Listeria monocytogenes Response to Propionate Is Differentially Modulated by Anaerobicity
Abstract
:1. Introduction
2. Results
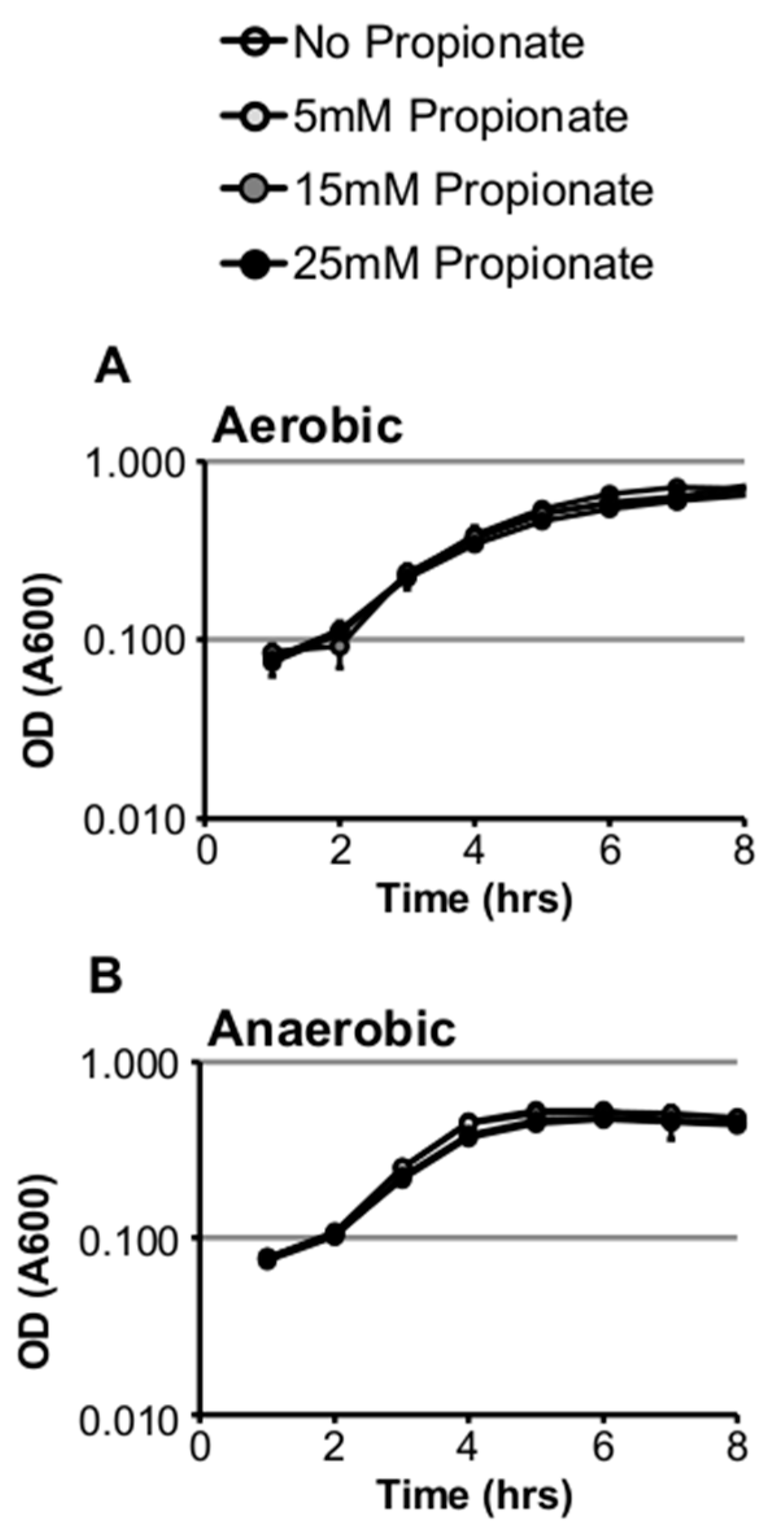
2.1. Propionate Perturbation on L. monocytogenes Planktonic Growth
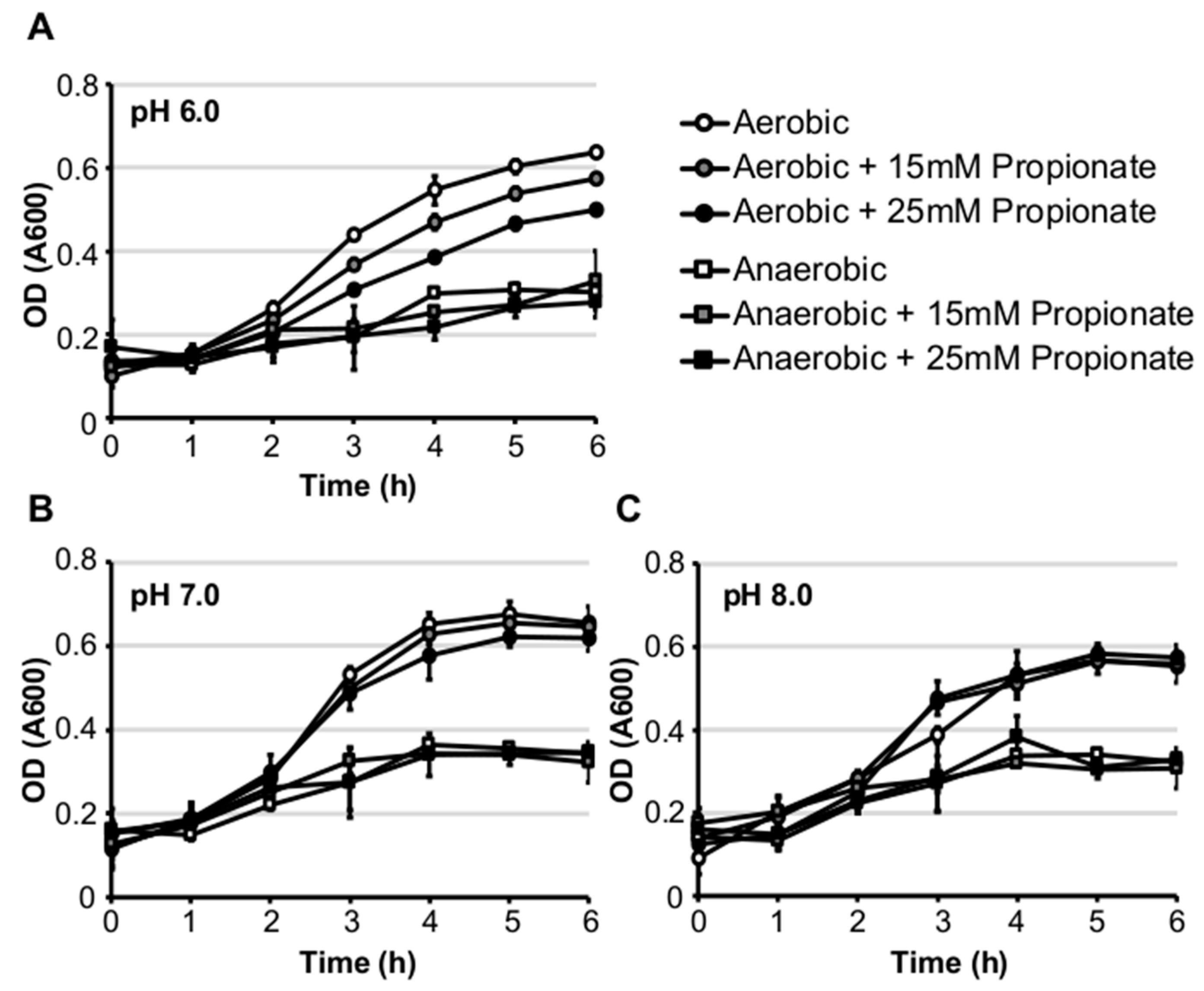
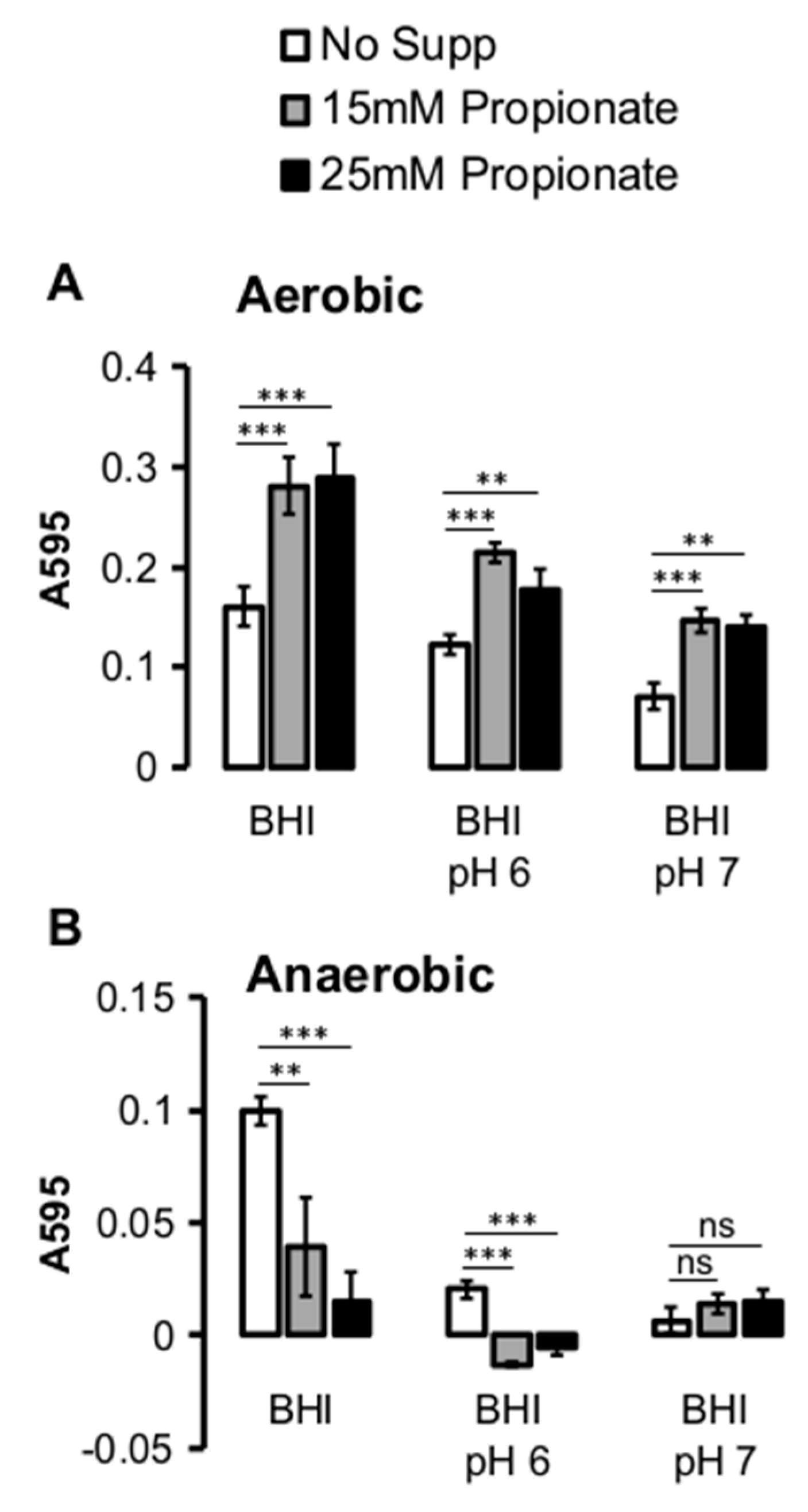
