1. Introduction
Legionellae are opportunistic pathogens that have been identified as a major public health concern with incidences of Legionellosis continuing to rise [
1]. The two clinical illnesses caused by
Legionella spp. are Legionnaires’ disease, a potentially fatal pneumonia; and Pontiac fever, a milder form that mimics the symptoms of influenza [
2]. The
Legionella species reside ubiquitously in built water systems favoring temperatures between 20 and 45 °C. Transmission occurs through inhalation or aspiration of aerosolized
Legionella spp. contaminated water particles [
3]. Cooling towers, warm water systems and spa systems are well-documented sources of exposure and outbreaks of disease [
4]. As a result, control and prevention measures have been established. However, little is documented on sporadic
Legionella cases where the cause of infection is often not identified [
5]. Household showers provide ideal environments for
Legionella proliferation and exposure, and have often been proposed as a source of sporadic, community-acquired legionellosis [
6].
Shower exposure as a potential route of transmission is a major concern for immunocompromised and elderly people that reside within the community as they are at the greatest risk of acquiring infection [
7]. This is particularly significant given our increasing aging population, with global estimates suggesting that by 2025 25% of the world’s population will be over 60 years old [
8].
The global demand for aged care has been rapidly increasing. In Australia, it is estimated that from 2010 to 2050 the Australian governments’ spending on aged care will double relative to national income [
