3. Comparative Study of the Different Techniques for Blastocystis Detections
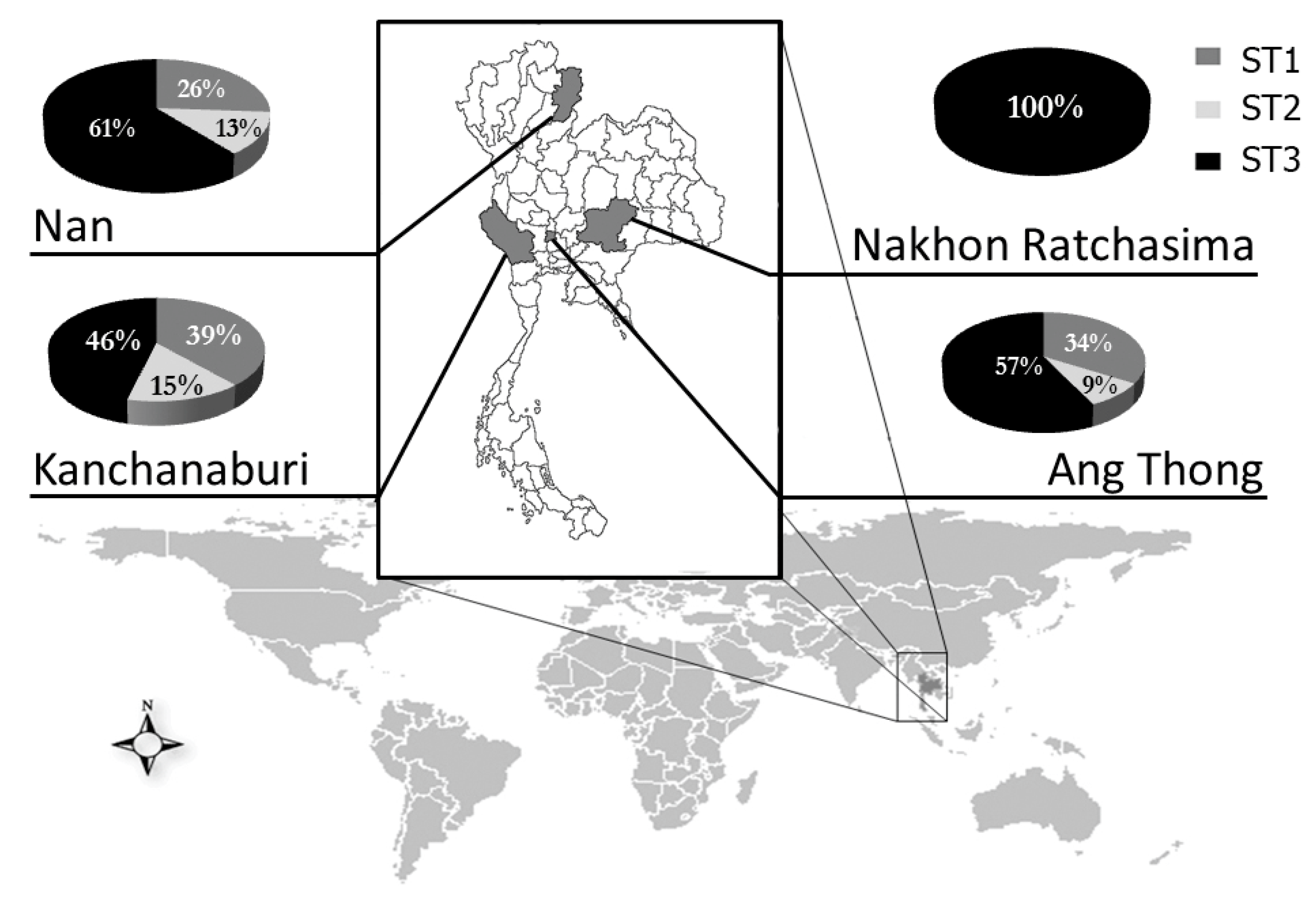
2.4. Distribution of Blastocystis Subtypes in Thai Students
3. Discussion
4. Materials and Methods
4.1. Population and Stool Samples
4.2. Stool Examination
4.3. DNA Extraction
4.4. Polymerase Chain Reaction (PCR)
4.5. Restriction Fragment Length Polymerase (RFLP)
4.6. DNA Sequencing and Phylogenetic Analysis
4.7. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garavelli, P.L.; Scaglione, L.; Bicocchi, R.; Libanore, M. Blastocystosis: A new disease in the acquired immunodeficiency syndrome? Int. J. STD AIDS 1990, 1, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Segui, R.; Klisiowicz, D.; Oishi, C.Y.; Toledo, R.; Esteban, J.G.; Munoz-Antoli, C. Intestinal symptoms and Blastocystis load in schoolchildren of Paranagua Bay, Parana, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e86. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Stark, D.; Harkness, J.; Ellis, J. Update on the pathogenic potential and treatment options for Blastocystis sp. Gut Pathog. 2014, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Nakamura, H.; Kinjo, N.; Hokama, A.; Kinjo, F.; Yamane, N.; Fujita, J. Prevalence of Blastocystis hominis and Strongyloides stercoralis infection in Okinawa, Japan. Parasitol. Res. 2007, 101, 1717–1719. [Google Scholar] [CrossRef] [PubMed]
- Amin, O.M. Seasonal prevalence of intestinal parasites in the United States during 2000. Am. J. Trop. Med. Hyg. 2002, 66, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Pegelow, K.; Gross, R.; Pietrzik, K.; Lukito, W.; Richards, A.L.; Fryauff, D.J. Parasitological and nutritional situation of school children in the Sukaraja district, West Java, Indonesia. Southeast Asian J. Trop. Med. Public Health 1997, 28, 173–190. [Google Scholar] [PubMed]
- Rayan, H.Z.; Ismail, O.A.; El Gayar, E.K. Prevalence and clinical features of Dientamoeba fragilis infections in patients suspected to have intestinal parasitic infection. J. Egypt Soc. Parasitol. 2007, 37, 599–608. [Google Scholar]
- El Safadi, D.; Gaayeb, L.; Meloni, D.; Cian, A.; Poirier, P.; Wawrzyniak, I.; Delbac, F.; Dabboussi, F.; Delhaes, L.; Seck, M.; et al. Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect. Dis. 2014, 14, 164. [Google Scholar] [CrossRef]
- Nuchprayoon, S.; Sanprasert, V.; Kaewzaithim, S.; Saksirisampant, W. Screening for intestinal parasitic infections among Myanmar migrant workers in Thai food industry: A high-risk transmission. J. Immigr. Minor. Health 2009, 11, 115–121. [Google Scholar] [CrossRef]
