Fungal Pathogens of Maize Gaining Free Passage Along the Silk Road
Abstract
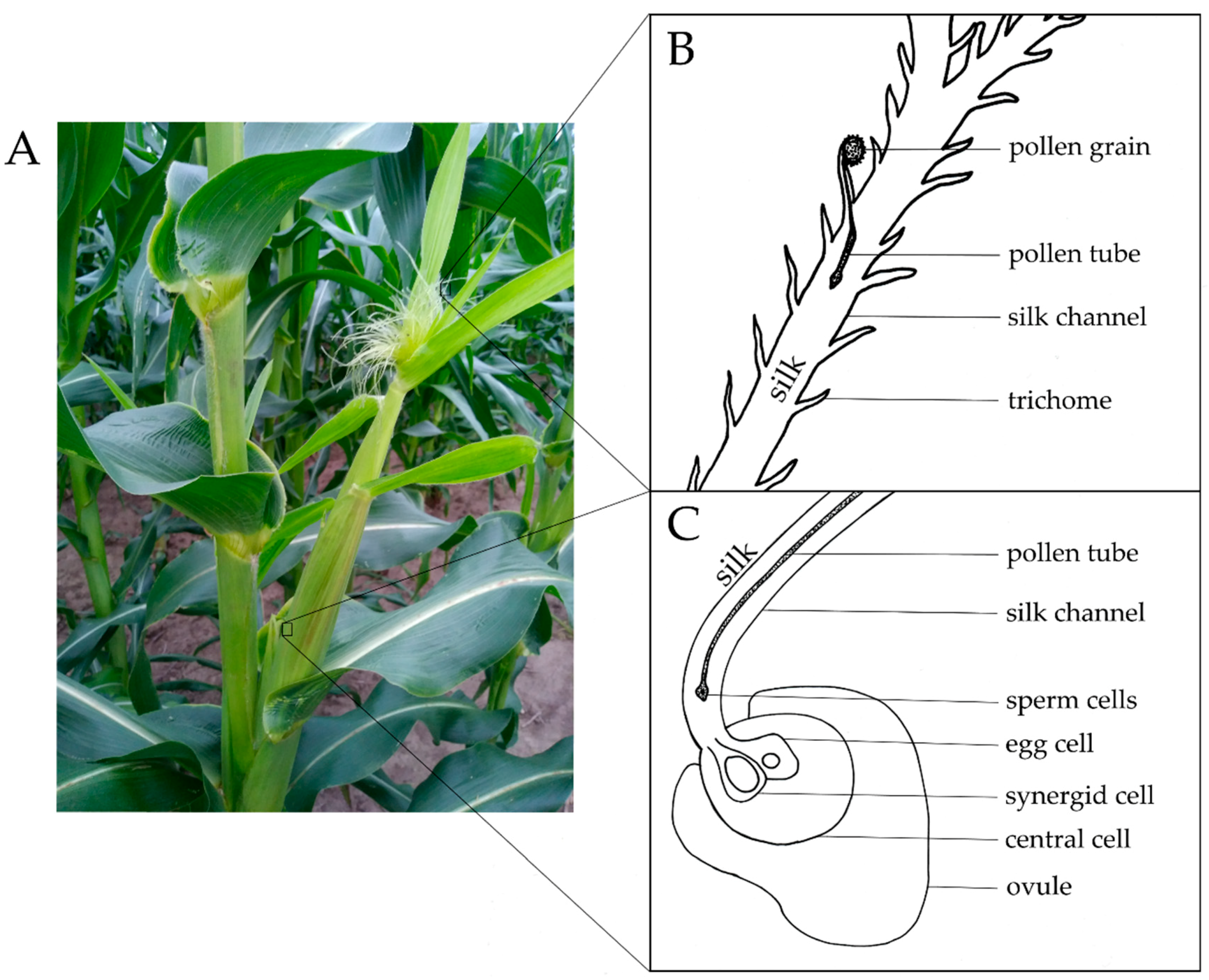
1. Introduction
2. Globally Important Pathogens That Infect Maize Silks and Subsequently Grain
2.1. Gibberella Ear Rot
2.2. Fusarium Ear Rot
2.3. Aspergillus Ear Rot
2.4. Corn Smut
2.5. Diplodia/Stenocarpella Ear Rot
3. Commonalities and Differences Amongst the Silk-Entering Pathogens
4. Strategies to Protect Silks from Ear Rot Pathogens—Current and Future
5. Concerns for the Future
6. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edgerton, M.D. Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiol. 2009, 149, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations FAOSTAT Data: Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 17 August 2018).
