Kinase Inhibitors that Increase the Sensitivity of Methicillin Resistant Staphylococcus aureus to β-Lactam Antibiotics
Abstract
:1. Introduction
2. Results
2.1. Kinase Inhibitors Increase Sensitivity of MRSA to β-Lactam Antibiotics, Such as Nafcillin
| MIC (µg/mL) | PEN | AMP | NAF | CXM | CAZ | FEP | IPM |
|---|---|---|---|---|---|---|---|
| Newman (MSSA) | 0.094 | 0.25 | 0.25 | 1.5 | 16 | 4 | 0.032 |
| MW2 | 48 | 24 | 32 | >256 | >256 | >256 | 1 |
| MW2∆stk1 | 12 | 12 | 2 | 6 | 32 | 8 | 0.125 |
| LAC | 48 | 32 | 16 | >256 | >256 | >256 | 0.75 |
| LAC∆stk1 | 24 | 24 | 4 | 6 | 12 | 4 | 0.06 |
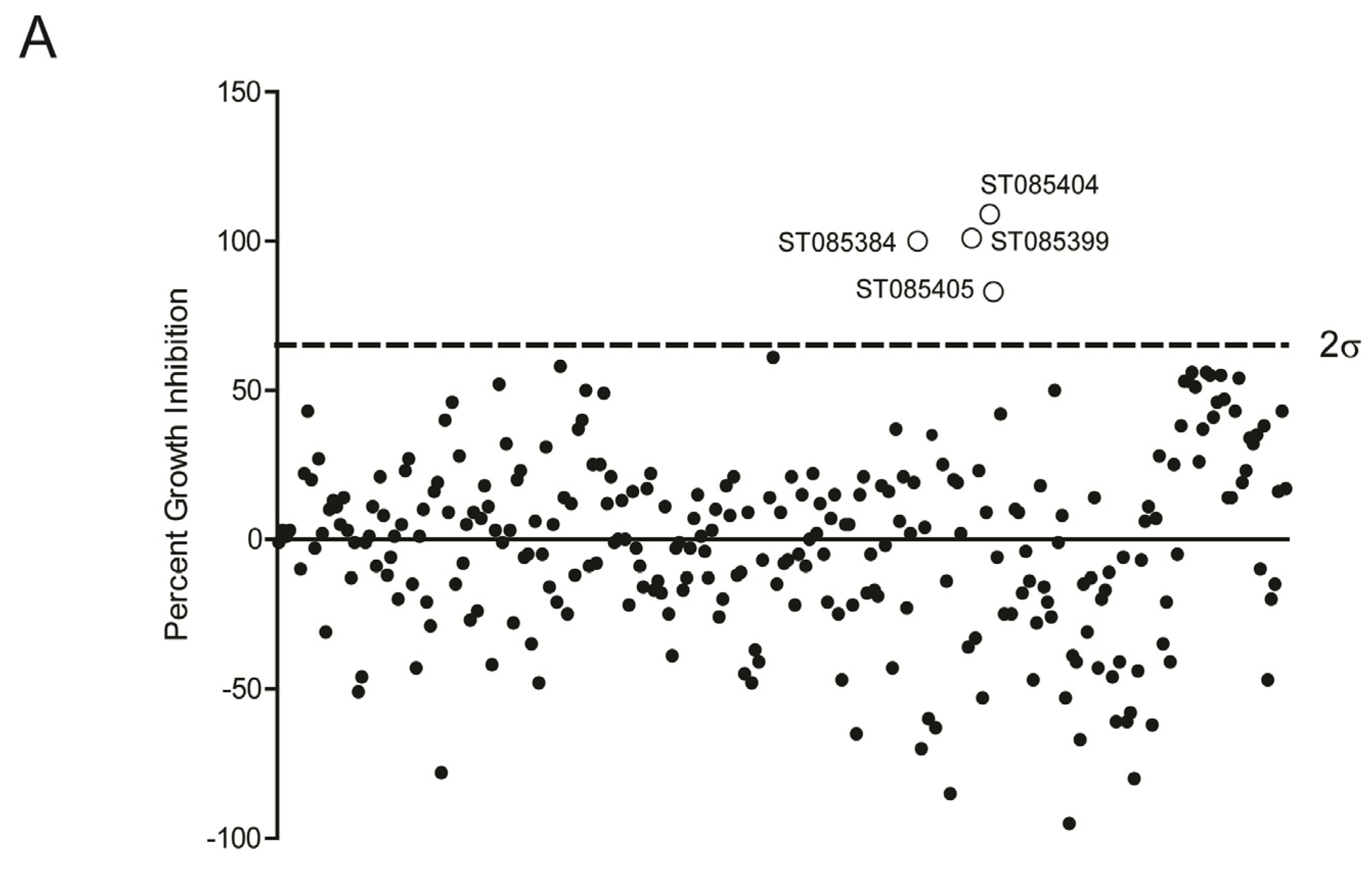

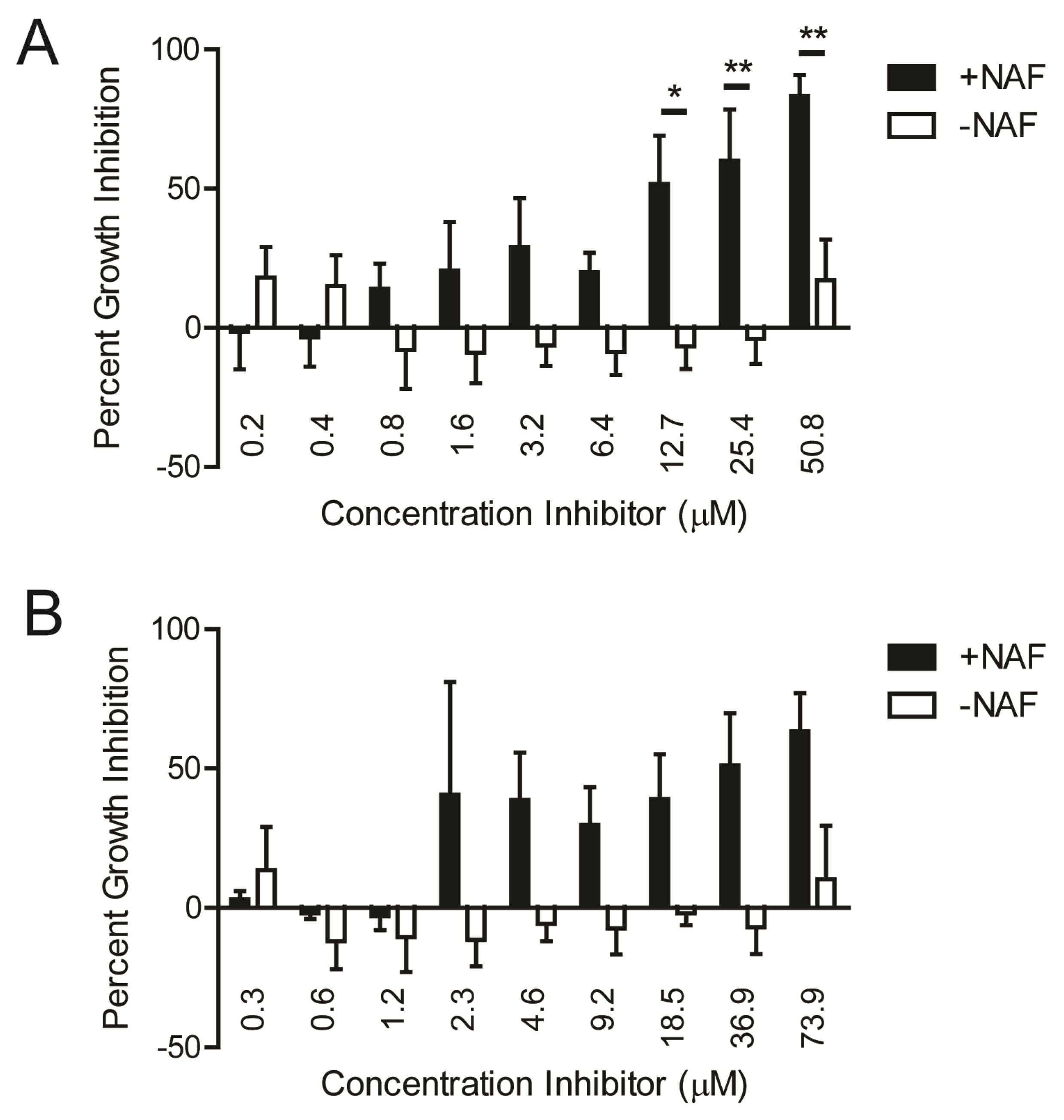
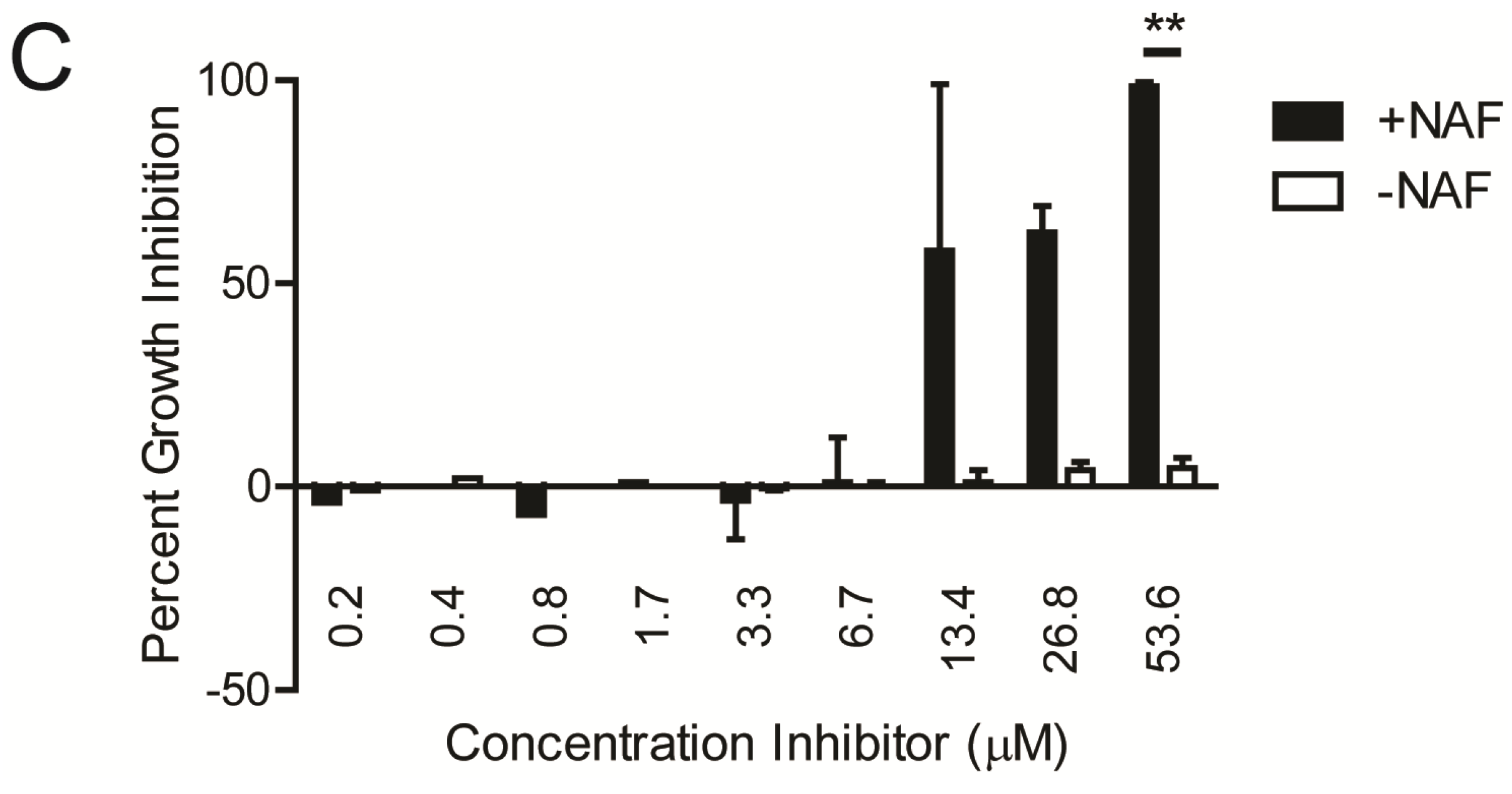
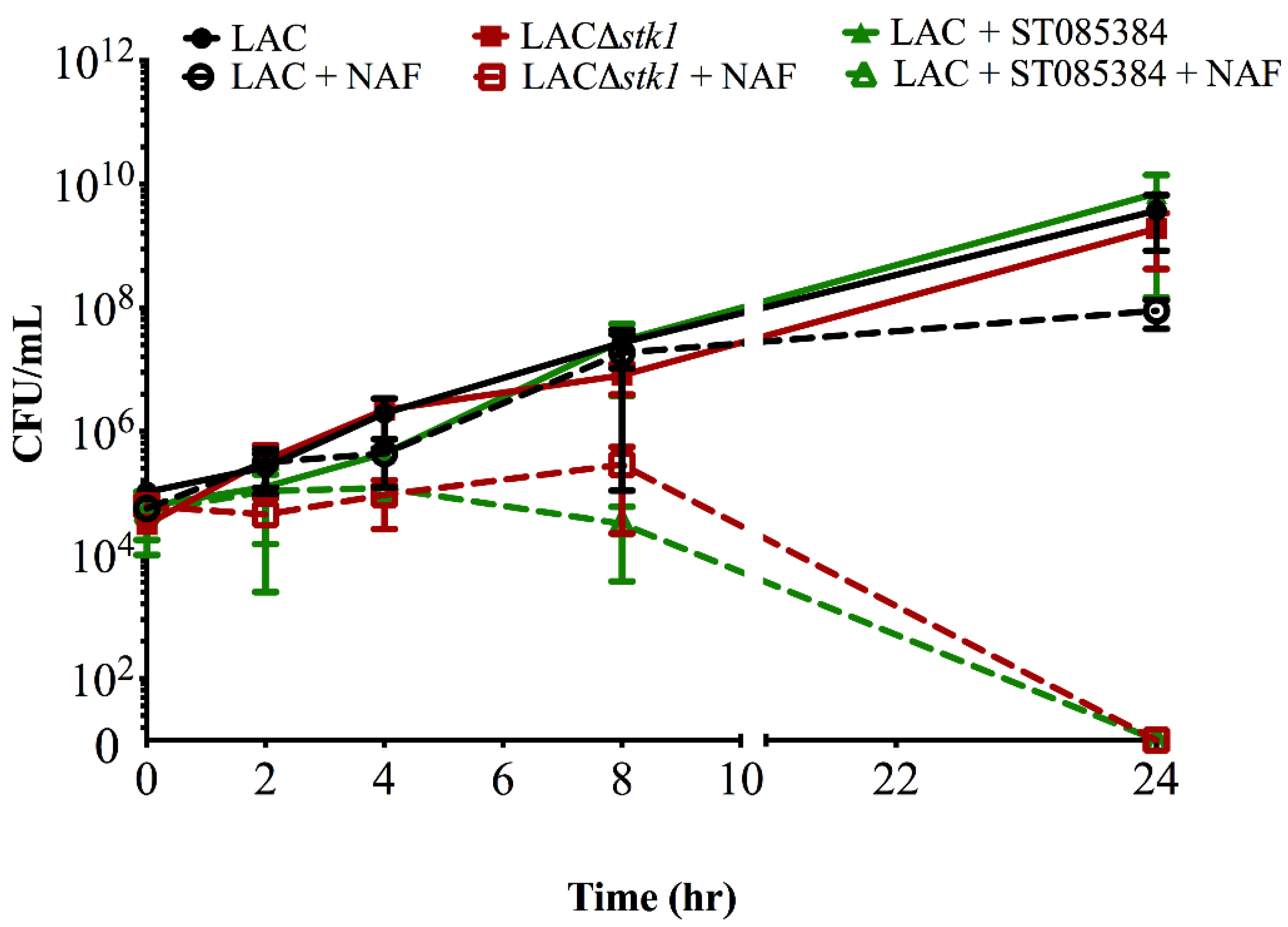
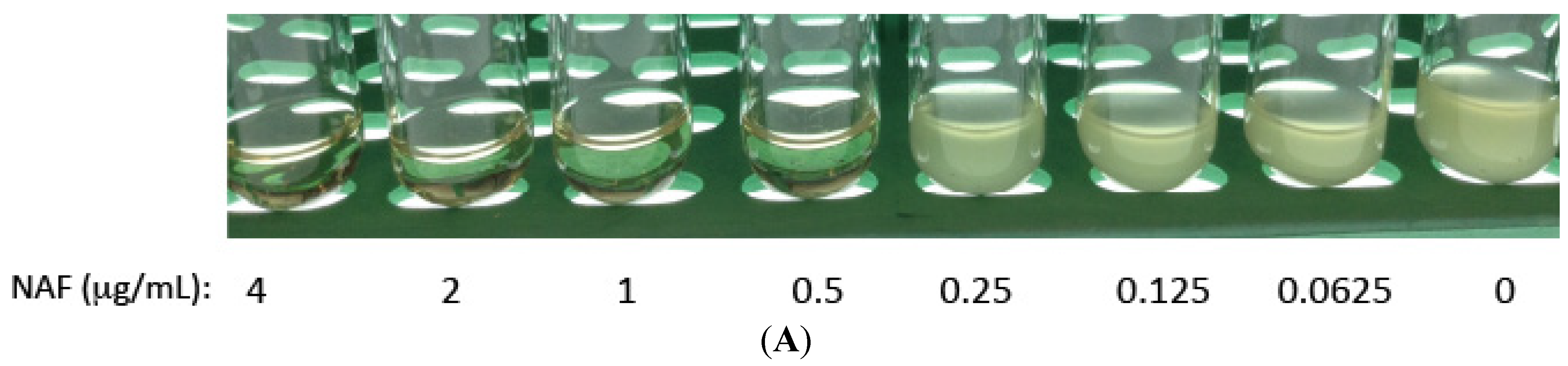