- Leelayoova, S.; Siripattanapipong, S.; Naaglor, T.; Taamasri, P.; Mungthin, M. Prevalence of intestinal parasitic infections in military personnel and military dogs, Thailand. J. Med. Assoc. Thail. 2009, 92 (Suppl. 1), S53–S59. [Google Scholar]
- Popruk, S.; Udonsom, R.; Koompapong, K.; Mahittikorn, A.; Kusolsuk, T.; Ruangsittichai, J.; Palasuwan, A. Subtype distribution of Blastocystis in Thai-Myanmar border, Thailand. Korean J. Parasitol. 2015, 53, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Yowang, A.; Tsaousis, A.D.; Chumphonsuk, T.; Thongsin, N.; Kullawong, N.; Popluechai, S.; Gentekaki, E. High diversity of Blastocystis subtypes isolated from asymptomatic adults living in Chiang Rai, Thailand. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 65, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Udonsom, R.; Prasertbun, R.; Mahittikorn, A.; Mori, H.; Changbunjong, T.; Komalamisra, C.; Pintong, A.R.; Sukthana, Y.; Popruk, S. Blastocystis infection and subtype distribution in humans, cattle, goats, and pigs in central and western Thailand. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 65, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Amin, O.M. Epidemiology of Blastocystis hominis in the United States. Res. J. Parasitol. 2006, 1, 1–10. [Google Scholar] [CrossRef]
- Armentia, A.; Mendez, J.; Gomez, A.; Sanchis, E.; Fernandez, A.; de la Fuente, R.; Sanchez, P. Urticaria by Blastocystis hominis. Successful treatment with paromomycin. Allergol. Immunopathol. (Madr.) 1993, 21, 149–151. [Google Scholar] [PubMed]
- Gupta, R.; Parsi, K. Chronic urticaria due to Blastocystis hominis. Australas. J. Dermatol. 2006, 47, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Katsarou-Katsari, A.; Vassalos, C.M.; Tzanetou, K.; Spanakos, G.; Papadopoulou, C.; Vakalis, N. Acute urticaria associated with amoeboid forms of Blastocystis sp. subtype 3. Acta Derm. Venereol. 2008, 88, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.; Suresh, K.G. Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients. Parasitol. Res. 2006, 98, 189–193. [Google Scholar] [CrossRef]
- Hussein, E.M.; Hussein, A.M.; Eida, M.M.; Atwa, M.M. Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol. Res. 2008, 102, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Olivo-Diaz, A.; Romero-Valdovinos, M.; Gudino-Ramirez, A.; Reyes-Gordillo, J.; Jimenez-Gonzalez, D.E.; Ramirez-Miranda, M.E.; Martinez-Flores, W.A.; Martinez-Hernandez, F.; Flisser, A.; Maravilla, P. Findings related to IL-8 and IL-10 gene polymorphisms in a Mexican patient population with irritable bowel syndrome infected with Blastocystis. Parasitol. Res. 2012, 111, 487–491. [Google Scholar] [CrossRef]