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef] [PubMed]
- NASS Online Database of the National Agricultural Statistics Service. Available online: https://www.nass.usda.gov/Statistics_by_Subject/index.php?sector=CROPS (accessed on 11 July 2018).
- USDA ERS Corn: Background. Available online: https://www.ers.usda.gov/topics/crops/corn-and-other-feedgrains/background/ (accessed on 11 July 2018).
- Alshannaq, A.; Yu, J.-H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 2003, 41, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.K.; Duncan, H.E.; Payne, G.A.; Leonard, K.J. Factors influencing infection by Aspergillus flavus in silk-inoculated corn. Plant Dis. 1980, 64, 859–863. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Mcgee, D.C.; Carlton, W.M. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Ecol. Epidemiol. 1997, 87, 209–217. [Google Scholar]
- Murillo-Williams, A.; Munkvold, G.P. Systemic infection by Fusarium verticillioides in maize plants grown under three temperature regimes. Plant Dis. 2008, 92, 1695–1700. [Google Scholar] [CrossRef]
- Kiesselbach, T. The Structure and Reproduction of Corn; Brown, D., Schaefer, S., Eds.; 50th Anniv.; Cold Spring Harbour Laboratory Press: Cold Spring Harbour, NY, USA, 1999. [Google Scholar]
- Sauter, M. A guided tour: Pollen tube orientation in flowering plants. Chin. Sci. Bull. 2009, 54, 2376–2382. [Google Scholar] [CrossRef]
- Doebley, J. The genetics of maize evolution. Annu. Rev. Genet. 2004, 38, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, P.; Westgate, M. Emergence, elongation, and senescence of maize silks. Crop Sci. 1993, 33, 271–275. [Google Scholar] [CrossRef]
- Turc, O.; Bouteillé, M.; Fuad-Hassan, A.; Welcker, C.; Tardieu, F. The growth of vegetative and reproductive structures (leaves and silks) respond similarly to hydraulic cues in maize. New Phytol. 2016, 212, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.A.; Wan Rosli, W.I. Nutritional compositions and antioxidative capacity of the silk obtained from immature and mature corn. J. King Saud Univ. Sci. 2014, 26, 119–127. [Google Scholar] [CrossRef]
- Miller, S.S.; Reid, L.M.; Harris, L.J. Colonization of maize silks by Fusarium graminearum, the causative organism of gibberella ear rot. Can. J. Bot. 2007, 85, 369–376. [Google Scholar] [CrossRef]
- Peters, B.M.; Palmer, G.E.; Nash, A.K.; Lilly, E.A.; Fidel, P.L.; Noverr, M.C. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect. Immun. 2014, 82, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.L.; Taylor, J.H.; Jie, L.; Sun, G.; William, M.; Kasha, K.J.; Reid, L.M.; Pauls, K.P. Molecular mapping of QTLs for resistance to Gibberella ear rot, in corn, caused by Fusarium graminearum. Genome 2005, 48, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Shier, W.T.; Plasencia, J.; Weaver, M.A.; Bellaloui, N.; Kotowicz, J.K.; Butler, A.M.; Accinelli, C.; de la Torre-Hernandez, M.E.; Zablotowicz, R.M. Mycotoxin contamination in corn smut (Ustilago maydis) galls in the field and in the commercial food products. Food Control 2017, 71, 57–63. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Dobson, A.D.W. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Vesonder, R.F.; Ellis, J.J.; Rohwedder, W.K. Elaboration of vomitoxin and zearalenone by Fusarium isolates and the biological activity of Fusarium-produced toxins. Appl. Environ. Microbiol. 1981, 42, 1132–1134. [Google Scholar] [PubMed]
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Mini-review Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat. Res./Rev. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Kalscheur, K.; Hippen, A.; Schingoethe, D. Mycotoxins in corn distillers grains: A concern in ruminants? SDSU Ext. Extra Arch. 2008, 1–3. Available online: https://openprairie.sdstate.edu/cgi/viewcontent.cgi?article=1134&context=extension_extra (accessed on 3 October 2018).