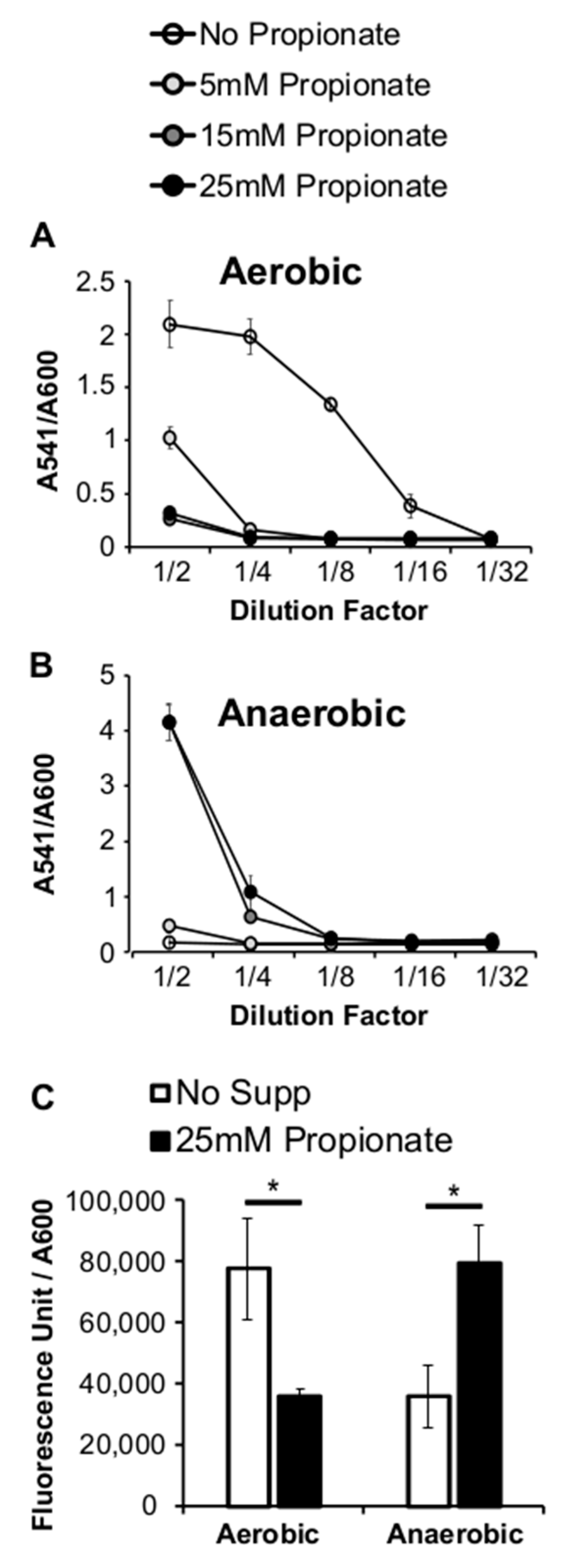
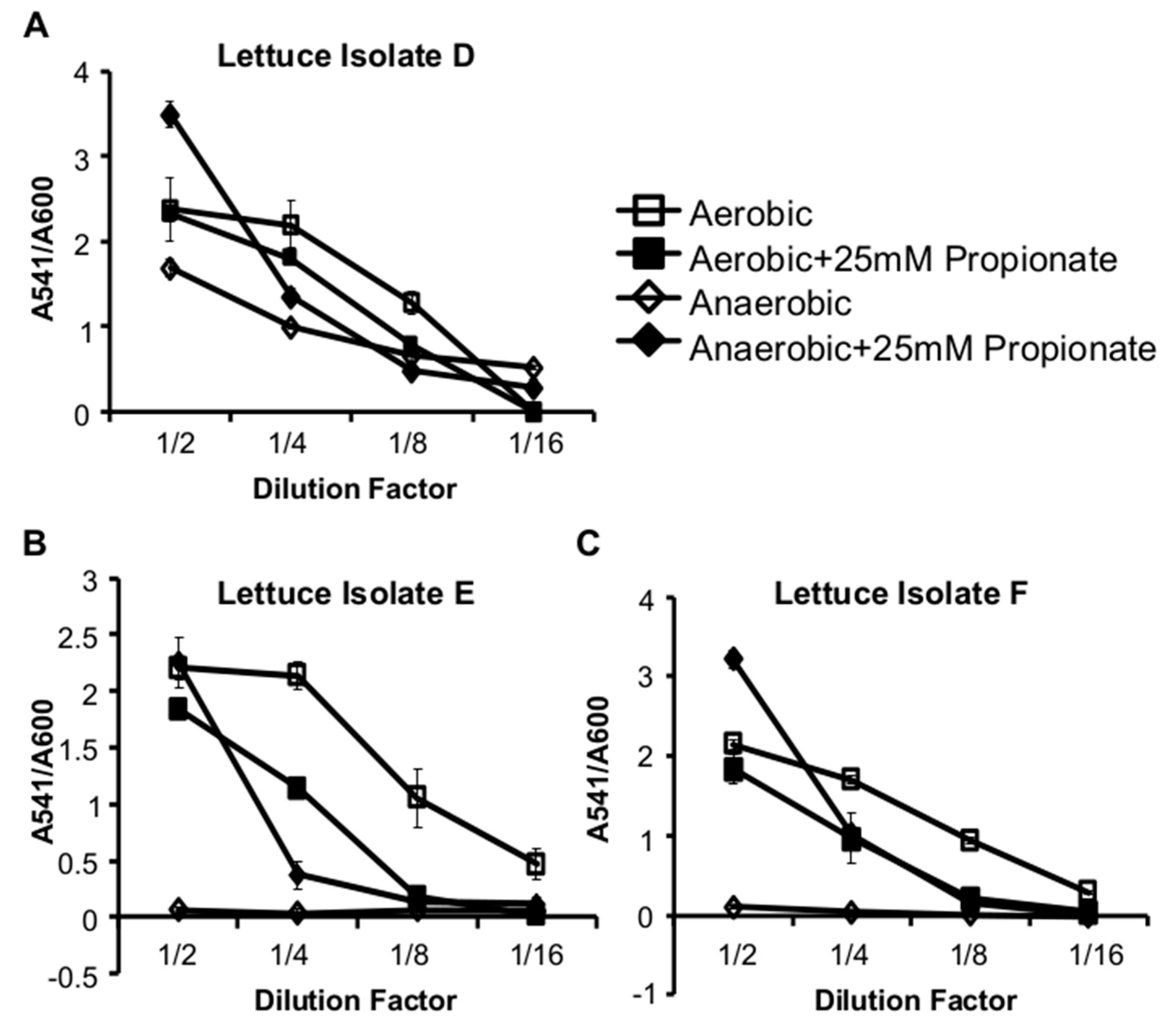
2.2. Propionate Perturbation on L. monocytogenes Adherent Growth
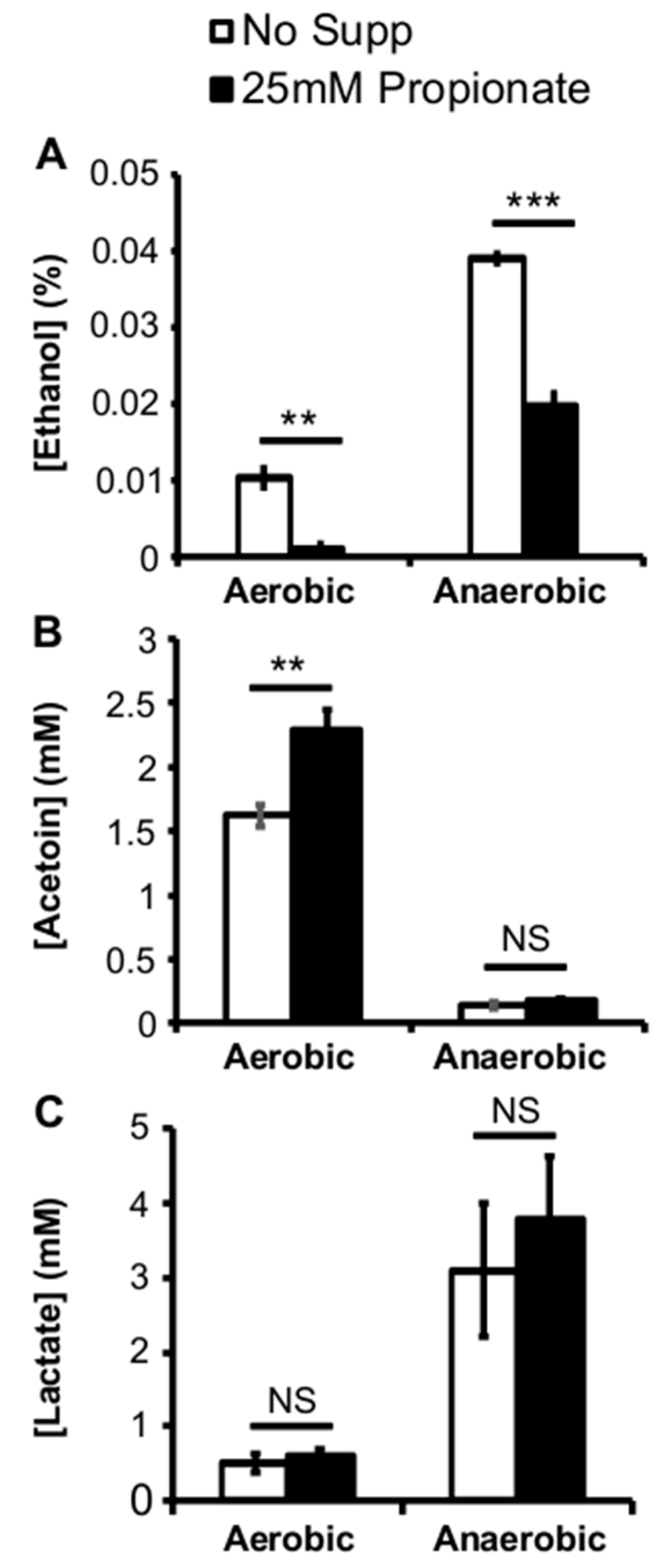
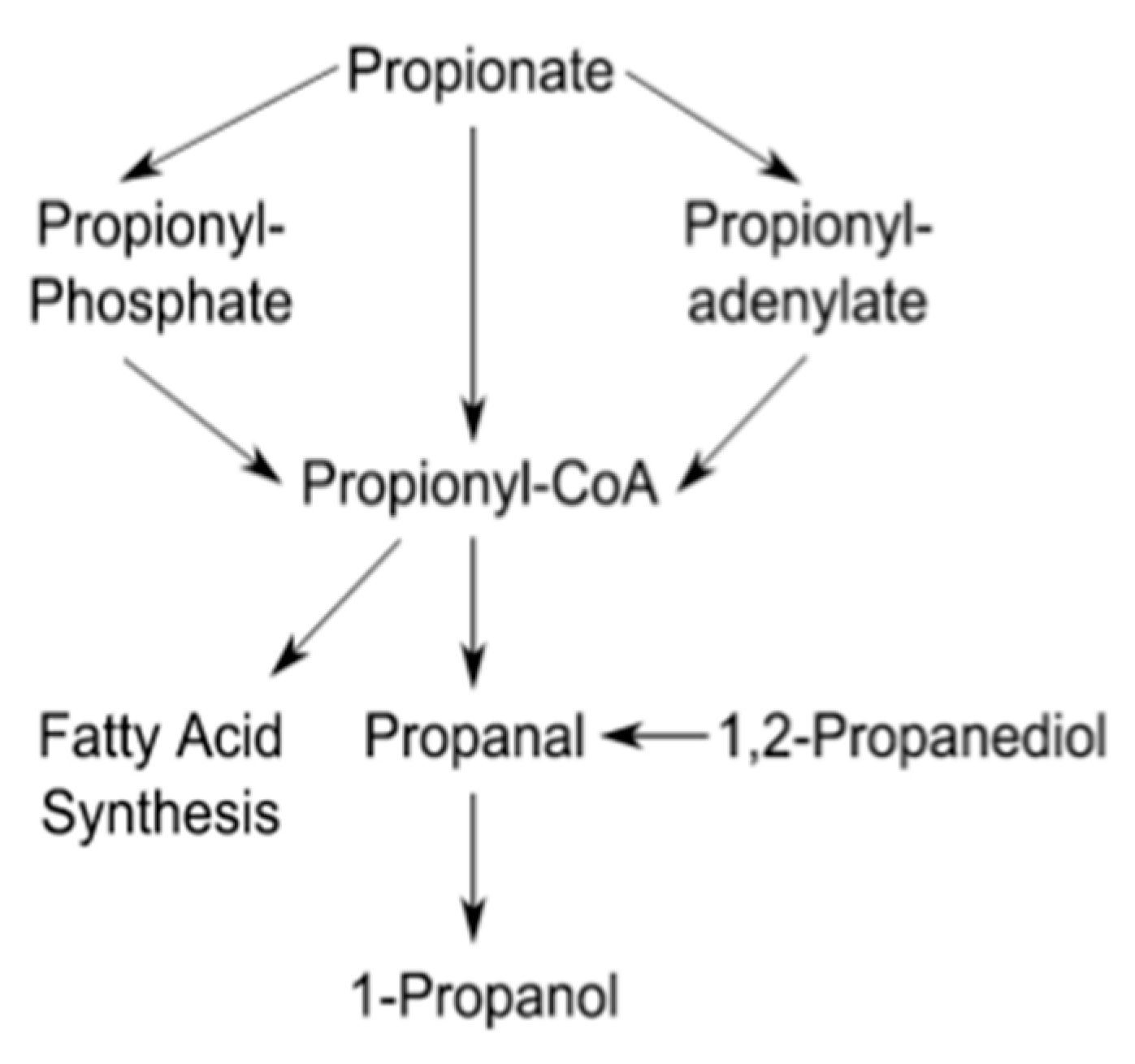
2.3. Propionate Perturbation on L. monocytogenes Metabolism
2.4. Propionate Perturbation on L. monocytogenes Fatty Acid Composition
2.5. Propionate Perturbation on L. monocytogenes LLO Production
3. Discussion
3.1. Propionate Perturbation on L. monocytogenes Growth
3.2. Propionate Perturbation on L. monocytogenes Metabolism
3.3. Propionate Perturbation on L. monocytogenes Pathogenesis
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Isolation of Listeria from Garden Vegetables
4.3. Adherent Growth Assay
4.4. Measurement of Acetoin, Ethanol, and Lactate Concentrations
4.5. FAME Analysis
4.6. Hemolytic Assay
4.7. MUG Assay
4.8. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lungu, B.; Ricke, S.C.; Johnson, M.G. Growth, survival, proliferation and pathogenesis of Listeria monocytogenes under low oxygen or anaerobic conditions: A review. Anaerobe 2009, 15, 7–17. [Google Scholar] [CrossRef] [PubMed]
- FDA CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1784 (accessed on 18 July 2016).