- Clark, C.G. Extensive genetic diversity in Blastocystis hominis. Mol. Biochem. Parasitol. 1997, 87, 79–83. [Google Scholar] [CrossRef]
- Alfellani, M.A.; Taner-Mulla, D.; Jacob, A.S.; Imeede, C.A.; Yoshikawa, H.; Stensvold, C.R.; Clark, C.G. Genetic diversity of blastocystis in livestock and zoo animals. Protist 2013, 164, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Alfellani, M.A.; Nørskov-Lauritsen, S.; Prip, K.; Victory, E.L.; Maddox, C.; Nielsen, H.V.; Clark, C.G. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int. J. Parasitol. 2009, 39, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Gomez-Munoz, M.T.; Solano-Aguilar, G.; Fayer, R. Development of a new PCR protocol to detect and subtype Blastocystis spp. from humans and animals. Parasitol. Res. 2011, 109, 205–212. [Google Scholar] [CrossRef]
- Fayer, R.; Elsasser, T.; Gould, R.; Solano, G.; Urban, J., Jr.; Santin, M. Blastocystis tropism in the pig intestine. Parasitol. Res. 2014, 113, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Parkar, U.; Traub, R.J.; Vitali, S.; Elliot, A.; Levecke, B.; Robertson, I.; Geurden, T.; Steele, J.; Drake, B.; Thompson, R.C.A. Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Vet. Parasitol. 2010, 169, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Noël, C.; Dufernez, F.; Gerbod, D.; Edgcomb, V.P.; Delgado-Viscogliosi, P.; Ho, L.C.; Singh, M.; Wintjens, R.; Sogin, M.L.; Capron, M.; et al. Molecular phylogenies of Blastocystis isolates from different hosts: Implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 2005, 43, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Sanpool, O.; Laymanivong, S.; Thanchomnang, T.; Rodpai, R.; Sadaow, L.; Phosuk, I.; Maleewong, W.; Intapan, P.M. Subtype identification of human Blastocystis spp. isolated from Lao People’s Democratic Republic. Acta Trop. 2017, 168, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Arendrup, M.C.; Nielsen, H.V.; Bada, A.; Thorsen, S. Symptomatic infection with Blastocystis sp. subtype 8 successfully treated with trimethoprim-sulfamethoxazole. Ann. Trop. Med. Parasitol. 2008, 102, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Stark, D.; Harkness, J.; Ellis, J. Subtype distribution of Blastocystis isolates identified in a Sydney population and pathogenic potential of Blastocystis. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 335–343. [Google Scholar] [CrossRef]
- Dominguez-Marquez, M.V.; Guna, R.; Munoz, C.; Gomez-Munoz, M.T.; Borras, R. High prevalence of subtype 4 among isolates of Blastocystis hominis from symptomatic patients of a health district of Valencia (Spain). Parasitol. Res. 2009, 105, 949–955. [Google Scholar] [CrossRef]