- Liu, K.-H.; Sun, X.-F.; Feng, Y.-Z.; Cheng, S.-F.; Li, B.; Li, Y.-P.; Shen, W.; Li, L. The impact of Zearalenone on the meiotic progression and primordial follicle assembly during early oogenesis. Toxicol. Appl. Pharmacol. 2017, 329, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Shier, W.T.; Shier, A.C.; Xie, W.; Mirocha, C.J. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 2001, 39, 1435–1438. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Voss, K.A. Toxicological highlight: A new perspective on deoxynivalenol and growth suppression. Toxicol. Sci. 2010, 113, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Windels, C.E. Economic and social impacts of Fusarium Head Blight: Changing farms and rural communities in the Northern Great Plains. Phytopathology 2000, 90, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Incremona, M.E.; del Pilar González, M.; Pioli, R.N.; Salinas, A.R. Infection of maize silks by a native Fusarium (Fusarium graminearum) isolate in Argentina. Chil. J. Agric. Anim. Sci. Agro-Cienc. 2014, 30, 203–211. [Google Scholar]
- Reid, L.M.; Bolton, A.T.; Hamilton, R.I.; Woldemariam, T.; Mather, D.E. Effect of silk age on resistance of maize to Fusarium graminearum. Can. J. Plant Pathol. 1992, 14, 293–298. [Google Scholar] [CrossRef]
- Reid, L.M.; Sinha, R.C. Maize maturity and the development of gibberella ear rot symptoms and deoxynivalenol after inoculation. Eur. J. Plant Pathol. 1998, 104, 147–154. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Nicol, R.W.; Reid, L.M. Evaluating commercial maize hybrids for resistance to gibberella ear rot. Eur. J. Plant Pathol. 1997, 103, 737–746. [Google Scholar] [CrossRef]
- Duncan, K.E.; Howard, R.J. Biology of maize kernel infection by Fusarium verticillioides. Mol. Plant-Microbe Interact. 2010, 23, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, E.; Ellner, F. Infection process and mycotoxin production in Fusarium culmorum-infected maize ears. Plant Breed. Seed Sci. 2011, 63, 59–65. [Google Scholar] [CrossRef]
- Lauren, D.R.; Di Menna, M.E. Fusaria and Fusarium mycotoxins in leaves and ears of maize plants 2. A time course study made in the Waikato region, New Zealand, in 1997. N. Z. J. Crop Hortic. Sci. 1999, 27, 215–223. [Google Scholar] [CrossRef]
- Oldenburg, E.; Höppner, F.; Ellner, F.; Weinert, J. Fusarium diseases of maize associated with mycotoxin contamination of agricultural products intended to be used for food and feed. Mycotoxin Res. 2017, 33, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, C.G.; Ojiambo, P.S.; Ekpo, E.J.A.; Menkir, A.; Bandyopadhyay, R. Evaluation of maize inbred lines for resistance to Fusarium Ear Rot and fumonisin accumulation in grain in tropical Africa. Plant Dis. 2007, 91, 279–286. [Google Scholar] [CrossRef]
- Marin, S.; Magan, N.; Ramos, A.J.; Sanchis, V. Fumonisin-producing strains of Fusarium: A review of their ecophysiology. J. Food Prot. 2004, 67, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ji, X.; Ni, X.; Fountain, J.C.; Li, H.; Abbas, H.K.; Lee, R.D.; Scully, B.T. Evaluation of maize inbred lines for resistance to pre-harvest aflatoxin and fumonisin contamination in the field. Crop J. 2017, 5, 259–264. [Google Scholar] [CrossRef]
- Kang’ethe, E.; Lang’a, K. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr. Health Sci. 2009, 9, 218–226. [Google Scholar] [PubMed]