- Dussault, D.; Vu, K.D.; Lacroix, M. Development of a model describing the inhibitory effect of selected preservatives on the growth of Listeria monocytogenes in a meat model system. Food Microbiol. 2016, 53, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Glass, K.A.; McDonnell, L.M.; Von Tayson, R.; Wanless, B.; Badvela, M. Inhibition of Listeria monocytogenes by propionic acid-based ingredients in cured deli-style Turkey. J. Food Prot. 2013, 76, 2074–2078. [Google Scholar] [CrossRef] [PubMed]
- Glass, K.A.; McDonnell, L.M.; Rassel, R.C.; Zierke, K.L. Controlling Listeria monocytogenes on sliced ham and turkey products using benzoate, propionate, and sorbate. J. Food Prot. 2007, 70, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Menconi, A.; Shivaramaiah, S.; Huff, G.R.; Prado, O.; Morales, J.E.; Pumford, N.R.; Morgan, M.; Wolfenden, A.; Bielke, L.R.; Hargis, B.M.; et al. Effect of different concentrations of acetic, citric, and propionic acid dipping solutions on bacterial contamination of raw chicken skin. Poult. Sci. 2013, 92, 2216–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samara, A.; Koutsoumanis, K.P. Effect of treating lettuce surfaces with acidulants on the behaviour of Listeria monocytogenes during storage at 5 and 20 °C and subsequent exposure to simulated gastric fluid. Int. J. Food Microbiol. 2009, 129, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.A.; Beirne, D.O. Effects of gas atmosphere, antimicrobial dip and temperature on the fate of Listeria innocua and Listeria monocytogenes on minimally processed lettuce. Int. J. Food Sci. Technol. 1997, 32, 141–151. [Google Scholar] [CrossRef]
- El-Shenawy, M.A.; Marth, E.H. Behavior of Listeria monocytogenes in the presence of sodium propionate. Int. J. Food Microbiol. 1989, 8, 85–94. [Google Scholar] [CrossRef]
- Romick, T.L.; Fleming, H.P. Acetoin production as an indicator of growth and metabolic inhibition of Listeria monocytogenes. J. Appl. Microbiol. 1998, 84, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Buazzi, M.M.; Marth, E.H. Sites of action by propionate on Listeria monocytogenes. Int. J. Food Microbiol. 1992, 15, 109–119. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.-C.; Wang, Y.; Wang, Z.-B.; Liu, W.-Y.; Sun, S.; Li, L.; Su, D.-F.; Zhang, L.-C. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, L.N.; Liyanage, R.; Lay, J.O.; Kwon, Y.M. Proteomic analysis of Salmonella enterica serovar Enteritidis following propionate adaptation. BMC Microbiol. 2010, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, L.N.; Kwon, Y.M. The effect of long-term propionate adaptation on the stress resistance of Salmonella Enteritidis. J. Appl. Microbiol. 2010, 109, 1294–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.-C.; Garner, C.D.; Slauch, J.M.; Dwyer, Z.W.; Lawhon, S.D.; Frye, J.G.; McClelland, M.; Ahmer, B.M.M.; Altier, C. The intestinal fatty acid propionate inhibits Salmonella invasion through the post-translational control of HilD. Mol. Microbiol. 2013, 87, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.A.; Zagory, D.; Kerbel, E.L. Modified atmosphere packaging of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1989, 28, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.J.; Sherry, K.M.; Thorpe, J.A. Changes in gastric tissue oxygenation during mobilisation for oesophageal replacement. Eur. J. Cardiothorac. Surg. 1995, 9, 158–160. [Google Scholar] [CrossRef]
- He, G.; Shankar, R.A.; Chzhan, M.; Samouilov, A.; Kuppusamy, P.; Zweier, J.L. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. USA 1999, 96, 4586–4591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheridan, W.G.; Lowndes, R.H.; Young, H.L. Intraoperative tissue oximetry in the human gastrointestinal tract. Am. J. Surg. 1990, 159, 314–319. [Google Scholar] [CrossRef]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H.; et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014, 147, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Karhausen, J.; Furuta, G.T.; Tomaszewski, J.E.; Johnson, R.S.; Colgan, S.P.; Haase, V.H. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Investig. 2004, 114, 1098–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marteyn, B.; West, N.P.; Browning, D.F.; Cole, J.A.; Shaw, J.G.; Palm, F.; Mounier, J.; Prévost, M.-C.; Sansonetti, P.; Tang, C.M. Modulation of Shigella virulence in response to available oxygen in vivo. Nature 2010, 465, 355–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julotok, M.; Singh, A.K.; Gatto, C.; Wilkinson, B.J. Influence of fatty acid precursors, including food preservatives, on the growth and fatty acid composition of Listeria monocytogenes at 37 and 10 °C. Appl. Environ. Microbiol. 2010, 76, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wilkinson, B.J.; Standiford, T.J.; Akinbi, H.T.; O’Riordan, M.X.D. Fatty acids regulate stress resistance and virulence factor production for Listeria monocytogenes. J. Bacteriol. 2012, 194, 5274–5284. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; O’Riordan, M.X.D. Branched-chain fatty acids promote Listeria monocytogenes intracellular infection and virulence. Infect. Immun. 2010, 78, 4667–4673. [Google Scholar] [CrossRef] [PubMed]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The Impact of the Level of the Intestinal Short Chain Fatty Acids in Inflammatory Bowel Disease Patients Versus Healthy Subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food additives; Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of propionic acid (E 280), sodium propionate (E 281), calcium propionate (E 282) and potassium propionate (E 283) as food additives. EFSA J. 2014, 12, 3779. [Google Scholar] [CrossRef]
- Pubchem Propionate|C3H5O2−—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/propionate (accessed on 17 July 2017).