- Dogruman-Al, F.; Dagci, H.; Yoshikawa, H.; Kurt, O.; Demirel, M. A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitol. Res. 2008, 103, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Nagano, I.; Wu, Z.; Yap, E.H.; Singh, M.; Takahashi, Y. Genomic polymorphism among Blastocystis hominis strains and development of subtype-specific diagnostic primers. Mol. Cell. Probes 1998, 12, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R. Comparison of sequencing (barcode region) and sequence-tagged-site PCR for Blastocystis subtyping. J. Clin. Microbiol. 2013, 51, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Wu, Z.; Kimata, I.; Iseki, M.; Ali, I.K.M.D.; Hossain, M.B.; Zaman, V.; Haque, R.; Takahashi, Y. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol. Res. 2004, 92, 22–29. [Google Scholar]
- Stensvold, C.R.; Alfellani, M.; Clark, C.G. Levels of genetic diversity vary dramatically between Blastocystis subtypes. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2012, 12, 263–273. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Stensvold, C.R.; Cotter, P.D. Development and Application of a Blastocystis Subtype-Specific PCR Assay Reveals that Mixed-Subtype Infections Are Common in a Healthy Human Population. Appl. Environ. Microbiol. 2015, 81, 4071–4076. [Google Scholar] [CrossRef] [PubMed]
- Thathaisong, U.; Siripattanapipong, S.; Mungthin, M.; Pipatsatitpong, D.; Tan-ariya, P.; Naaglor, T.; Leelayoova, S. Identification of Blastocystis subtype 1 variants in the Home for Girls, Bangkok, Thailand. Am. J. Trop. Med. Hyg. 2013, 88, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Rivera, W.L. Phylogenetic analysis of Blastocystis isolates from animal and human hosts in the Philippines. Vet. Parasitol. 2008, 156, 178–182. [Google Scholar] [CrossRef]
- Parkar, U.; Traub, R.J.; Kumar, S.; Mungthin, M.; Vitali, S.; Leelayoova, S.; Morris, K.; Thompson, R.C. Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 2007, 134, 359–367. [Google Scholar] [CrossRef]
- Termmathurapoj, S.; Leelayoova, S.; Aimpun, P.; Thathaisong, U.; Nimmanon, T.; Taamasri, P.; Mungthin, M. The usefulness of short-term in vitro cultivation for the detection and molecular study of Blastocystis hominis in stool specimens. Parasitol. Res. 2004, 93, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Init, I.; Foead, A.L.; Fong, M.Y.; Yamazaki, H.; Rohela, M.; Yong, H.S.; Mak, J.W. Restriction enzyme digestion analysis of PCR-amplified DNA of Blastocystis hominis isolates. Southeast Asian J. Trop. Med. Public Health 2007, 38, 991–997. [Google Scholar] [PubMed]