- Leslie, J.F.; Zeller, K.A.; Lamprecht, S.C.; Rheeder, J.P.; Marasas, W.F.O. Toxicity, pathogenicity, and genetic differentiation of five species of Fusarium from sorghum and millet. Phytopathology 2005, 95, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Pan, J.J.; May, G. Endophytic Fusarium verticillioides reduces disease severity caused by Ustilago maydis on maize. FEMS Microbiol. Lett. 2009, 299, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Estrada, A.E.; Jonkers, W.; Corby Kistler, H.; May, G. Interactions between Fusarium verticillioides, Ustilago maydis, and Zea mays: An endophyte, a pathogen, and their shared plant host. Fungal Genet. Biol. 2012, 49, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Estrada, A.E.; Hegeman, A.; Kistler, H.C.; May, G. In vitro interactions between Fusarium verticillioides and Ustilago maydis through real-time PCR and metabolic profiling. Fungal Genet. Biol. 2011, 48, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, Á.; Lemmens, M.; Reid, L.M. Breeding for resistance to ear rots caused by Fusarium spp. in maize—A review. Plant Breed. 2012, 131, 1–19. [Google Scholar] [CrossRef]
- Maiorano, A.; Reyneri, A.; Sacco, D.; Magni, A.; Ramponi, C. A dynamic risk assessment model (FUMAgrain) of fumonisin synthesis by Fusarium verticillioides in maize grain in Italy. Crop Prot. 2009, 28, 243–256. [Google Scholar] [CrossRef]
- Sancho, A.M.; Moschini, R.C.; Filippini, S.; Rojas, D.; Ricca, A. Weather-based logistic models to estimate total fumonisin levels in maize kernels at export terminals in Argentina. Trop. Plant Pathol. 2018, 43, 99–108. [Google Scholar] [CrossRef]
- Lanubile, A.; Maschietto, V.; Borrelli, V.M.; Stagnati, L.; Logrieco, A.F.; Marocco, A. Molecular basis of resistance to Fusarium Ear Rot in maize. Front. Plant Sci. 2017, 8, 1774. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium verticillioides: Managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Grace, D.; Mahuku, G.; Hoffmann, V.; Atherstone, C.; Upadhyaya, H.D.; Bandyopadhyay, R. International agricultural research to reduce food risks: Case studies on aflatoxins. Food Secur. 2015, 7, 569–582. [Google Scholar] [CrossRef]
- Marsh, S.F.; Payne, G.A. Preharvest infection of corn silks and kernels by Aspergillus flavus. Phytopathology 1984, 74, 1284–1289. [Google Scholar] [CrossRef]
- Peethambaran, B.; Hawkins, L.; Windham, G.L.; Williams, W.P.; Luthe, D.S. Anti-fungal activity of maize silk proteins and role of chitinases in Aspergillus flavus resistance. Toxin Rev. 2010, 29, 27–39. [Google Scholar] [CrossRef]
- Balconi, C.; Motto, M.; Mazzinelli, G.; Berardo, N. Ear secondary traits related to aflatoxin accumulation in commercial maize hybrids under artificial field inoculation. World Mycotoxin J. 2010, 3, 239–250. [Google Scholar] [CrossRef]
- Magbanua, Z.V.; Williams, W.P.; Luthe, D.S. The maize rachis affects Aspergillus flavus spread during ear development. Maydica 2013, 58, 182–188. [Google Scholar]
- Chavan, S.; Smith, S.M. A rapid and efficient method for assessing pathogenicity of Ustilago maydis on maize and teosinte lines. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- du Toit, L.J.; Pataky, J.K. Variation associated with silk channel inoculation for Common Smut of sweet corn. Plant Dis. 1999, 83, 727–732. [Google Scholar] [CrossRef]
- du Toit, L.J.; Pataky, J.K. Effects of silk maturity and pollination on infection of maize ears by Ustilago maydis. Plant Dis. 1999, 83, 621–626. [Google Scholar] [CrossRef]
