The Optimisation of Pseudotyped Viruses for the Characterisation of Immune Responses to Equine Influenza Virus
Abstract
:1. Introduction
2. Results
2.1. The Effect of Different Proteases on Equine Influenza H3 PV Titre
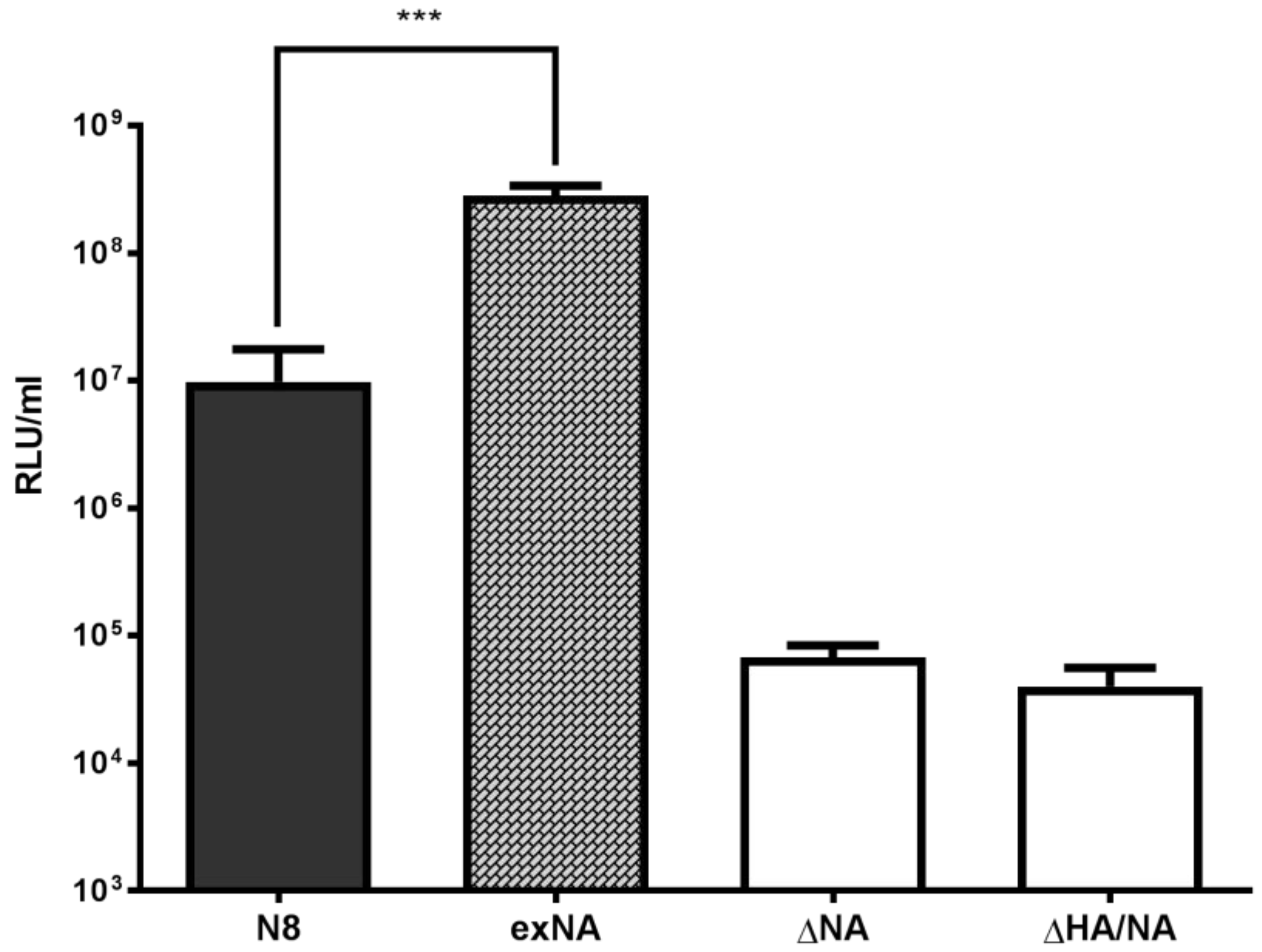
2.2. Influence of Source of Neuraminidase on PV Titre
2.3. Repeatability of Pseudotyped Virus Neutralisation Tests (PVNTs)
3. Discussion
4. Materials and Methods
4.1. Generation of H3 Pseudotyped Viruses
4.2. Pseudotyped Virus Neutralisation Test (PVNT)
4.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EIV | equine influenza virus |
| HA | haemagglutinin |
| HAT | human airway trypsin-like protease |
| NA | neuraminidase |
| PV | pseudotyped virus |
| PVNT | pseudotyped virus neutralisation test |
| TMPRSS2/4 | Transmembrane Protease Serine S1 member 2/4 |
References
- Carnell, G.W.; Ferrara, F.; Grehan, K.; Thompson, C.P.; Temperton, N.J. Pseudotype-based neutralization assays for influenza: A systematic analysis. Front. Immunol. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Bottcher-Friebertshauser, E.; Klenk, H.D.; Garten, W. Activation of influenza viruses by proteases from host cells and bacteria in the human airway epithelium. Pathog. Dis. 2013, 69, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, E.; Matrosovich, T.; Beyerle, M.; Klenk, H.-D.; Garten, W.; Matrosovich, M. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 2006, 80, 9896–9898. [Google Scholar] [CrossRef] [PubMed]
- Kinsley, R.; Scott, S.D.; Daly, J.M. Controlling equine influenza: Traditional to next generation serological assays. Vet. Microbiol. 2016, 187, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.; Molesti, E.; Temperton, N.; Ferrara, F.; Bottcher-Friebertshauser, E.; Daly, J. The use of equine influenza pseudotypes for serological screening. J. Mol. Gen. Med. 2012, 6, 304–308. [Google Scholar] [CrossRef]
- Memoli, M.J.; Shaw, P.A.; Han, A.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Fargis, S.; Risos, K.; Powers, J.H.; et al. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. MBio 2016, 7, e00417-16. [Google Scholar] [CrossRef] [PubMed]
- Hai, R.; Krammer, F.; Tan, G.S.; Pica, N.; Eggink, D.; Maamary, J.; Margine, I.; Albrecht, R.A.; Palese, P. Influenza viruses expressing chimeric hemagglutinins: Globular head and stalk domains derived from different subtypes. J. Virol. 2012, 86, 5774–5781. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Voss, J.; Zhang, G.; Buchy, P.; Zuo, T.; Wang, L.; Wang, F.; Zhou, F.; Wang, G.; Tsai, C.; et al. A human antibody recognizing a conserved epitope of H5 hemagglutinin broadly neutralizes highly pathogenic avian influenza H5N1 viruses. J. Virol. 2012, 86, 2978–2989. [Google Scholar] [CrossRef] [PubMed]
- Molesti, E.; Wright, E.; Terregino, C.; Rahman, R.; Cattoli, G.; Temperton, N.J. Multiplex evaluation of influenza neutralizing antibodies with potential applicability to in-field serological studies. J. Immunol. Res. 2014, 2014, 457932. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Huang, Y.; Wang, Q.; Tian, D.; Zhang, W.; Hu, Y.; Yuan, Z.; Zhang, X.; Xu, J. Boosting heterosubtypic neutralization antibodies in recipients of 2009 pandemic H1N1 influenza vaccine. Clin. Infect. Dis. 2012, 54, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, G.; Buchy, P.; Cai, Z.; Chen, H.; Chen, Z.; Cheng, G.; Wan, X.F.; Deubel, V.; Zhou, P. A triclade DNA vaccine designed on the basis of a comprehensive serologic study elicits neutralizing antibody responses against all clades and subclades of highly pathogenic avian influenza H5N1 viruses. J. Virol. 