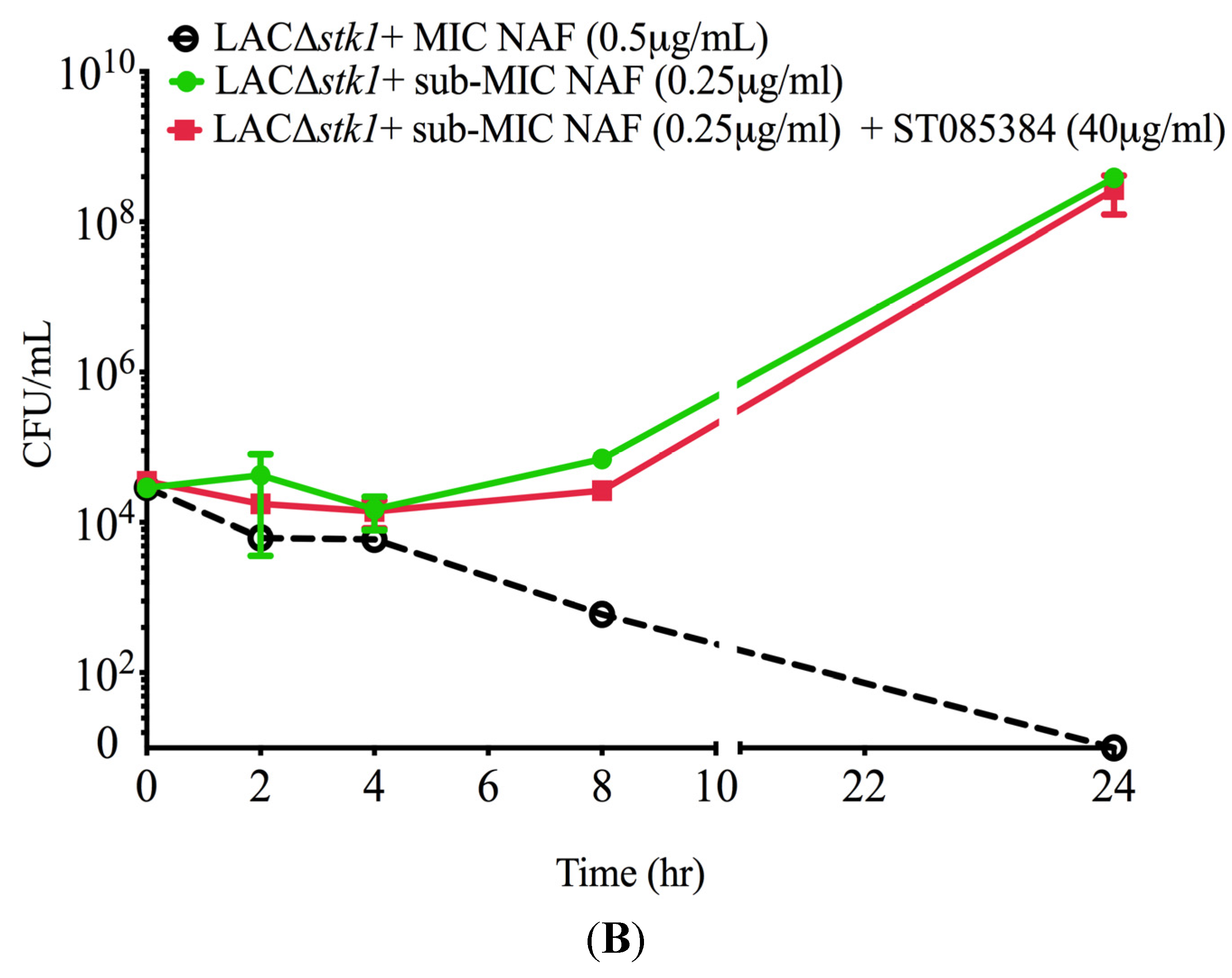
2.2. Kinase Inhibitors Decrease Stk1 Autophosphorylation

2.3. The Kinase Inhibitor ST085384 Is Tolerated in Mice
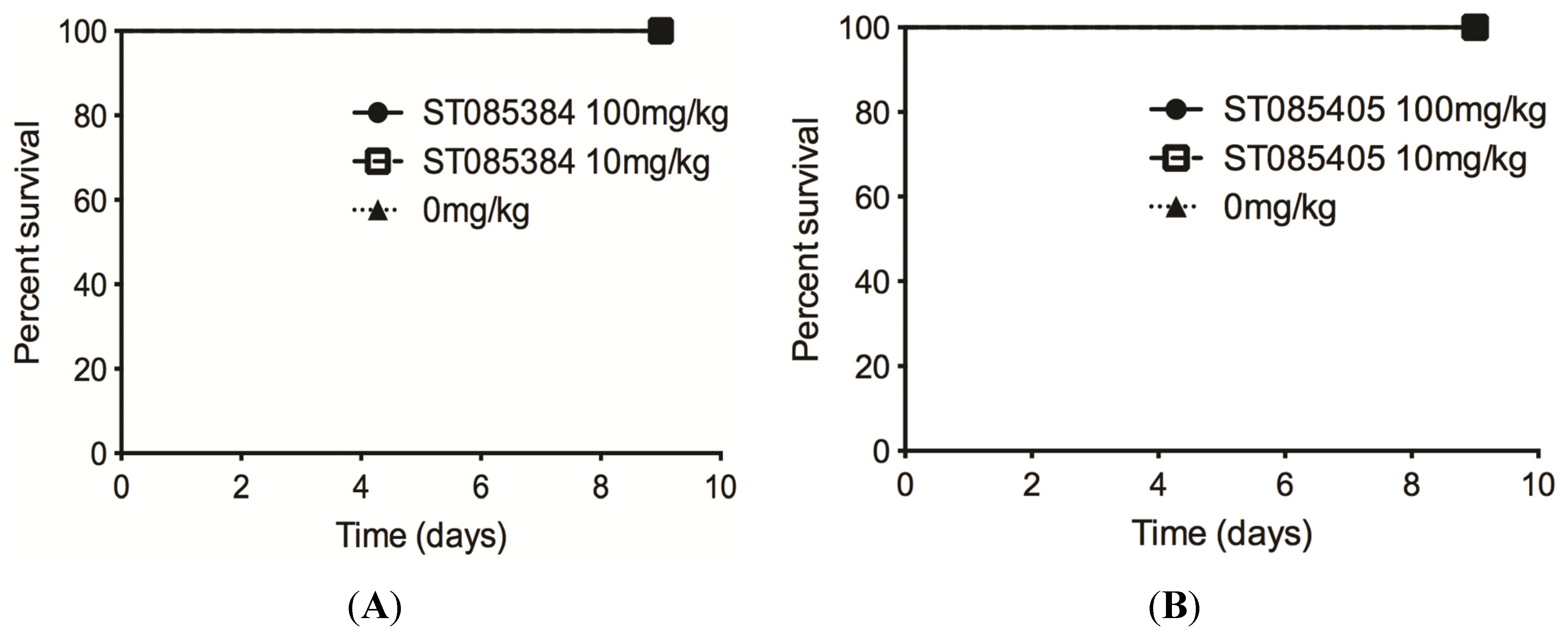
3. Discussion
4. Experimental Section
4.1. Strains and Chemicals
4.2. STK Inhibitor Library Screen
4.3. Time-to-Kill Assay
4.4. Protein Purification and in Vitro Phosphorylation Assays
Primer Sequence
- 6His-SaSTKBamH1F: 5′ TAGAGGATCCATGATAGGTAAAATAATAAATGAAC 3′
- 6His-SaSTKXhoIR: 5′ TGAACTCGAGTACATCATCATAGCTGACTTCTT 3′
4.5. Animal Studies
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Styers, D.; Sheehan, D.J.; Hogan, P.; Sahm, D.F. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann. Clin. Microbiol. Antimicrob. 2006. [Google Scholar] [CrossRef] [PubMed]