- Pataky, J.K.; Chandler, M.A. Production of huitlacoche, Ustilago maydis: Timing inoculation and controlling pollination. Mycologia 2003, 95, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Tracy, W.F.; Vargas, C.; Zepeda, L.; Pataky, J.K.; Chandler, M.A. Production and marketing of Huitlacoche. In Issues in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2007; pp. 233–236. [Google Scholar]
- Abbas, H.K.; Zablotowicz, R.M.; Shier, W.T.; Johnson, B.J.; Phillips, N.A.; Weaver, M.A.; Abel, C.A.; Bruns, H.A. Aflatoxin and fumonisin in corn (Zea mays) infected by Common Smut Ustilago maydis. Plant Dis. 2015, 99, 1236–1240. [Google Scholar] [CrossRef]
- Valdez-Morales, M.; Barry, K.; Fahey, G.C., Jr.; Domínguez, J.; de Mejia, E.G.; Valverde, M.E.; Paredes-López, O. Effect of maize genotype, developmental stage, and cooking process on the nutraceutical potential of huitlacoche (Ustilago maydis). Food Chem. 2010, 119, 689–697. [Google Scholar] [CrossRef]
- Snetselaar, K.M.; Carfioli, M.A.; Cordisco, K.M. Pollination can protect maize ovaries from infection by Ustilago maydis, the corn smut fungus. Can. J. Bot. 2001, 79, 1390–1399. [Google Scholar] [CrossRef]
- Snetselaar, K.M.; Mims, C.W. Infection of maize stigmas by Ustilago maydis: Light and electron microscopy. Phytopathology 1993, 83, 843–850. [Google Scholar] [CrossRef]
- Pataky, J.K.; Richter, P.M. Silk abscission in two sweet corn (Zea mays L.) hybrids that differ in susceptibility to Common Smut infection of ears. HortScience 2007, 42, 1409–1412. [Google Scholar]
- Dorrance, A.E.; Hinkelmann, K.H.; Warren, H.L. Diallel analysis of Diplodia Ear Rot resistance in maize. Plant Dis. 1998, 82, 699–703. [Google Scholar] [CrossRef]
- Masango, M.G.; Flett, B.C.; Ellis, C.E.; Botha, C.J. Stenocarpella maydis and its toxic metabolites: A South African perspective on diplodiosis. World Mycotoxin J. 2015, 8, 341–350. [Google Scholar] [CrossRef]
- Koehler, B. Natural mode of entrance of fungi into corn ears and some symptoms that indicate infection. J. Agric. Res. 1942, 64, 421–442. [Google Scholar]
- Bensch, M.J.; Staden, J.; Rijkenberg, F.H.J. Time and site of inoculation of maize for optimum infection of ears by Stenocarpella maydis. J. Phytopathol. 1992, 136, 265–269. [Google Scholar] [CrossRef]
- Bensch, M.J. Stenocarpella maydis (Berk.) sutton colonization of maize ears. J. Phytopathol. 1995, 143, 597–599. [Google Scholar] [CrossRef]
- Reid, L.M.; Zhu, X.; Parker, A.; Yan, W. Increased resistance to Ustilago zeae and Fusarium verticilliodes in maize inbred lines bred for Fusarium graminearum resistance. Euphytica 2009, 165, 567–578. [Google Scholar] [CrossRef]
- Ni, X.; Krakowsky, M.D.; Buntin, G.D.; Rector, B.G.; Guo, B.; Snook, M.E. Identification of multiple ear-colonizing insect and disease resistance in CIMMYT maize inbred lines with varying levels of silk maysin. J. Econ. Entomol. 2008, 101, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, B.R.; Snook, M.E. Effect of corn silk age on flavone content and development of corn earworm (Lepidoptera: Noctuidae) larvae. J. Econ. Entomol. 1995, 88, 1795–1800. [Google Scholar] [CrossRef]
- Widstrom, N.W.; Snook, M.E.; Wilson, D.M.; Cleveland, T.E.; McMillan, W.W. Silk maysin content and resistance of commercial corn [maize] hybrids to kernel contamination by aflatoxin. J. Sci. Food Agric. 1995, 67, 317–321. [Google Scholar] [CrossRef]