- Gahan, C.G.; O’Driscoll, B.; Hill, C. Acid adaptation of Listeria monocytogenes can enhance survival in acidic foods and during milk fermentation. Appl. Environ. Microbiol. 1996, 62, 3128–3132. [Google Scholar] [PubMed]
- Bonnet, M.; Montville, T.J. Acid-tolerant Listeria monocytogenes persist in a model food system fermented with nisin-producing bacteria. Lett. Appl. Microbiol. 2005, 40, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, G.; Conte, M.P.; Chiarini, F.; Seganti, L.; Ammendolia, M.G.; Superti, F.; Longhi, C. Acid adaptation and survival of Listeria monocytogenes in Italian-style soft cheeses. J. Appl. Microbiol. 2007, 103, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Skandamis, P.N.; Gounadaki, A.S.; Geornaras, I.; Sofos, J.N. Adaptive acid tolerance response of Listeria monocytogenes strains under planktonic and immobilized growth conditions. Int. J. Food Microbiol. 2012, 159, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Skandamis, P.N.; Yoon, Y.; Stopforth, J.D.; Kendall, P.A.; Sofos, J.N. Heat and acid tolerance of Listeria monocytogenes after exposure to single and multiple sublethal stresses. Food Microbiol. 2008, 25, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Sewell, D.; Allen, S.C.; Phillips, C.A. Oxygen limitation induces acid tolerance and impacts simulated gastro-intestinal transit in Listeria monocytogenes J0161. Gut Pathog. 2015, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Brissonnet, F.; Trotier, E.; Briandet, R. The Biofilm Lifestyle Involves an Increase in Bacterial Membrane Saturated Fatty Acids. Front. Microbiol. 2016, 7, 1673. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, A.; Serrazanetti, D.; Sado Kamdem, S.; Guerzoni, M.E. Involvement of cell fatty acid composition and lipid metabolism in adhesion mechanism of Listeria monocytogenes. Int. J. Food Microbiol. 2008, 123, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.R.; den Besten, H.M.W.; van der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Borucki, M.K.; Peppin, J.D.; White, D.; Loge, F.; Call, D.R. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7336–7342. [Google Scholar] [CrossRef] [PubMed]
- Lundén, J.M.; Miettinen, M.K.; Autio, T.J.; Korkeala, H.J. Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. J. Food Prot. 2000, 63, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Ingram, L.O.; Chevalier, L.S.; Gabba, E.J.; Ley, K.D.; Winters, K. Propionate-induced synthesis of odd-chain-length fatty acids by Escherichia coli. J. Bacteriol. 1977, 131, 1023–1025. [Google Scholar] [PubMed]
- Sirobhushanam, S.; Galva, C.; Sen, S.; Wilkinson, B.J.; Gatto, C. Broad substrate specificity of phosphotransbutyrylase from Listeria monocytogenes: A potential participant in an alternative pathway for provision of acyl CoA precursors for fatty acid biosynthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.P.; Sen, S.; Wilkinson, B.J.; Gatto, C. Insights into the Mechanism of Homeoviscous Adaptation to Low Temperature in Branched-Chain Fatty Acid-Containing Bacteria through Modeling FabH Kinetics from the Foodborne Pathogen Listeria monocytogenes. Front. Microbiol. 2016, 7, 1386. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Zhang, Y.-M.; Zhu, K.; Subramanian, C.; Li, Z.; Jayaswal, R.K.; Gatto, C.; Rock, C.O.; Wilkinson, B.J. FabH selectivity for anteiso branched-chain fatty acid precursors in low-temperature adaptation in Listeria monocytogenes. FEMS Microbiol. Lett. 2009, 301, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Romick, T.L.; Fleming, H.P.; McFeeters, R.F. Aerobic and anaerobic metabolism of Listeria monocytogenes in defined glucose medium. Appl. Environ. Microbiol. 1996, 62, 304–307. [Google Scholar] [PubMed]
- Stritzker, J.; Janda, J.; Schoen, C.; Taupp, M.; Pilgrim, S.; Gentschev, I.; Schreier, P.; Geginat, G.; Goebel, W. Growth, Virulence, and Immunogenicity of Listeria monocytogenes aro Mutants. Infect. Immun. 2004, 72, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Wallace, N.; Newton, E.; Abrams, E.; Zani, A.; Sun, Y. Metabolic determinants in Listeria monocytogenes anaerobic listeriolysin O production. Arch. Microbiol. 2017, 199, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Stasiewicz, M.J.; Wiedmann, M.; Bergholz, T.M. The transcriptional response of Listeria monocytogenes during adaptation to growth on lactate and diacetate includes synergistic changes that increase fermentative acetoin production. Appl. Environ. Microbiol. 2011, 77, 5294–5306. [Google Scholar] [CrossRef] [PubMed]
- Milenbachs, A.A.; Brown, D.P.; Moors, M.; Youngman, P. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 1997, 23, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Behari, J.; Youngman, P. Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA. Infect. Immun. 1998, 66, 3635–3642. [Google Scholar] [PubMed]
- Poncet, S.; Milohanic, E.; Mazé, A.; Nait Abdallah, J.; Aké, F.; Larribe, M.; Deghmane, A.-E.; Taha, M.-K.; Dozot, M.; De Bolle, X.; et al. Correlations between carbon metabolism and virulence in bacteria. Contrib. Microbiol. 2009, 16, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Scortti, M.; Monzo, H.J.; Lacharme-Lora, L.; Lewis, D.A.; Vazquez-Boland, J.A. The PrfA virulence regulon. Microbes Infect. 2007, 9, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, W.; Dandekar, T.; Heesemann, J.; Goebel, W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat. Rev. Microbiol. 2010, 8, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, E.; Kern, T.; Felsl, A.; Eisenreich, W.; Fuchs, T.M. Isotopologue profiling of the listerial N-metabolism. Mol. Microbiol. 2016, 100, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Schär, J.; Stoll, R.; Schauer, K.; Loeffler, D.I.M.; Eylert, E.; Joseph, B.; Eisenreich, W.; Fuchs, T.M.; Goebel, W. Pyruvate carboxylase plays a crucial role in carbon metabolism of extra- and intracellularly replicating Listeria monocytogenes. J. Bacteriol. 2010, 192, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- FDA Laboratory Methods—BAM Media M52: Buffered Listeria Enrichment Broth (BLEB). Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm064305.htm (accessed on 3 July 2017).