- Seybold, U.; Kourbatova, E.V.; Johnson, J.G.; Halvosa, S.J.; Wang, Y.F.; King, M.D.; Ray, S.M.; Blumberg, H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 2006, 42, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Noskin, G.A.; Rubin, R.J.; Schentag, J.J.; Kluytmans, J.; Hedblom, E.C.; Jacobson, C.; Smulders, M.; Gemmen, E.; Bharmal, M. National trends in Staphylococcus aureus infection rates: Impact on economic burden and mortality over a 6-year period (1998–2003). Clin. Infect. Dis. 2007, 45, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Hansford, K.A.; Blaskovich, M.A.; Halai, R.; Cooper, M.A. Glycopeptide antibiotics: Back to the future. J. Antibiot. (Tokyo). 2014, 67, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Beltramini, A.M.; Mukhopadhyay, C.D.; Pancholi, V. Modulation of cell wall structure and antimicrobial susceptibility by a Staphylococcus aureus eukaryote-like serine/threonine kinase and phosphatase. Infect. Immun. 2009, 77, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Tamber, S.; Schwartzman, J.; Cheung, A.L. Role of PknB kinase in antibiotic resistance and virulence in community-acquired methicillin-resistant Staphylococcus aureus strain USA300. Infect. Immun. 2010, 78, 3637–3646. [Google Scholar] [CrossRef] [PubMed]
- Burnside, K.; Lembo, A.; de Los Reyes, M.; Iliuk, A.; Binhtran, N.T.; Connelly, J.E.; Lin, W.J.; Schmidt, B.Z.; Richardson, A.R.; Fang, F.C.; et al. Regulation of hemolysin expression and virulence of Staphylococcus aureus by a serine/threonine kinase and phosphatase. PLoS ONE 2010, 5, e11071. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Chambers, H.F. