Studies of Immune Responses in Candida vaginitis
Abstract
:1. Introduction
2. Summary of Our Studies to Identify the Proper Targets for New Strategies for Vaccination or Immunotherapy of Vaginal Candidiasis
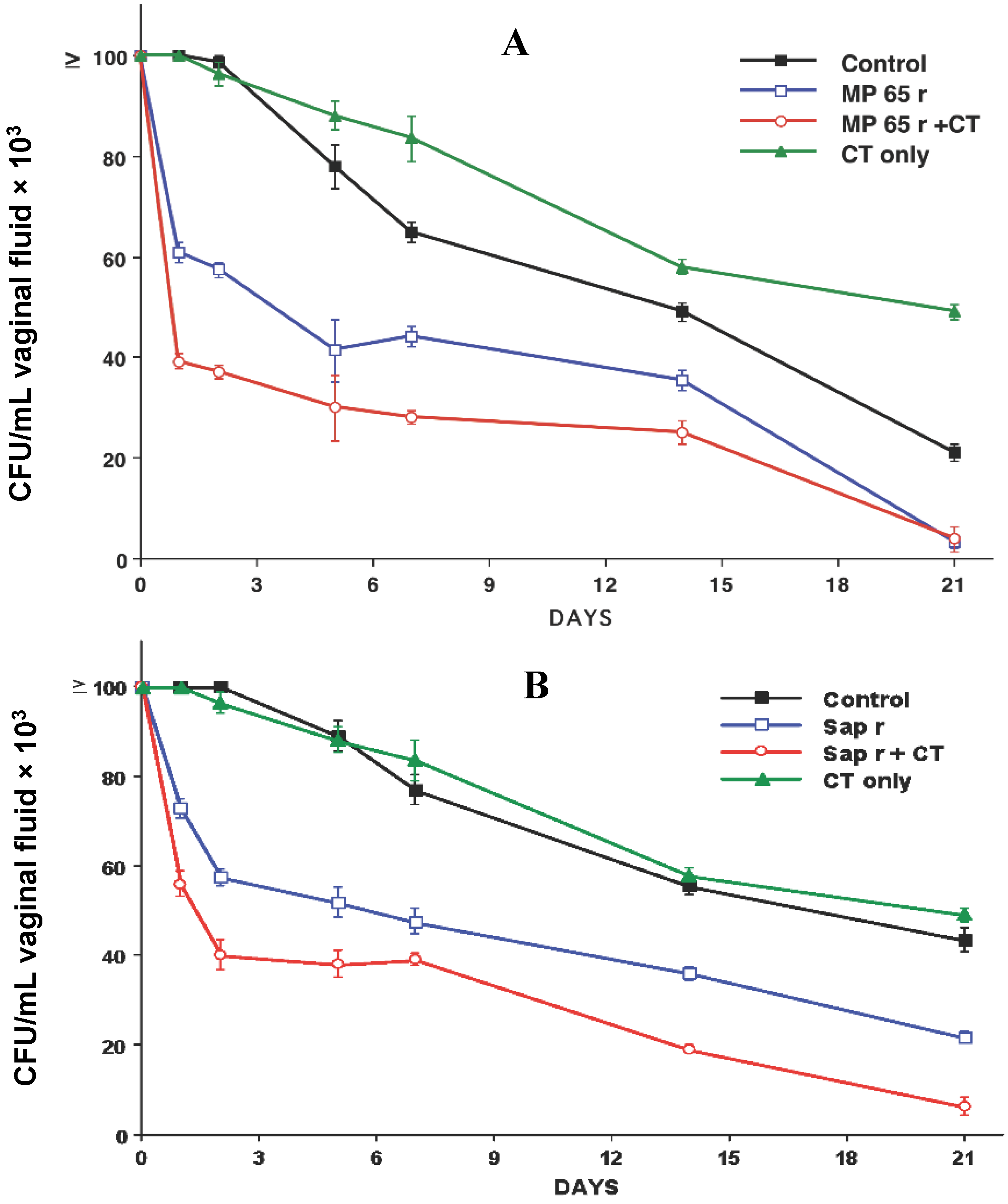
3. Summary of Our Studies for the Development of a Protective Vaccine for C. albicans Vaginal Infections
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nucci, M.; Marr, K.A. Emerging Fungal Diseases. Clin. Inf. Dis. 2005, 41, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Pathogenesis of Recurrent Vulvovaginal Candidiasis. Curr. Infect. Dis. Rep. 2002, 4, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Nyirjesy, P.; Sobel, J.D. Vuvovaginal candidiasis. Obstet. Gynecol. Clin. N. Am. 2003, 30, 671–684. [Google Scholar] [CrossRef]
- Sobel, J.D.; Wiesenfield, H.C.; Martens, M.; Danna, P.; Hooton, T.M.; Rompalo, A.; Sperling, M.; Livengood, C., 3rd; Horowitz, B.; von Thron, J.; et al. Maintenance therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004, 351, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Management of recurrent vulvovaginal candidiasis: Unresolved issues. Curr. Infect. Dis. Rep. 2006, 8, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Muraglia, R.; Dietz, J.P.; Sobel, J.D.; Wagner, J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: Results from an internet panel survey. J. Low. Genit. Tract Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Bauters, T.G.; Dhont, M.A.; Temmerman, M.I.; Nelis, H.J. Prevalence of vulvovaginal candidiasis and susceptibility to fluconazole in women. Am. J. Obstet. Gynecol. 2002, 187, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Cernicka, J.; Subik, J. Resistance mechanisms in fluconazole resistant Candida albicans isolates from vaginal candidiasis. Int. J. Antimicrob. Agents 2006, 27, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.T.; Mullings, A.M.; Rainford, L.; Miller, A. The epidemiology of mycotic vulvovaginitis and the use of antifungal agents in suspected mycotic vulvovaginitis and its implications for clinical practice. West Indian Med. J. 2005, 54, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.S.; Galask, R.P.; Messer, S.A.; Hollis, R.J.; Diekema, D.J.; Pfaller, M.A. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J. Clin. Microbiol. 2005, 43, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Ringdahl, E.N. Recurrent vulvovaginal candidiasis. Mol. Med. 2006, 103, 165–168. [Google Scholar]
- Ventolini, G.; Baggish, M.S.; Walsh, P.M. Vulvovaginal candidiasis from non-albicans species: retrospective study of recurrence rate after fluconazole therapy. J. Reprod. Med. 2006, 51, 475–478. [Google Scholar] [PubMed]
- Shahid, Z.; Sobel, J.D. Reduced fluconazole susceptibility of Candida albicans isolates in women with recurrent vulvovaginal candidiasis: Effects of long-term fluconazole therapy. Diagn. Microbiol. Infect. Dis. 2009, 64, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Marchaim, D.; Lemanek, L.; Bheemreddy, S.; Kaye, K.S.; Sobel, J.D. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet. Gynecol. 2012, 20, 1407–1414. [Google Scholar]
- Kennedy, M.A.; Sobel, J.D. Vulvovaginal Candidiasis Caused by Non-albicans Candida Species: New Insights. Curr. Infect. Dis. Rep. 2010, 12, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Chronic mucocutaneous candidosis. In Candida and Candidosis; University Park Press: Baltimore, MD, USA, 1988; pp. 104–110. [Google Scholar]
- De Bernardis, F.; Cassone, A.; Sturtevant, J.; Calderone, R. Expression of Candida albicans SAP1 and SAP2 in experimental vaginitis. Infect. Immun. 1995, 6, 1887–1892. [Google Scholar]