- Hoevers, J.; Holman, P.; Logan, K.; Hommel, M.; Ashford, R.; Snowden, K. Restriction-fragment-length polymorphism analysis of small-subunit rRNA genes of Blastocystis hominis isolates from geographically diverse human hosts. Parasitol. Res. 2000, 86, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Wu, Z.; Yoshikawa, H. Molecular characterization of Blastocystis isolates from birds by PCR with diagnostic primers and restriction fragment length polymorphism analysis of the small subunit ribosomal RNA gene. Parasitol. Res. 2003, 89, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Wu, Z.; Yoshikawa, H. Zoonotic genotypes of Blastocystis hominis detected in cattle and pigs by PCR with diagnostic primers and restriction fragment length polymorphism analysis of the small subunit ribosomal RNA gene. Parasitol. Res. 2003, 90, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Jantermtor, S.; Pinlaor, P.; Sawadpanich, K.; Pinlaor, S.; Sangka, A.; Wilailuckana, C.; Wongsena, W.; Yoshikawa, H. Subtype identification of Blastocystis spp. isolated from patients in a major hospital in northeastern Thailand. Parasitol. Res. 2013, 112, 1781–1786. [Google Scholar] [CrossRef]
- Arisue, N.; Hashimoto, T.; Yoshikawa, H. Sequence heterogeneity of the small subunit ribosomal RNA genes among blastocystis isolates. Parasitology 2003, 126, 1–9. [Google Scholar] [CrossRef]
- Abe, N. Molecular and phylogenetic analysis of Blastocystis isolates from various hosts. Vet. Parasitol. 2004, 120, 235–242. [Google Scholar] [CrossRef]
- Ozyurt, M.; Kurt, O.; Molbak, K.; Nielsen, H.V.; Haznedaroglu, T.; Stensvold, C.R. Molecular epidemiology of Blastocystis infections in Turkey. Parasitol. Int. 2008, 57, 300–306. [Google Scholar] [CrossRef]
- Leipe, D.D.; Tong, S.M.; Goggin, C.L.; Slemenda, S.B.; Pieniazek, N.J.; Sogin, M.L. 16S-like rDNA sequences from Developayella elegans, Labyrinthuloides haliotidis, and Proteromonas lacertae confirm that the stramenopiles are a primarily heterotrophic group. Eur. J. Protistol. 1996, 32, 449–458. [Google Scholar] [CrossRef]
- Noel, C.; Peyronnet, C.; Gerbod, D.; Edgcomb, V.P.; Delgado-Viscogliosi, P.; Sogin, M.L.; Capron, M.; Viscogliosi, E.; Zenner, L. Phylogenetic analysis of Blastocystis isolates from different hosts based on the comparison of small-subunit rRNA gene sequences. Mol. Biochem. Parasitol. 2003, 126, 119–123. [Google Scholar] [CrossRef]