- FDA Laboratory Methods—BAM: Detection and Enumeration of Listeria monocytogenes. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071400.htm (accessed on 3 January 2018).
- FDA Laboratory Methods—BAM Protocol: Simultaneous Confirmation of Listeria Species and L. monocytogenes Isolates by Real-Time PCR. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm279532.htm (accessed on 3 July 2017).
- Taylor, K.A.C.C. A simple colorimetric assay for muramic acid and lactic acid. Appl. Biochem. Biotechnol. 1996, 56, 49–58. [Google Scholar] [CrossRef]
| Aerobic | Aerobic +25 mM Propionate | Anaerobic | Anaerobic +25 mM Propionate | |
|---|---|---|---|---|
| Doubling Time (Minutes) at 37 °C | 78.06 ± 5.05 | 85.22 ± 4.83 | 73.51 ± 0.53 | 86.37 ± 4.96 * |
| Doubling Time (Minutes) at Room Temperature | 170.60 ± 0.56 | 157.66 ± 2.11 ** | 119.48 ± 5.48 | 123.64 ± 10.84 |
| Culture pH after Overnight Growth at 37 °C | 5.28 ± 0.02 | 5.57 ± 0.01 *** | 4.90 ± 0.02 | 5.29 ± 0.01 *** |
| Aerobic | Aerobic +25 mM Propionate | Anaerobic | Anaerobic +25 mM Propionate | |
|---|---|---|---|---|
| 15:0 Anteiso | 46.24 | 42.15 | 35.65 | 31.8 |
| 17:0 Anteiso | 36.92 | 26.8 | 24.35 | 12.27 |
| Anteiso Total | 83.16 | 68.95 | 60 | 44.07 |
| 14:0 Iso | 0.41 | 0.5 | 0.89 | 0.74 |
| 15:0 Iso | 9.53 | 7.96 | 11.76 | 5.97 |
| 16:0 Iso | 2.19 | 1.92 | 3.21 | 1.33 |
| 17:0 Iso | 3.28 | 2.38 | 4.78 | 1.4 |
| Iso Total | 15.41 | 12.76 | 20.64 | 9.44 |
| Anteiso: Iso Ratio | 5.40 | 5.40 | 2.90 | 4.67 |
| 13 straight | 0 | 0.95 | 0 | 1.71 |
| 15 straight | 0 | 14.27 | 0 | 22.87 |
| 16 straight | 0.66 | 0.65 | 2.21 | 1.81 |
| 17 straight | 0 | 1.38 | 0 | 2.23 |
| 18 straight | 0 | 0.16 | 0.44 | 0.59 |
| Straight Total | 0.66 | 17.41 | 2.65 | 29.21 |
| Branched: Straight Ratio | 149.35 | 4.69 | 30.43 | 1.83 |
| 18:1 w9c | 0 | 0.21 | 6.18 | 7.4 |
| 18:2 w6,9c | 0 | 0.21 | 1.72 | 1.88 |
| Unsaturated Total | 0 | 0.42 | 7.9 | 9.28 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinehart, E.; Newton, E.; Marasco, M.A.; Beemiller, K.; Zani, A.; Muratore, M.K.; Weis, J.; Steinbicker, N.; Wallace, N.; Sun, Y. Listeria monocytogenes Response to Propionate Is Differentially Modulated by Anaerobicity. Pathogens 2018, 7, 60. https://doi.org/10.3390/pathogens7030060
Rinehart E, Newton E, Marasco MA, Beemiller K, Zani A, Muratore MK, Weis J, Steinbicker N, Wallace N, Sun Y. Listeria monocytogenes Response to Propionate Is Differentially Modulated by Anaerobicity. Pathogens. 2018; 7(3):60. https://doi.org/10.3390/pathogens7030060
Chicago/Turabian StyleRinehart, Erica, Eric Newton, Megan A. Marasco, Kaitlin Beemiller, Ashley Zani, Melani K. Muratore, John Weis, Nicole Steinbicker, Nathan Wallace, and Yvonne Sun. 2018. "Listeria monocytogenes Response to Propionate Is Differentially Modulated by Anaerobicity" Pathogens 7, no. 3: 60. https://doi.org/10.3390/pathogens7030060
APA StyleRinehart, E., Newton, E., Marasco, M. A., Beemiller, K., Zani, A., Muratore, M. K., Weis, J., Steinbicker, N., Wallace, N., & Sun, Y. (2018). Listeria monocytogenes Response to Propionate Is Differentially Modulated by Anaerobicity. Pathogens, 7(3), 60. https://doi.org/10.3390/pathogens7030060





