Sociability Linked to Reproductive Status Affects Intestinal Parasite Infections in the Red-Billed Chough
Abstract
1. Introduction
2. Materials and Methods
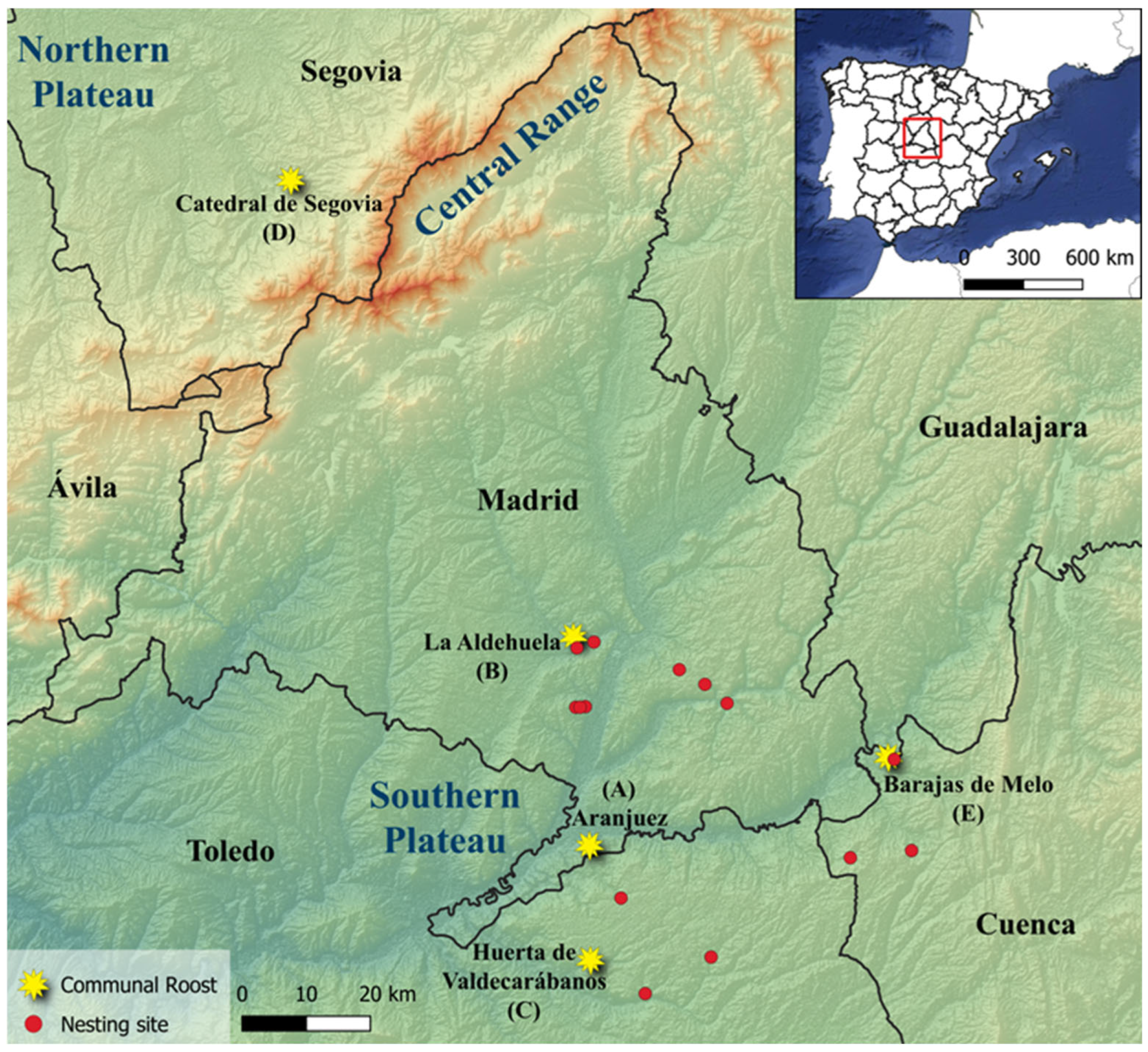
2.1. Study Areas
2.2. Fieldwork
2.3. Parasitological Analysis
2.4. Statistical Analysis
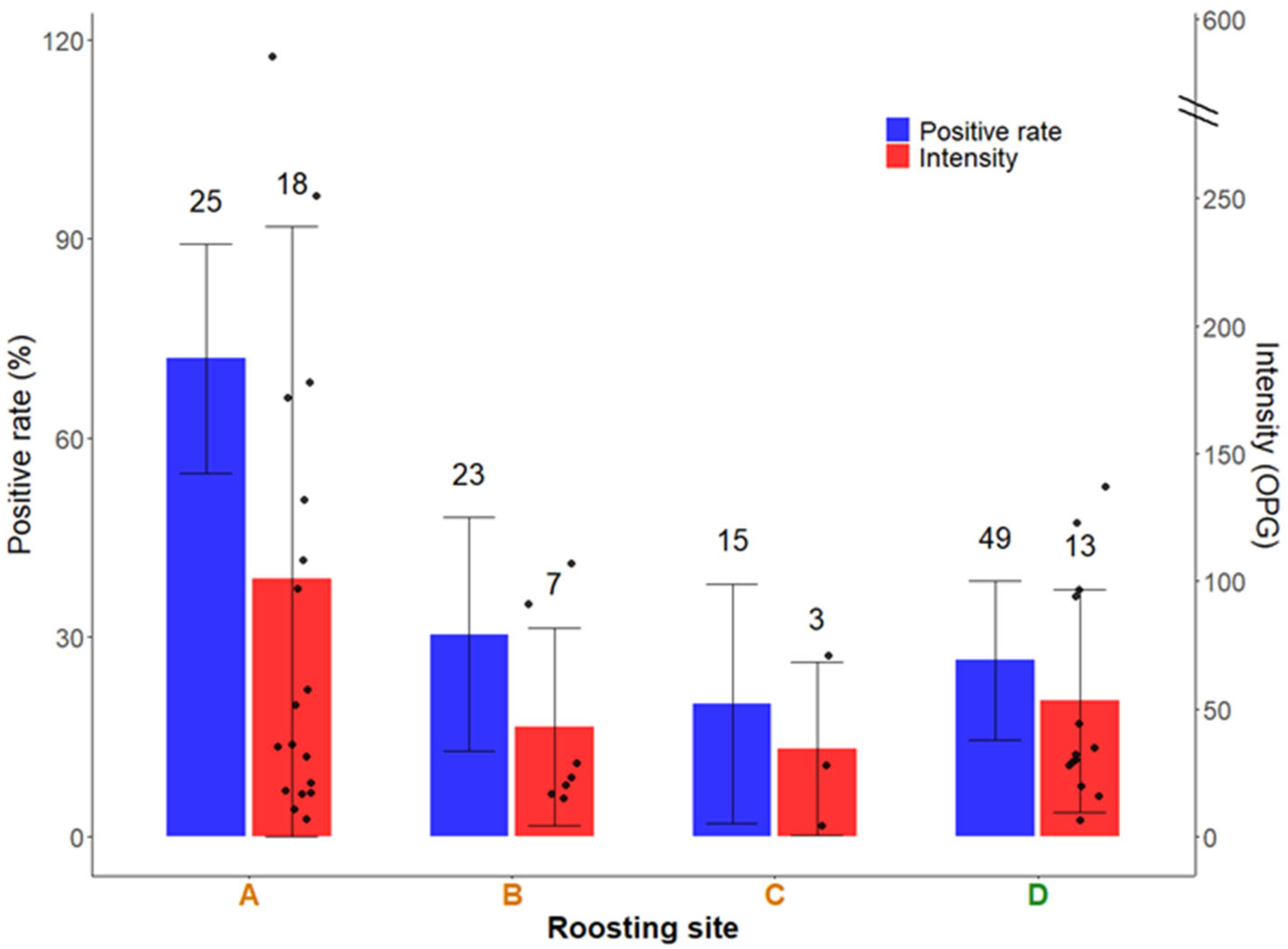
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kappeler, P.M.; Cremer, S.; Nunn, C.L. Sociality and Health: Impacts of Sociality on Disease Susceptibility and Transmission in Animal and Human Societies. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140116. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Parasites and Their Social Hosts. Trends Parasitol. 2017, 33, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Moore, J. Parasites and the Behavior of Animals; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Ward, A.; Webster, M. Sociality: The Behaviour of Group-Living Animals; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Lindholm, A.K.; Clayton, D.H.; Moore, J. Host-Parasite Evolution: General Principles and Avian Models. J. Anim. Ecol. 1997, 66, 913. [Google Scholar] [CrossRef]
- Botzler, R.G.; Brown, R.N. Foundations of Wildlife Diseases; University of California Press: Oakland, CA, USA, 2014. [Google Scholar]