- Pipatsatitpong, D.; Leelayoova, S.; Mungthin, M.; Aunpad, R.; Naaglor, T.; Rangsin, R. Prevalence and Risk Factors for Blastocystis Infection among Children and Caregivers in a Child Care Center, Bangkok, Thailand. Am. J. Trop. Med. Hyg. 2015, 93, 310–315. [Google Scholar] [CrossRef]
- Saksirisampant, W.; Nuchprayoon, S.; Wiwanitkit, V.; Yenthakam, S.; Ampavasiri, A. Intestinal parasitic infestations among children in an orphanage in Pathum Thani province. J. Med. Assoc. Thail. 2003, 86 (Suppl. 2), S263–S270. [Google Scholar]
- Soriano, S.V.; Barbieri, L.M.; Pierangeli, N.B.; Giayetto, A.L.; Manacorda, A.M.; Castronovo, E.; Pezzani, B.C.; Minvielle, M.; Basualdo, J.A. Intestinal parasites and the environment: Frequency of intestinal parasites in children of Neuquen, Patagonia, Argentina. Rev. Latinoam. Microbiol. 2001, 43, 96–101. [Google Scholar] [PubMed]
- Leelayoova, S.; Siripattanapipong, S.; Thathaisong, U.; Naaglor, T.; Taamasri, P.; Piyaraj, P.; Mungthin, M. Drinking water: A possible source of blastocystis spp. subtype 1 infection in schoolchildren of a rural community in central Thailand. Am. J. Trop. Med. Hyg. 2008, 79, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Zhou, X.N.; Du, Z.W.; Wang, X.Z.; Wang, L.B.; Jiang, J.Y.; Yoshikawa, H.; Steinmann, P.; Utzinger, J.; Wu, Z.; et al. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol. Int. 2007, 56, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kitvatanachai, S.; Boonslip, S.; Watanasatitarpa, S. Intestinal parasitic infections in Srimum suburban area of Nakhon Ratchasima Province, Thailand. Trop. Biomed. 2008, 25, 237–242. [Google Scholar] [PubMed]
- Boonjaraspinyo, S.; Boonmars, T.; Kaewsamut, B.; Ekobol, N.; Laummaunwai, P.; Aukkanimart, R.; Wonkchalee, N.; Juasook, A.; Sriraj, P. A cross-sectional study on intestinal parasitic infections in rural communities, northeast Thailand. Korean J. Parasitol. 2013, 51, 727–734. [Google Scholar] [CrossRef]
- Roberts, T.; Barratt, J.; Harkness, J.; Ellis, J.; Stark, D. Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of blastocystis sp. in clinical stool samples. Am. J. Trop. Med. Hyg. 2011, 84, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Forsell, J.; Koskiniemi, S.; Hedberg, I.; Edebro, H.; Evengard, B.; Granlund, M. Evaluation of factors affecting real-time PCR performance for diagnosis of Entamoeba histolytica and Entamoeba dispar in clinical stool samples. J. Med. Microbiol. 2015, 64, 1053–1062. [Google Scholar] [CrossRef]
- Oikarinen, S.; Tauriainen, S.; Viskari, H.; Simell, O.; Knip, M.; Virtanen, S.; Hyoty, H. PCR inhibition in stool samples in relation to age of infants. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2009, 44, 211–214. [Google Scholar] [CrossRef]