- De Bernardis, F.; Sullivan, P.A.; Cassone, A. Aspartyl proteinases of Candida albicans and their role in pathogenicity. Med. Mycol. 2001, 39, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Calderone, R.; Fonzi, W. Virulence factors of Candida albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef]
- Hube, B. From commensal to pathogen: stage and tissue specific gene expression of Candida albicans. Curr. Opin. Microbiol. 2004, 7, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [PubMed]
- Thewes, S.; Kretschmar, M.; Park, H.; Schaller, M.; Filler, S.G.; Hube, B. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol. Microbiol. 2007, 63, 1606–1628. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 15, 119–128. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Arancia, S.; Morelli, L.; Hube, B.; Sanglard, D.; Schafer, W.; Cassone, A. Evidence that members of the secretory aspartyl proteinases gene family (SAP), in particular SAP2, are virulence factors for Candida vaginitis. J. Infect. Dis. 1999, 179, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, L.L. The ALS gene family of Candida albicans. Trends Microbiol. 2001, 9, 176–180. [Google Scholar] [CrossRef]
- Kumamoto, C.A.; Vinces, M.D. Contribution of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell. Microbiol. 2005, 7, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, P. Adhesion in Candida spp. Cell. Microbiol. 2002, 4, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.J.; Ibrahim, A.S.; Avanesian, V.; Fu, Y.; Myers, C.; Phan, Q.T.; Filler, S.G.; Yeaman, M.R.; Edwards, J.E. Efficacy of the anti-Candida rAls3p-N or Als1p-N vaccines against disseminated and mucosal candidiasis. J. Infect. Dis. 2006, 194, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Zordan, R.; Cormack, B. Adhesins on Opportunistic Fungal Pathogens. In Candida and Candidiasis; Calderone, R.A., Clancy, C.J., Eds.; ASM Press: Washington, DC, USA, 2012; pp. 243–259. [Google Scholar]
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Wilson, D.; Wächtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Saville, S.P.; Lazell, A.L.; Monteagudo, C.; Lopez-Ribot, J.L. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell. 2003, 2, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Sandini, S.; la Valle, R.; de Bernardis, F.; Macri, C.; Cassone, A. The 65-kilodalton mannoprotein gene of Candida albicans encodes a putative glucanase adhesin required for hyphal morphogenesis and experimental pathogenicity. Cell. Microbiol. 2007, 9, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Molinari, A.; Boccanera, M.; Stringaro, A.; Robert, R.; Senet, J.M.; Arancia, G.; Cassone, A. Modulation of cell surface-associated mannoprotein antigen expression in experimental candidal vaginitis. Infect. Immun. 1994, 62, 509–519. [Google Scholar] [PubMed]
- De Bernardis, F.; Liu, H.; O’Mahony, R.; la Valle, R.; Bartollino, S.; Sandini, S.; Grant, S.; Brewis, N.; Tomlinson, I.; Basset, R.C.; et al. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J. Infect. Dis. 2007, 195, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.; Kholer, J.R.; di Domenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [PubMed]
- Peters, B.M.; Palmer, G.E.; Nash, A.K.; Lilly, E.A.; Fidel, P.L., Jr.; Noverr, M.C. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect. Immun. 2014, 82, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Murciano, C.; Runglall, M.; Islam, A.; Thavaraj, S.; Naglik, J.R. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A.; Boccanera, M.; Adriani, D.; Santoni, G.; de Bernardis, F. Rat clearing a vagina infection by Candida albicans acquired specific antibody-mediated resistance to vaginal reinfection. Infect. Immun. 1995, 63, 2619–2625. [Google Scholar] [PubMed]