9]. There is also an increase in the number of individuals wishing to remain in their own homes and part of the community for longer. From June 2017 to June 2018, there was a 28.6% increase in the number of home care packages provided by the Australian government to individuals wishing to remain living independently in their own homes [
10]. Consequently, there is an increasing number of elderly individuals and vulnerable people residing within their own homes, with showers that are not regulated for the control of
Legionella.Exposure of people over 65 years to Legionella in their homes raises concern about possible public health implications. The aim of this study was to investigate the presence of Legionella spp. and Legionella pneumophila in domestic showers and identify factors that may increase the likelihood of contamination. Additionally, the general public’s awareness of Legionella control within the home was investigated. Increased awareness may help to reduce the risk of Legionella exposure in the domestic environment, protecting our vulnerable populations.
2. Results
Of the 68 shower samples enumerated using qPCR, 74.6% (n = 50) were positive for
Legionella spp. and 64.2% (n = 43) were positive for
L. pneumophila. The concentration of those positive samples ranged from 2.5 copies/mL to 110,000 copies/mL and the mean concentration was 7603 copies/mL and 4295 copies/mL for
Legionella spp. and
L. pneumophila, respectively. No statistically significant association (
p > 0.05) with temperature, type of hot water system, age of system, age of house or frequency of use and
L. pneumophila concentrations were observed. However, there was a statistically significant association (
p = 0.000) between
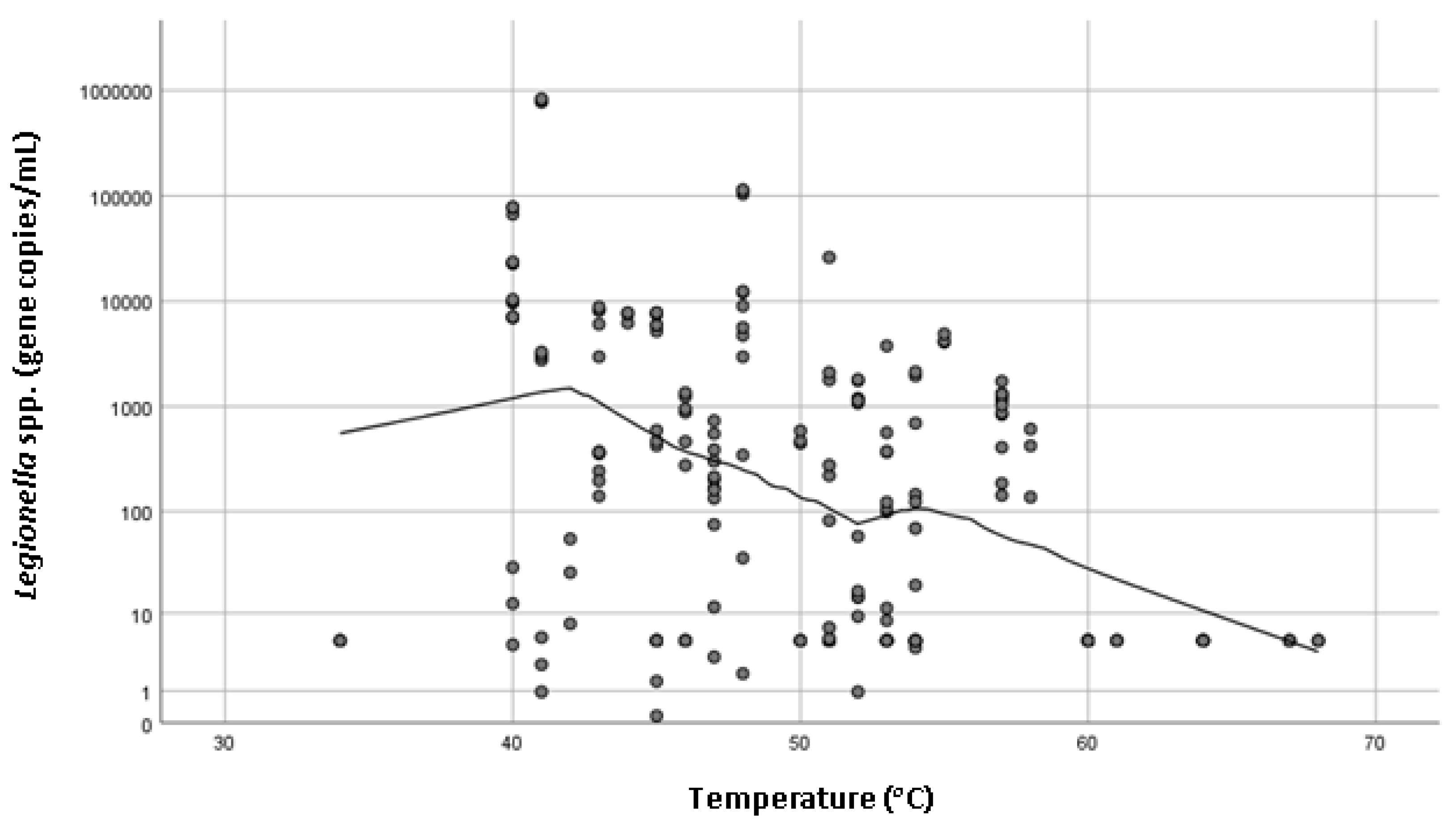
Legionella spp. concentration and the temperature of hot water measured at the outlet (
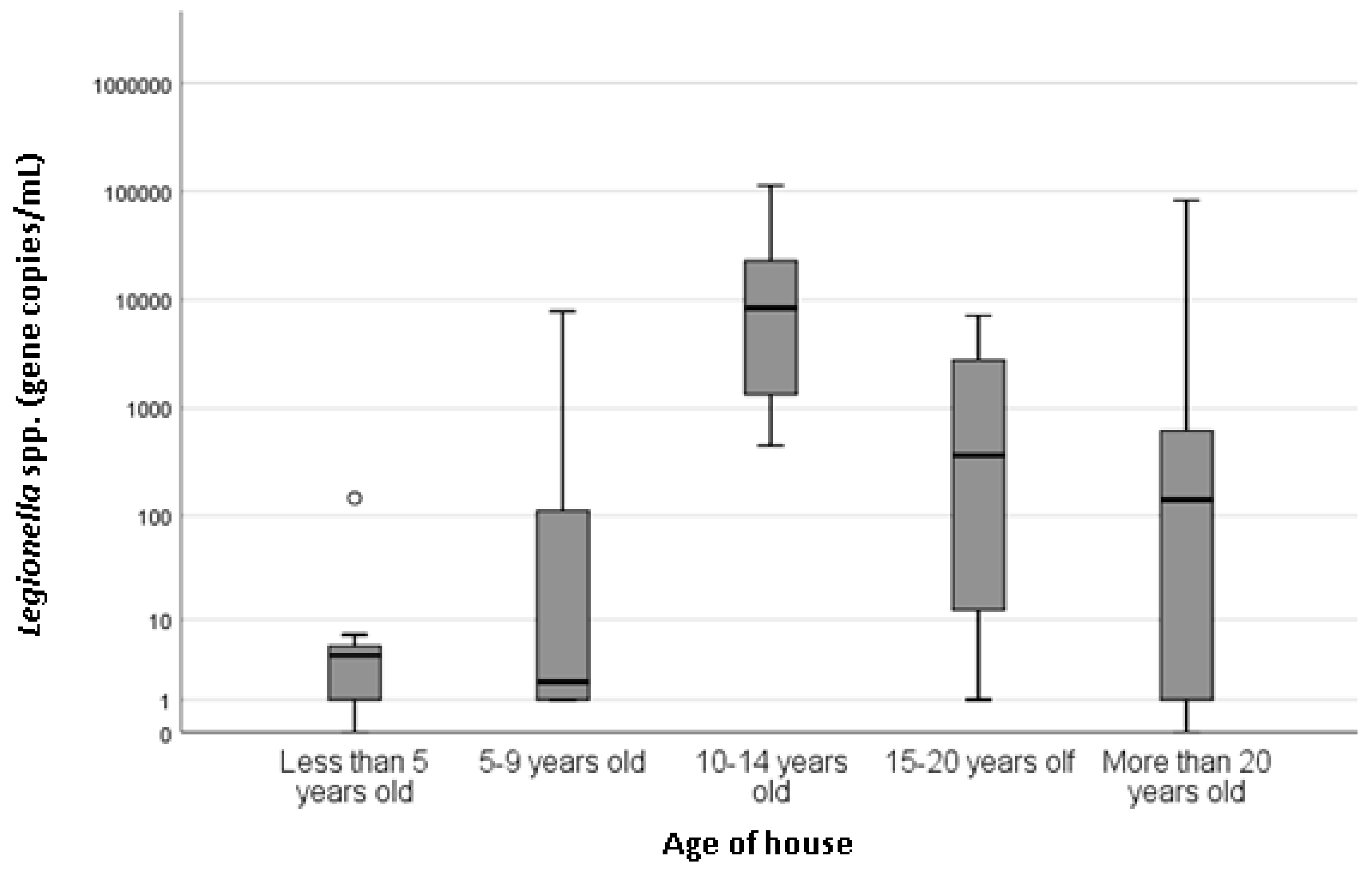
Figure 1). Recorded hot water temperatures ranged from 34 to 68 °C, and the mean was 50 °C. Property age ranged between less than 5 years old to more than 20 years old. There was a statistically significant association with the age of the house (
p = 0.037) and
Legionella spp. concentration (
Figure 2), with houses less than 5 years old associated with the lowest
Legionella spp. concentration. There was also a statistically significant (
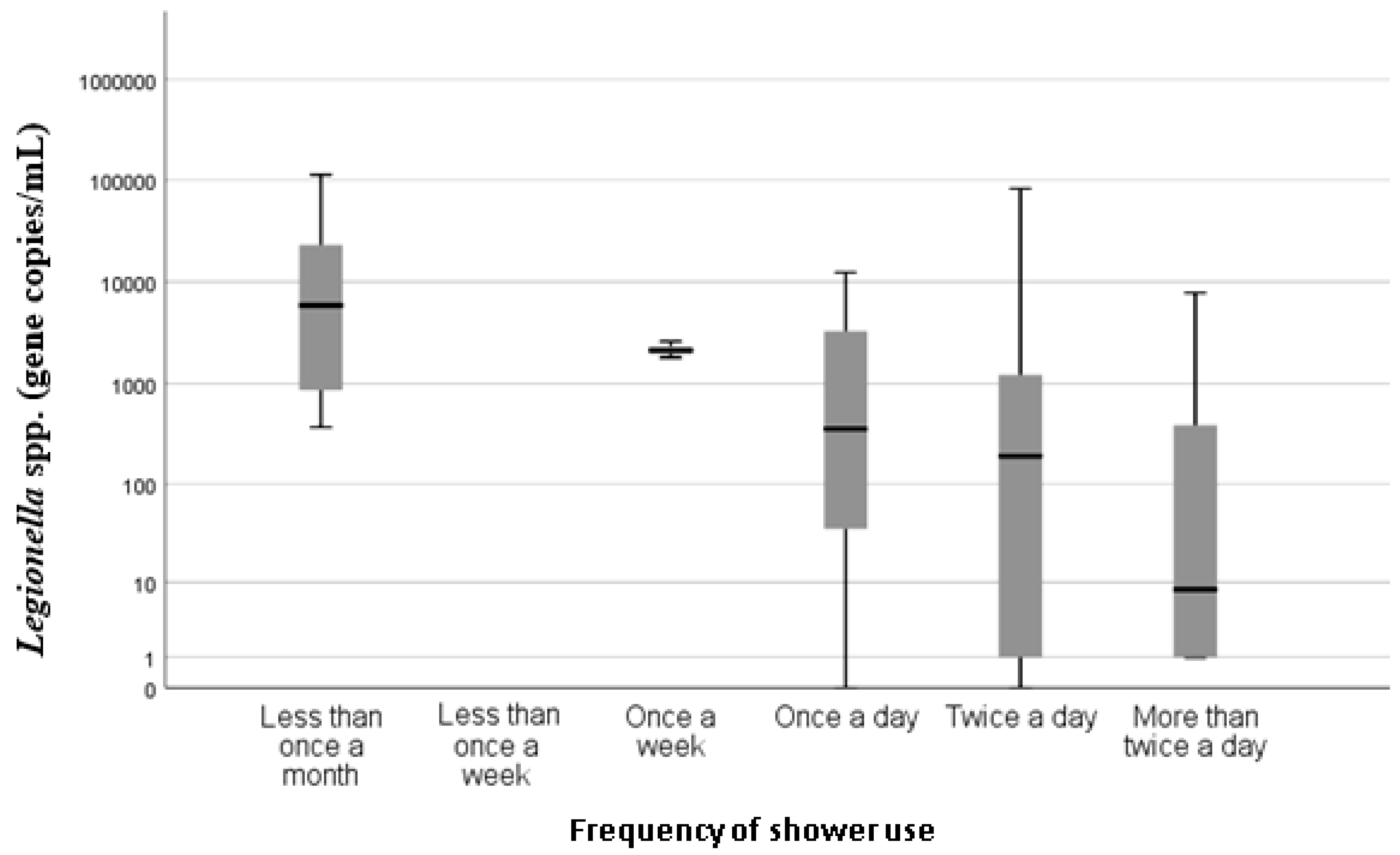
p = 0.000) association between the frequencies of shower usage and
Legionella spp. concentration, with showers used less than once a month having higher concentrations of
Legionella spp. compared with showers that were used once a week or more frequently (
Figure 3).
There was no statistically significant association between Legionella spp. and other household variables measured. The majority of hot water systems were gas (73.8%), followed by electric (15.4%) and solar (4.6%) (6.2% of participants did not know). Hot water storage was also examined, 58% were reported to be instantaneous, 28% stored in a tank and 14% of the respondents did not know. The number of showers in the 50 homes surveyed ranged from 1 to 4, with a mean of 1.72 showers per home. Age of hot water system was also investigated, 34% of hot water units were less than 5 years old, 12% were 5–9 years old, 14% were 10–14 years old, 4% were 15–20 years old, 10% were more than 20 years old and 38% did not know how old their hot water system was.
When survey participants were asked if they knew what the temperature of the hot water system was set at, 70% of participants did not know, indicating the lack of awareness of bacterial and Legionella control in the home. Those that did know gave answers as to why including “safety-burning,” “kids showering themselves,” “cost,” and personal preference to “hot showers”. No answers gave any indication that the temperature was set to reduce Legionella or microbial contamination.
3. Discussion
This study demonstrates that domestic household showers located in South Australia are a significant source of
Legionella spp. and
L. pneumophila. These findings support a previous study conducted in the UK by Collins et al. [
5] that detected
Legionella spp. and
L. pneumophila in household showers located in South England and a recent study that detected
Legionella along the South Australian municipal potable water distribution pipeline [
11]. This study used qPCR and found that 74.6% and 64.2% of showers positive for
Legionella spp. and
L. pneumophila, respectively. This is significantly higher than the findings of the UK study, which reported 31% of showers to be positive for
Legionella spp. using qPCR and only 6% positive using culture methods of detection [
5]. This may possibly be linked to climatic differences in South Australia [
4]. The most significant variables associated with increased
Legionella concentrations found in this study were temperature and frequency of shower use, followed by the age of the house.