- Schmid-Hempel, P. Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics, 2nd ed.; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Friend, M.; McLean, R.G.; Dein, F.J. Disease Emergence in Birds: Challenges for the Twenty-First Century. Auk 2001, 118, 290–303. [Google Scholar] [CrossRef]
- Gottdenker, N.L.; Streicker, D.G.; Faust, C.L.; Carroll, C.R. Anthropogenic Land Use Change and Infectious Diseases: A Review of the Evidence. Ecohealth 2014, 11, 619–632. [Google Scholar] [CrossRef]
- Riley, S.P.; Serieys, L.E.; Moriarty, J.G. Infectious Disease and Contaminants in Urban Wildlife: Unseen and Often Overlooked Threats. In Urban Wildlife Conservation; Springer: Boston, MA, USA, 2014; pp. 175–215. [Google Scholar]
- Scott, M.E. The Impact of Infection and Disease on Animal Populations: Implications for Conservation Biology. Conserv. Biol. 1988, 2, 40–56. [Google Scholar] [CrossRef]
- Cleaveland, S.; Hess, G.R.; Dobson, A.P.; Laurenson, M.K.; McCallum, H.I.; Roberts, M.G.; Woodroffe, R. The Role of Pathogens in Biological Conservation. In The Ecology of Wildlife Diseases; Hudson, P.J., Rizzoli, A., Grenfell, B.T., Heesterbeek, H., Dobson, A.P., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 139–150. [Google Scholar]