2012, 86, 6970–6978. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, G.; Locher, S.; Berger Rentsch, M.; Halbherr, S.J. Pseudotyping of vesicular stomatitis virus with the envelope glycoproteins of highly pathogenic avian influenza viruses. J. Gen. Virol. 2014, 95, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Molesti, E.; Böttcher-Frieberthäuser, E.; Cattoli, G.; Corti, D.; Scott, S.D.; Temperton, N.J. The human transmembrane protease serine 2 is necessary for the production of group 2 influenza a virus pseudotypes. J. Mol. Gen. Med. 2013, 7, 309–314. [Google Scholar] [CrossRef]
- Wang, W.; Butler, E.N.; Veguilla, V.; Vassell, R.; Thomas, J.T.; Moos, M., Jr.; Ye, Z.; Hancock, K.; Weiss, C.D. Establishment of retroviral pseudotypes with influenza hemagglutinins from H1, H3, and H5 subtypes for sensitive and specific detection of neutralizing antibodies. J. Virol. Meth. 2008, 153, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Chaipan, C.; Kobasa, D.; Bertram, S.; Glowacka, I.; Steffen, I.; Tsegaye, T.S.; Takeda, M.; Bugge, T.H.; Kim, S.; Park, Y.; et al. Proteolytic activation of the 1918 influenza virus hemagglutinin. J. Virol. 2009, 83, 3200–3211. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Wang, S.Y.; Shie, J.J.; Jeng, K.S.; Temperton, N.J.; Fang, J.M.; Wong, C.H.; Cheng, Y.S. In vitro evaluation of neuraminidase inhibitors using the neuraminidase-dependent release assay of hemagglutinin-pseudotyped viruses. Antivir. Res. 2008, 79, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Neverov, A.D.; Kryazhimskiy, S.; Plotkin, J.B.; Bazykin, G.A. Coordinated evolution of influenza A surface proteins. PLoS Genet. 2015, 11, e1005404. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.J.; Lycett, S.J.; Avila, D.; Bollback, J.P.; Leigh Brown, A.J. Evolutionary interactions between haemagglutinin and neuraminidase in avian influenza. BMC Evol. Biol. 2013, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, Q.; Su, X.; Teng, Q.; Bao, D.; Che, G.; Chen, H.; Cui, H.; Ruan, T.; Li, X.; et al. Pathogenicity of reassortant H9 influenza viruses with different NA genes in mice and chickens. Vet. Res. 2016, 47, 67. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, S.D.; Kinsley, R.; Temperton, N.; Daly, J.M. The Optimisation of Pseudotyped Viruses for the Characterisation of Immune Responses to Equine Influenza Virus. Pathogens 2016, 5, 68. https://doi.org/10.3390/pathogens5040068
Scott SD, Kinsley R, Temperton N, Daly JM. The Optimisation of Pseudotyped Viruses for the Characterisation of Immune Responses to Equine Influenza Virus. Pathogens. 2016; 5(4):68. https://doi.org/10.3390/pathogens5040068
Chicago/Turabian StyleScott, Simon D., Rebecca Kinsley, Nigel Temperton, and Janet M. Daly. 2016. "The Optimisation of Pseudotyped Viruses for the Characterisation of Immune Responses to Equine Influenza Virus" Pathogens 5, no. 4: 68. https://doi.org/10.3390/pathogens5040068
APA StyleScott, S. D., Kinsley, R., Temperton, N., & Daly, J. M. (2016). The Optimisation of Pseudotyped Viruses for the Characterisation of Immune Responses to Equine Influenza Virus. Pathogens, 5(4), 68. https://doi.org/10.3390/pathogens5040068