- Elitsur, Y.; Jackman, S.; Neace, C.; Keerthy, S.; Liu, X.; Dosescu, J.; Moshier, J.A. Gen. Diagn. Pathol. 1998, 143, 271–277.
- De Bernardis, F.; Boccanera, M.; Adriani, D.; Spreghini, E.; Santoni, G.; Cassone, A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 1997, 65, 3399–3405. [Google Scholar] [PubMed]
- De Bernardis, F.; Santoni, G.; Boccanera, M.; Spreghini, E.; Adriani, D.; Morelli, L.; Cassone, A. Local anticandidal immune responses in a rat model of vaginal infection by and protection, Candida albicans. Infect. Immun. 2000, 68, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Boccanera, M.; Adriani, D.; Lucciarini, R.; Amantini, C.; Morrone, S.; Cassone, A.; de Bernardis, F. Immune cell-mediated protection against vaginal candidiasis: Evidence for a mayor role of vaginal CD4+ T cells and possible participation of other local lymphocyte effectors. Infect. Immun. 2002, 70, 4791–4797. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Santoni, G.; Boccanera, M.; Lucciarini, R.; Arancia, S.; Sandini, S.; Amantini, C.; Cassone, A. Protection against rat vaginal Candidiasis by adoptive transfer of vaginal B lymphocytes. FEMS Yeast Res. 2010, 10, 432–440. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Lucciarini, R.; Boccanera, M.; Amantini, C.; Arancia, S.; Morrone, S.; Mosca, M.; Cassone, A.; Santoni, G. Phenotypic and functional characterization of vaginal dendritic cells in a rat model of Candida albicans vaginitis. Infect. Immun. 2006, 74, 4282–4294. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A. Fungal vaccines: Real progress from real challenges. Lancet Infect. Dis. 2008, 8, 114–124. [Google Scholar] [CrossRef]
- Cassone, A.; Casadevall, A. Recent progress in vaccines against fungal diseases. Curr. Opin. Microbiol. 2012, 15, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A. Development of vaccines for Candida albicans: Fighting a skilled transformer. Nat. Rev. Microbiol. 2013, 11, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E., Jr. Fungal cell wall vaccines: an update. J. Med. Microbiol. 2012, 61, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, R.G.; Carvalho, A.; Romani, L. From memory to antifungal vaccine design. Trends Immunol. 2012, 33, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Moragues, M.D.; Rementeria, A.; Sevilla, M.J.; Eraso, E.; Quindos, G. Candida antigens and immune responses: Implications for a vaccine. Expert Rev. Vaccines 2014, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Yano, J.; Noverr, M.C.; Fidel, P.L., Jr. Candida vaginitis: When Opportunism Knocks, the Host Responds. PLoS Pathog. 2014, 10, e1003965. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Boccanera, M.; Adriani, D.; Girolamo, A.; Cassone, A. Intravaginal and intranasal immunizations are equally effective in inducing vaginal antibodies and conferring protection against vaginal candidiasis. Infect. Immun. 2002, 70, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Sandini, S.; la Valle, R.; Deaglio, S.; Malavasi, F.; Cassone, A.; de Bernardis, F. A highly immunogenic recombinant and truncated protein of the secreted aspartic proteases family (rSap2t) of Candida albicans as a mucosal anticandidal vaccine. FEMS Immunol. Med. Microbiol. 2011, 62, 215–224. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Amacker, M.; Arancia, S.; Sandini, S.; Gremion, C.; Zurbriggen, R.; Moser, C.; Cassone, A. A virosomal vaccine against candidal vaginitis: Immunogenicity, efficacy and safety profile in animal models. Vaccine 2012, 30, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- PEV7 Clinical Trial. Available online: http://www.clinicaltrials.gov/ct2/show/NCT01067131 (accessed on 8 October 2015).