- Yan, Y.; Su, S.; Lai, R.; Liao, H.; Ye, J.; Li, X.; Luo, X.; Chen, G. Genetic variability of Blastocystis hominis isolates in China. Parasitol. Res. 2006, 99, 597–601. [Google Scholar] [CrossRef]
- Abdulsalam, A.M.; Ithoi, I.; Al-Mekhlafi, H.M.; Al-Mekhlafi, A.M.; Ahmed, A.; Surin, J. Subtype distribution of Blastocystis isolates in Sebha, Libya. PLoS ONE 2013, 8, e84372. [Google Scholar] [CrossRef]
- Boondit, J.; Pipatsatitpong, D.; Mungthin, M.; Taamasri, P.; Tan-ariya, P.; Naaglor, T.; Leelayoova, S. Incidence and risk factors of blastocystis infection in orphans at the Babies’ Home, Nonthaburi Province, Thailand. J. Med. Assoc. Thail. 2014, 97 (Suppl. 2), S52–S59. [Google Scholar]
- Sanpool, O.; Laoraksawong, P.; Janwan, P.; Intapan, P.M.; Sawanyawisuth, K.; Thanchomnang, T.; Changtrakul, Y.; Maleewong, W. Genetic Subtypes of Blastocystis Isolated from Thai Hospitalized Patients in Northeastern Thailand. Southeast Asian J. Trop. Med. Public Health 2015, 46, 184–190. [Google Scholar]
- Yoshikawa, H.; Abe, N.; Wu, Z. PCR-based identification of zoonotic isolates of Blastocystis from mammals and birds. Microbiology 2004, 150, 1147–1151. [Google Scholar] [CrossRef]

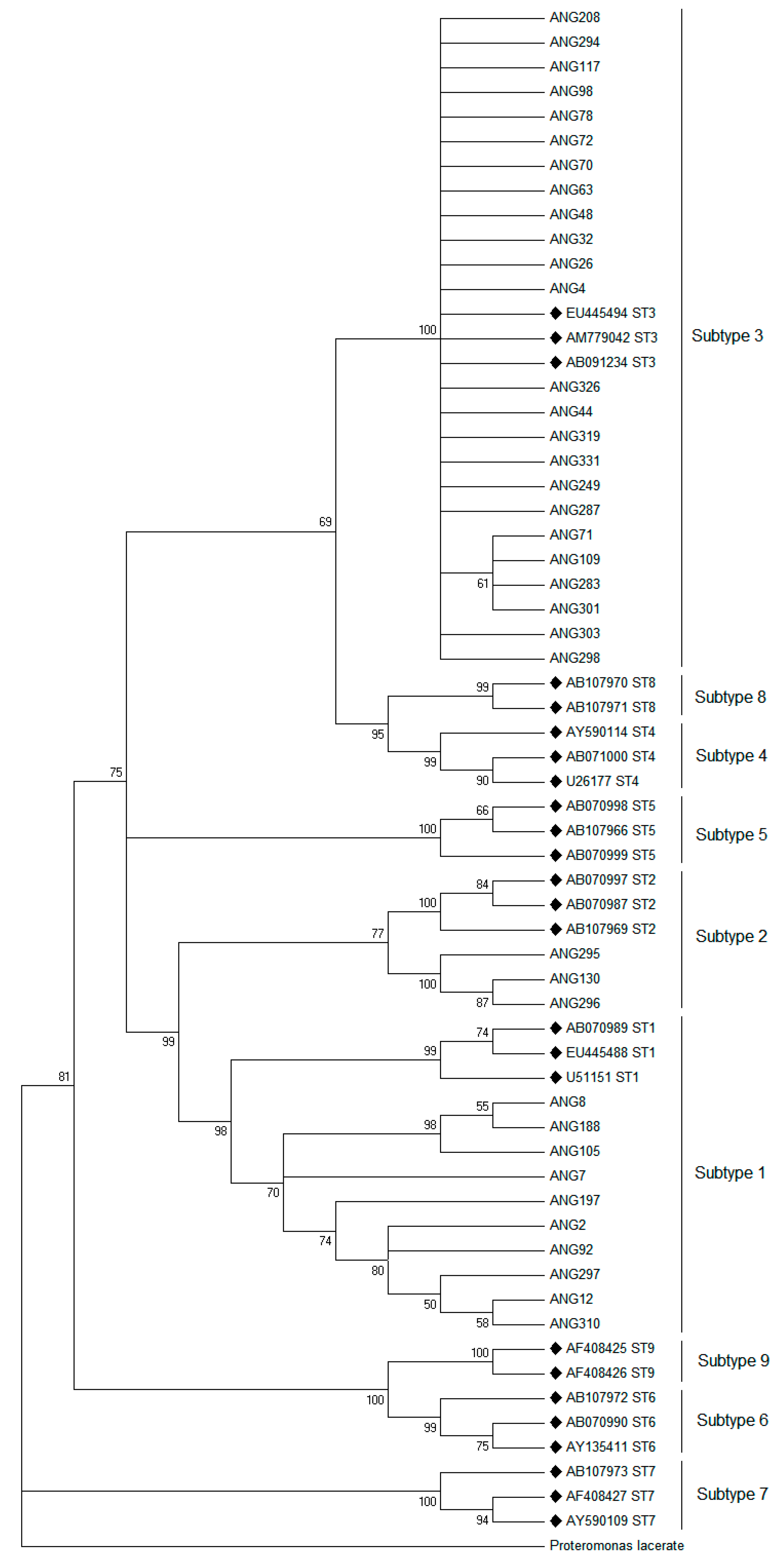
| Subtype | Accession Number | Country | Host | Reference |
|---|---|---|---|---|
| ST1 | AB070989 | Japan | Human | [47] |
| EU445488 | Philippines | Monkey | [39] | |
| U51151 | USA | Human | [47] | |
| ST2 | AB070997 | Japan | Monkey | [47] |
| AB107969 | Japan | Monkey | [48] | |
| AB070987 | Japan | Human | [47] | |
| ST3 | AB091234 | Japan | Quail | [47] |
| AM779042 | Turkey | Human | [49] | |
| EU445494 | Philippines | Human | [39] | |
| ST4 | AB071000 | Japan | Rat | [47] |
| AY590114 | Singapore | Rat | [27] | |
| U26177 | USA | Guinea pig | [50] | |
| ST5 | AB070998 | Japan | Pig | [47] |
| AB070999 | Japan | Pig | [47] | |
| AB107966 | Japan | Cattle | [48] | |
| ST6 | AB070990 | Japan | Human | [47] |
| AB107972 | Japan | Partridge | [48] | |
| AY135411 | France | Turkey | [51] | |
| ST7 | AB107973 | Japan | Goose | [48] |
| AF408427 | Singapore | Human | [47] | |
| AY590109 | Singapore | Human | [27] | |
| ST8 | AB107970 | Japan | Primate | [48] |
| AB107971 | Japan | Bird | [48] | |
| ST9 | AF408425 | Japan | Human | [35] |
| AF408426 | Japan | Human | [35] |
| Age | No. | Infected Patients |
|---|---|---|
| 1–6 | ||
| Male | 78 (52%) | 25 (56.8%) |
| Female | 72 (48%) | 19 (43.2) |
| Total | 150 (100%) | 44 (100%) |
| 7–12 | ||
| Male | 96 (46.25%) | 153 (47.5%) |
| Female | 344 (53.75%) | 169 (52.5%) |
| Total | 640 (100%) | 322 (100%) |
| 13–23 | ||