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J. Clin. Investig. 2009, 119, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Wikler, M.A. Performance standards for antimicrobial susceptibility testing: 18th informational supplement M100-Sl8. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Pankey, G.; Ashcraft, D.; Kahn, H.; Ismail, A. Time-kill assay and Etest evaluation for synergy with polymyxin B and fluconazole against Candida glabrata. Antimicrob. Agents Chemother. 2014, 58, 5795–5800. [Google Scholar] [CrossRef] [PubMed]
- Vasicek, E.M.; Berkow, E.L.; Bruno, V.M.; Mitchell, A.P.; Wiederhold, N.P.; Barker, K.S.; Rogers, P.D. Disruption of the transcriptional regulator Cas5 results in enhanced killing of Candida albicans by Fluconazole. Antimicrob. Agents Chemother. 2014, 58, 6807–6818. [Google Scholar] [CrossRef] [PubMed]
- Seepersaud, R.; Needham, R.H.; Kim, C.S.; Jones, A.L. Abundance of the delta subunit of RNA polymerase is linked to the virulence of Streptococcus agalactiae. J. Bacteriol. 2006, 188, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Memmi, G.; Hernandez, D.; Bard, J.; Beaume, M.; Gill, S.; Francois, P.; Cheung, A.L. Whole-genome sequencing of Staphylococcus aureus strain RN4220, a key laboratory strain used in virulence research, identifies mutations that affect not only virulence factors but also the fitness of the strain. J. Bacteriol. 2011, 193, 2332–2335. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Gill, S.R.; Chang, R.F.; Phan, T.H.; Chen, J.H.; Davidson, M.G.; Lin, F.; Lin, J.; Carleton, H.A.; Carleton, E.F.; et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006, 367, 731–739. [Google Scholar] [CrossRef]
- Lagorce, D.; Sperandio, O.; Galons, H.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs2: Free ADME/tox filtering tool to assist drug discovery and chemical biology projects. BMC Bioinform. 2008. [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchya, H.; Takahashi, Y.; Masuma, R. A new alkaloid AM-2282 OF Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. (Tokyo) 1977, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.B.; Hayes, L.D.; Brown, K.; Hoo, E.C.; Ethier, K.A. CDC National Health Report: Leading Causes of Morbidity and Mortality and Associated Behavioral Risk and Protective Factors—United States, 2005–2013. Available online: http://www.cdc.gov/mmwr/preview/mmwrhtml/su6304a2.htm (accessed on 20 October 2015).