The hot water delivery temperature of shower taps ranged between 34 and 68 °C, with higher
Legionella spp. concentrations statistically significantly associated with lower temperatures (
p = 0.000). It is advised by the World Health Organization that hot water systems should ideally maintain a temperature above 50 °C to reduce microbial colonization [
7] and previous studies have also demonstrated that buildings with hot water systems set under 60 °C were more likely to harbor Legionella [
12]. The mean maximum water temperature recorded in this study was below 50 °C which could explain the high incidence of
Legionella positive samples. These finding are supported by previous study conducted in Germany that identified temperature of hot water to be the most important determinant for
Legionella growth in domestic residences [
13]. Interestingly, the UK study did not find a statistically significant association between the presence of
Legionella spp. and water temperature [
5].
A statistically significant association between
Legionella spp. concentrations and frequency of shower use (
p = 0.000) was found. Higher concentrations of
Legionella spp. were found in showers that were used on average less than once per month than those used once per day or more. This indicates that increased shower use is potentially protective against
Legionella positivity. These findings are consistent with the current understanding that stagnant or low turnover of hot water are recognized as risk factors for
Legionella colonization and proliferation [
7], and also support the findings of the UK study [
5].
Property and hot water system age have often been found to influence the occurrence of
Legionella in showers [
5,
14]. This study found a statistically significant association between the age of house and
Legionella spp. concentration (
p = 0.037). Older houses were generally considered to harbor greater amounts of
Legionella due to older infrastructure and more time to enable biofilm formation [
5,
13]. In this study, the highest concentrations of
Legionella spp. were found in homes 10–14 years old and the lowest concentrations were seen in homes less than 5 years of old, which could be attributed to limited time for biofilm formation and colonization. This supports the findings of the UK study that found
Legionella spp. was associated with the age of the property (
p = 0.02), with newer houses associated with lower incidence of
Legionella spp., followed by a steady increase in
Legionella numbers until a decrease was observed for properties over 30 years old. In this South Australian study, houses over 30 years old were not identified; however, there was a large variation seen in houses over 20 years and a decrease in the mean concentration compared with houses 10–14 years old. It is difficult to determine possible factors related to higher positivity in relatively young properties (10–14 years old); however, differences in plumbing material (i.e., copper or PEX piping) have previously been suggested to influence
Legionella colonization [
5].
In this study, the type of hot water system was not statistically significantly associated with increased
Legionella concentrations. Instantaneous hot water systems are generally considered a lower risk for
Legionella contamination as they produce hot water instantaneously without storage [
5,
13,
15]. However, this study found 74.6% of showers were positive for
Legionella spp. and 64% of positive samples were from instantaneous systems, although this association was not statistically significant. The configuration of instantaneous water systems is such that temperatures above 50 °C are rarely achieved and residence time of water at elevated temperatures is minimal. This may negate the effects of temperature in controlling legionella and other bacteria entering the building’s water system. In turn, this may permit direct colonization of outlets.
The lack of public awareness regarding the risks associated with domestic hot water system identified in this study raises concerns. This is the first time in Australia that public perception regarding Legionella risks in the home has been quantified, and there is an apparent need for simple public health advice such as increasing hot water temperature or running showers every week to reduce the risk of a Legionella contamination. This is particularly significant for the elderly or immunocompromised who are most vulnerable to Legionella infection.
Whilst this study has demonstrated
Legionella contamination is common in South Australian household showers, these sources are not routinely sampled during sporadic Legionnaires’ disease investigations. As a consequence, clinical significance and evidence to indicate a public health burden is yet to be determined. It is also important to note that although
Legionella were detected in over two-thirds of the samples collected, the detection method does not differentiate between viable and non-viable bacteria. There is also no established equivalent value for the detection of
Legionella using qPCR, and we are unable to align our results to current legislation to determine what showers may pose a public health threat. This is also highlighted by a UK study investigating the presence of
Legionella contaminated aerosols from showers known to be positive for
Legionella, which failed to detect viable
Legionella using culture. However, the study did detect
Legionella DNA in the aerosols using qPCR [
5], and as such the negative culture results could be attributed to the presence of viable but non-culturable cells contained within the aerosols [
16]. There is a need for further research to explore the role of domestic shower aerosols as a potential route of transmission for Legionnaires’ disease.