- Haslina, H.; Praseptiangga, D.; Bintoro, V.P.; Pujiasmanto, B. Chemical and phytochemical characteristics of local corn silk powder of three different varieties. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 1957. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Bot. 1996, 74, 1195–1202. [Google Scholar] [CrossRef]
- Williams, W.P.; Krakowsky, M.D.; Scully, B.T.; Brown, R.L.; Menkir, A.; Warburton, M.L.; Windham, G.L. Identifying and developing maize germplasm with resistance to accumulation of aflatoxins. World Mycotoxin J. 2015, 8, 193–209. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y.; Heslop-Harrison, J.; Reger, B.J. The pollen-stigma interaction in the grasses. 7. Pollen-tube guidance and the regulation of tube number in Zea mays L. Acta Bot. Neerl. 1985, 34, 193–211. [Google Scholar] [CrossRef]
- Borrás, L.; Vitantonio-Mazzini, L.N. Maize reproductive development and kernel set under limited plant growth environments. J. Exp. Bot. 2018, 69, 3235–3243. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, J.; Edmeades, G.O. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crop. Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Jurado, M.; Marín, P.; Magan, N.; González-Jaén, M.T. Relationship between solute and matric potential stress, temperature, growth, and FUM1 gene expression in two Fusarium verticillioides strains from Spain. Appl. Environ. Microbiol. 2008, 74, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Xu, W.; Blanco, M.H.; Wilson, J.P. Evaluation of corn germplasm lines for multiple ear-colonizing insect and disease resistance. J. Econ. Entomol. 2012, 105, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Waiss, A.C.J.; Chan, B.G.; Elliger, C.A.; Wiseman, B.R.; McMillian, W.W.; Widstrom, N.W.; Zuber, M.S.; Keaster, A.J. Maysin, a flavone glycoside from corn silks with antibiotic activity toward corn earworm. J. Econ. Entomol. 1979, 72, 256–258. [Google Scholar] [CrossRef]
- Reid, L.M.; Mather, D.E.; Arnason, J.T.; Hamilton, R.I.; Bolton, A.T. Changes in phenolic constituents of maize silk infected with Fusarium graminearum. Can. J. Bot. 1992, 70, 1697–1702. [Google Scholar] [CrossRef]
- El-Ghorab, A.; El-Massry, K.F.; Shibamoto, T. Chemical composition of the volatile extract and antioxidant activities of the volatile and nonvolatile extracts of Egyptian corn silk (Zea mays L.). J. Agric. Food Chem. 2007, 55, 9124–9127. [Google Scholar] [CrossRef] [PubMed]
- Zeringue, H.J. Identification and effects of maize silk volatiles on cultures of Aspergillus flavus. J. Agric. Food Chem. 2000, 48, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Sarepoua, E.; Tangwongchai, R.; Suriharn, B.; Lertrat, K. Relationships between phytochemicals and antioxidant activity in corn silk. Int. Food Res. J. 2013, 20, 2073–2079. [Google Scholar]
- Neucere, J.N. Inhibition of Aspergillus flavus growth by silk extracts of resistant and susceptible corn. J. Agric. Food Chem. 1996, 44, 1982–1983. [Google Scholar] [CrossRef]