- Pensinger, D.A.; Aliota, M.T.; Schaenzer, A.J.; Boldon, K.M.; Ansari, I.U.; Vincent, W.J.; Knight, B.; Reniere, M.L.; Striker, R.; Sauer, J.-D. Selective pharmacologic inhibition of a PASTA kinase increases Listeria monocytogenes susceptibility to beta-lactam antibiotics. Antimicrob. Agents Chemother. 2014, 58, 4486–4494. [Google Scholar] [CrossRef] [PubMed]
- Magnet, S.; Hartkoorn, R.C.; Szekely, R.; Pato, J.; Triccas, J.A.; Schneider, P.; Szántai-Kis, C.; Orfi, L.; Chambon, M.; Banfi, D.; et al. Leads for antitubercular compounds from kinase inhibitor library screens. Tuberculosis (Edinburgh) 2010, 90, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Lougheed, K.E.; Osborne, S.A.; Saxty, B.; Whalley, D.; Chapman, T.; Bouloc, N.; Chugh, J.; Nott, T.J.; Patel, D.; Spivey, V.L.; et al. Effective inhibitors of the essential kinase PknB and their potential as anti-mycobacterial agents. Tuberculosis (Edinburgh) 2011, 91, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Turapov, O.; Loraine, J.; Jenkins, C.H.; Barthe, P.; McFeely, D.; Forti, F.; Daniela, G.; Dusan, H.; Mijoon, L.; Andrew, R.; et al. The external PASTA domain of the essential serine/threonine protein kinase PknB regulates mycobacterial growth. Open Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, L.; Vo, A.; Silvestroni, A.; Rubens, C.E. Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Mol. Microbiol. 2006, 62, 941–957. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vornhagen, J.; Burnside, K.; Whidbey, C.; Berry, J.; Qin, X.; Rajagopal, L. Kinase Inhibitors that Increase the Sensitivity of Methicillin Resistant Staphylococcus aureus to β-Lactam Antibiotics. Pathogens 2015, 4, 708-721. https://doi.org/10.3390/pathogens4040708
Vornhagen J, Burnside K, Whidbey C, Berry J, Qin X, Rajagopal L. Kinase Inhibitors that Increase the Sensitivity of Methicillin Resistant Staphylococcus aureus to β-Lactam Antibiotics. Pathogens. 2015; 4(4):708-721. https://doi.org/10.3390/pathogens4040708
Chicago/Turabian StyleVornhagen, Jay, Kellie Burnside, Christopher Whidbey, Jessica Berry, Xuan Qin, and Lakshmi Rajagopal. 2015. "Kinase Inhibitors that Increase the Sensitivity of Methicillin Resistant Staphylococcus aureus to β-Lactam Antibiotics" Pathogens 4, no. 4: 708-721. https://doi.org/10.3390/pathogens4040708
APA StyleVornhagen, J., Burnside, K., Whidbey, C., Berry, J., Qin, X., & Rajagopal, L. (2015). Kinase Inhibitors that Increase the Sensitivity of Methicillin Resistant Staphylococcus aureus to β-Lactam Antibiotics. Pathogens, 4(4), 708-721. https://doi.org/10.3390/pathogens4040708