- ClinicalTrials.gov Identifier: NCT01926028. Available online: https://clinicaltrials.gov/ct2/show/NCT01926028 (accessed on 8 October 2015).
- Luo, G.; Ibrahim, A.S.; Spellberg, B.; Nobile, C.J.; Mitchell, A.P.; Fu, Y. Candida albicans Hyr1p Confers Resistance to Neutrophil Killing and Is a Potential Vaccine Target. J. Infect. Dis. 2010, 201, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ibrahim, A.S.; French, S.W.; Edwards, J.E., Jr.; Fu, Y. Active and Passive Immunization with rHyr1p-N Protects Mice against Hematogenously Disseminated Candidiasis. PLoS ONE 2011, 6, e25909. [Google Scholar] [CrossRef] [PubMed]
- Nitz, M.; Ling, C.; Otter, A.; Cutler, J.E.; Bundle, D.R. The Unique Solution Structure and Immunochemistry of the Candida albicans β-1,2-Mannopyranan Cell Wall Antigens. J. Biol. Chem. 2002, 277, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Magliani, W.; Conti, S.; de Bernardis, F.; Cassone, A.; Polonelli, L. New immunotherapeutic strategies to control vaginal candidiasis. Trends Mol. Med. 2002, 8, 121–126. [Google Scholar] [CrossRef]
- Ostrowski-Zeichner, L.; Casadevall, A.; Galgiani, J.N.; Odds, F.C.; Rex, J.H. An insight into the antifungal pipeline: selected new molecules and beyond. Nat. Rev. Drug Discov. 2010, 9, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.E.; Deepe, G.S.; Klein, D. Advances in combating fungal diseases: Vaccines on the threshold. Nat. Rev. Microbiol. 2007, 5, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.S.; White, C.J.; Ibrahim, A.S.; Filler, S.G.; Fu, Y.; Yeaman, M.R.; Edwards, J.E., Jr.; Hennessey, J.P., Jr. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012, 30, 7594–7600. [Google Scholar] [CrossRef] [PubMed]
- Saville, S.P.; Lazzell, A.J.; Chaturvedi, A.; Monteagudo, C.; Lopez-Ribot, J.L. Efficacy of a genetically engineered Candida albicans tet-NRG1strain as an experimental live attenuated vaccine against hemathogeneously disseminated candidiasis. Clin. Vaccine Immunol. 2009, 16, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13526–13531. [Google Scholar] [CrossRef] [PubMed]
- Bromuro, C.; Romano, M.; Chiani, P.; Berti, F.; Tontini, M.; Proietti, D.; Mori, E.; Torosantucci, A.; Costantino, P.; Rappuoli, R.; et al. Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine 2010, 28, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A.; Cauda, R. Candida and candidiasis in HIV-infected subjects. Where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS 2012, 26, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bernardis, F.; Arancia, S.; Sandini, S.; Graziani, S.; Norelli, S. Studies of Immune Responses in Candida vaginitis. Pathogens 2015, 4, 697-707. https://doi.org/10.3390/pathogens4040697
De Bernardis F, Arancia S, Sandini S, Graziani S, Norelli S. Studies of Immune Responses in Candida vaginitis. Pathogens. 2015; 4(4):697-707. https://doi.org/10.3390/pathogens4040697
Chicago/Turabian StyleDe Bernardis, Flavia, Silvia Arancia, Silvia Sandini, Sofia Graziani, and Sandro Norelli. 2015. "Studies of Immune Responses in Candida vaginitis" Pathogens 4, no. 4: 697-707. https://doi.org/10.3390/pathogens4040697
APA StyleDe Bernardis, F., Arancia, S., Sandini, S., Graziani, S., & Norelli, S. (2015). Studies of Immune Responses in Candida vaginitis. Pathogens, 4(4), 697-707. https://doi.org/10.3390/pathogens4040697