| Male | 111 (51.4%) | 18 (44%) |
| Female | 105 (48.6%) | 23 (56%) |
| Total | 216 (100%) | 41 (100%) |
| Unknown | 19 | 9 |
| Total | 1025 | 416 (40.5%) |
| Province | N | No. of Positive | Diagnostic Method | Blastocystis Subtype | |||||
|---|---|---|---|---|---|---|---|---|---|
| DS | FECT | LES | PCR | 1 | 2 | 3 | |||
| Ang Thong | |||||||||
| Male | 179 | 43 | 4 | 12 | ND | 33 | 12 | 4 | 18 |
| Female | 151 | 42 | 5 | 7 | ND | 35 | 11 | 2 | 21 |
| Total | 330 | 85 | 9 | 19 | ND | 68 | 23 | 6 | 39 |
| Nakhon Ratchasima | |||||||||
| Male | 91 | 3 | 1 | 0 | ND | 2 | 0 | 0 | 2 |
| Female | 87 | 2 | 0 | 0 | ND | 2 | 0 | 0 | 2 |
| Unknown | 10 | 1 | 0 | 0 | ND | 1 | 0 | 0 | 1 |
| Total | 188 | 6 | 1 | 0 | ND | 5 | 0 | 0 | 5 |
| Nan | |||||||||
| Male | 115 | 50 | 21 | 17 | 39 | 7 | 3 | 2 | 2 |
| Female | 116 | 54 | 12 | 14 | 40 | 15 | 3 | 1 | 11 |
| Unknown | 9 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Total | 240 | 105 | 33 | 32 | 79 | 23 | 6 | 3 | 14 |
| Kanchanaburi | |||||||||
| Male | 122 | 100 | 34 | 31 | 89 | 6 | 2 | 1 | 3 |
| Female | 138 | 113 | 27 | 37 | 103 | 7 | 3 | 1 | 3 |
| Unknown | 7 | 7 | 1 | 3 | 6 | 0 | 0 | 0 | 0 |
| Total | 267 | 220 | 62 | 71 | 198 | 13 | 5 | 2 | 6 |
| Total | 1025 | 416 | 105 | 122 | 277 | 109 | 34 | 11 | 64 |
| (40.6%) | (25.2%) | (29.3%) | (66.6%) * | (26.2%) | (31.2%) | (10.1%) | (58.7%) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srichaipon, N.; Nuchprayoon, S.; Charuchaibovorn, S.; Sukkapan, P.; Sanprasert, V. A Simple Genotyping Method for Rapid Differentiation of Blastocystis Subtypes and Subtype Distribution of Blastocystis spp. in Thailand. Pathogens 2019, 8, 38. https://doi.org/10.3390/pathogens8010038
Srichaipon N, Nuchprayoon S, Charuchaibovorn S, Sukkapan P, Sanprasert V. A Simple Genotyping Method for Rapid Differentiation of Blastocystis Subtypes and Subtype Distribution of Blastocystis spp. in Thailand. Pathogens. 2019; 8(1):38. https://doi.org/10.3390/pathogens8010038
Chicago/Turabian StyleSrichaipon, Nittaya, Surang Nuchprayoon, Sarit Charuchaibovorn, Pattadon Sukkapan, and Vivornpun Sanprasert. 2019. "A Simple Genotyping Method for Rapid Differentiation of Blastocystis Subtypes and Subtype Distribution of Blastocystis spp. in Thailand" Pathogens 8, no. 1: 38. https://doi.org/10.3390/pathogens8010038
APA StyleSrichaipon, N., Nuchprayoon, S., Charuchaibovorn, S., Sukkapan, P., & Sanprasert, V. (2019). A Simple Genotyping Method for Rapid Differentiation of Blastocystis Subtypes and Subtype Distribution of Blastocystis spp. in Thailand. Pathogens, 8(1), 38. https://doi.org/10.3390/pathogens8010038