- Alessandra, L.; Luca, P.; Adriano, M. Differential gene expression in kernels and silks of maize lines with contrasting levels of ear rot resistance after Fusarium verticillioides infection. J. Plant Physiol. 2010, 167, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Campos-Bermudez, V.A.; Fauguel, C.M.; Tronconi, M.A.; Casati, P.; Presello, D.A.; Andreo, C.S. Transcriptional and metabolic changes associated to the infection by Fusarium verticillioides in maize inbreds with contrasting ear rot resistance. PLoS ONE 2013, 8, e61580. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.M.; Mather, D.E.; Bolton, A.T.; Hamilton, R.I. Evidence for a gene for silk resistance to Fusarium graminearum Schw. ear rot of maize. J. Hered. 1994, 85, 118–121. [Google Scholar] [CrossRef]
- Brefort, T.; Doehlemann, G.; Mendoza-Mendoza, A.; Reissmann, S.; Djamei, A.; Kahmann, R. Ustilago maydis as a pathogen. Annu. Rev. Phytopathol. 2009, 47, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.-B.; Wu, P.-T.; Sun, S.-K.; Li, X.-L.; Wang, Y.-B.; Gao, X.-R. Impact of land use change on hydrologic processes in a large plain irrigation district. Water Resour. Manag. 2018, 32, 3203–3217. [Google Scholar] [CrossRef]
- Ouyang, Y.; Feng, G.; Leininger, T.D.; Read, J.; Jenkins, J.N. Pond and irrigation model (PIM): A tool for simultaneously evaluating pond water availability and crop irrigation demand. Water Resour. Manag. 2018, 32, 2969–2983. [Google Scholar] [CrossRef]
- Mansfield, B.D.; Mumm, R.H. Survey of plant density tolerance in U.S. maize germplasm. Crop Sci. 2014, 54, 157. [Google Scholar] [CrossRef]
- Reid, A.; Gonzalez, V.; Sikkema, P.H.; Lee, E.A.; Lukens, L.; Swanton, C.J. Delaying weed control lengthens the anthesis-silking interval in maize. Weed Sci. 2014, 62, 326–337. [Google Scholar] [CrossRef]
- Šimić, B.; Ćosić, J.; Poštić, J.; Beraković, I.; Tucak, M. Higher rates of potassium fertilizer improve economic efficiency of sweet maize (Zea mays saccharata). Rom. Agric. Res. 2012, 29, 185–188. [Google Scholar]
- Shahzad, A.N.; Fatima, A.; Sarwar, N.; Bashir, S.; Rizwan, M.; Qayyum, M.F.; Qureshi, M.K.; Javaid, M.H.; Ahmad, S. Foliar application of potassium sulfate partially alleviates pre-anthesis drought-induced kernel abortion in maize. Int. J. Agric. Biol. 2017, 19, 495. [Google Scholar] [CrossRef]
- Kostandi, S.F.; Soliman, M.F. The role of calcium in mediating Smut Disease severity and salt tolerance in corn under chloride and sulphate salinity. J. Phytopathol. 1998, 146, 191–195. [Google Scholar] [CrossRef]
- Mól, R.; Filek, M.; Dumas, C.; Matthys-Rochon, E. Cytoplasmic calcium in silk trichomes after pollen grain reception and post-pollination changes of the electric potential in pistil tissues of maize. Plant Sci. 2004, 166, 1461–1469. [Google Scholar] [CrossRef]
- Andric, L.; Kovacevic, V.; Kadar, I.; Jambrovic, A.; Plavsic, H.; Simic, D. Genotypic effects on boron concentrations and response on boron fertilization in maize inbred lines. Genetika 2016, 48, 297–305. [Google Scholar] [CrossRef]
- Lordkaew, S.; Dell, B.; Jamjod, S.; Rerkasem, B. Boron deficiency in maize. Plant Soil 2011, 342, 207–220. [Google Scholar] [CrossRef]
- Habibi, G. Effect of foliar-applied silicon on photochemistry, antioxidant capacity and growth in maize plants subjected to chilling stress. Acta Agric. Slov. 2016, 107, 33. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Foliar application of silica nanoparticles on the phytochemical responses of maize (Zea mays L.) and its toxicological behavior. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2014, 44, 1128–1131. [Google Scholar] [CrossRef]
- Hou, Y.-P.; Qu, X.-P.; Mao, X.-W.; Kuang, J.; Duan, Y.-B.; Song, X.; Wang, J.-X.; Chen, C.-J.; Zhou, M.-G. Resistance mechanism of Fusarium fujikuroi to phenamacril in the field. Pest Manag. Sci. 2018, 74, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Falcão, V.C.A.; Ono, M.A.; de Ávila Miguel, T.; Vizoni, E.; Hirooka, E.Y.; Ono, E.Y.S. Fusarium verticillioides: Evaluation of fumonisin production and effect of fungicides on in vitro inhibition of mycelial growth. Mycopathologia 2011, 171, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Sivparsad, B.J.; Laing, M.D. Pre-harvest silk treatment with Trichoderma harzianum reduces aflatoxin contamination in sweetcorn. J. Plant Dis. Prot. 2016, 123, 285–293. [Google Scholar] [CrossRef]
- Mousa, W.K.; Shearer, C.R.; Limay-Rios, V.; Zhou, T.; Raizada, M.N. Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front. Plant Sci. 2015, 6, 805. [Google Scholar] [CrossRef] [PubMed]
- Czembor, E.; Stępień, Ł.; Waśkiewicz, A. Effect of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in Poland. PLoS ONE 2015, 10, e0133644. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D. Changing patterns of fungal toxins in crops: Challenges for analysts. J. AOAC Int. 2016, 99, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Mitchell, N.J. How climate change and regulations can affect the economics of mycotoxins. World Mycotoxin J. 2016, 9, 653–663. [Google Scholar] [CrossRef]
- Powlson, D.S.; Stirling, C.M.; Jat, M.L.; Gerard, B.G.; Palm, C.A.; Sanchez, P.A.; Cassman, K.G. Limited potential of no-till agriculture for climate change mitigation. Nat. Clim. Chang. 2014, 4, 678–683. [Google Scholar] [CrossRef]
- Mikel, M.A.; D’arcy, C.J.; Ford, R.E. Seed transmission of maize dwarf mosaic virus in sweet corn. Phytopathol. Z. 1984, 110, 185–191. [Google Scholar] [CrossRef]
| Disease Common Name | Organism | Major Mycotoxins | Silk Susceptible Period |
|---|---|---|---|
| Gibberella Ear Rot | Fusarium graminearum Schw. | Deoxynivalenol, Zearalenone | After pollination, beginning of senescence, begin browning |
| Fusarium Ear Rot | Fusarium verticillioides (Sacc.) Nirenberg, F. proliferatum (Matsush.) Nirenberg, F. subglutinans (Wollenw. & Reinking) | Fumonisin B1 | 4–6 days after pollination |
| Aspergillus Ear Rot | Aspergillus flavus Link, Aspergillus parasiticus Speare | Aflatoxins | After pollination, beginning of senescence, yellow-brown |
| Corn Smut/Common Smut/Boil Smut | Ustilago maydis (DC) Corda, also known as Ustilago zeae, Ustilago zeae mays | No toxins 1 | Maximum occurrence at six days after mid-silking, incidence decreasing thereafter |
| Diplodia Ear Rot/Stenocarpella Ear Rot | Stenocarpella maydis (Berk.) Sutton, previously known as Diplodia maydis | Diplodiatoxin | More research needed 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, M.E.H.; Raizada, M.N. Fungal Pathogens of Maize Gaining Free Passage Along the Silk Road. Pathogens 2018, 7, 81. https://doi.org/10.3390/pathogens7040081
Thompson MEH, Raizada MN. Fungal Pathogens of Maize Gaining Free Passage Along the Silk Road. Pathogens. 2018; 7(4):81. https://doi.org/10.3390/pathogens7040081
Chicago/Turabian StyleThompson, Michelle E. H., and Manish N. Raizada. 2018. "Fungal Pathogens of Maize Gaining Free Passage Along the Silk Road" Pathogens 7, no. 4: 81. https://doi.org/10.3390/pathogens7040081
APA StyleThompson, M. E. H., & Raizada, M. N. (2018). Fungal Pathogens of Maize Gaining Free Passage Along the Silk Road. Pathogens, 7(4), 81. https://doi.org/10.3390/pathogens7040081