- Smith, K.F.; Sax, D.F.; Lafferty, K.D. Evidence for the Role of Infectious Disease in Species Extinction and Endangerment. Conserv. Biol. 2006, 20, 1349–1357. [Google Scholar] [CrossRef]
- Thompson, R.C.A.; Lymbery, A.J.; Smith, A. Parasites, Emerging Disease and Wildlife Conservation. Int. J. Parasitol. 2010, 40, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Friend, M.; Franson, J.C. Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds; Biological Resources Division Information and Technology Report 1999-001; U.S. Geological Survey: Reston, VA, USA, 1999.
- Isomursu, M.; Rätti, O.; Helle, P.; Hollmén, T. Sex and Age Influence Intestinal Parasite Burden in Three Boreal Grouse Species. J. Avian Biol. 2006, 37, 516–522. [Google Scholar] [CrossRef]
- Bandelj, P.; Blagus, R.; Trilar, T.; Vengust, M.; Rataj, A.V. Influence of Phylogeny, Migration and Type of Diet on the Presence of Intestinal Parasites in the Faeces of European Passerine Birds (Passeriformes). Wildl. Biol. 2015, 21, 227–233. [Google Scholar] [CrossRef]
- Blanco, G.; Cardells, J.; Garijo-Toledo, M.M. Supplementary Feeding and Endoparasites in Threatened Avian Scavengers: Coprologic Evidence from Red Kites in Their Wintering Stronghold. Environ. Res. 2017, 155, 22–30. [Google Scholar] [CrossRef]
- Arneberg, P.; Skorping, A.; Grenfell, B.; Read, A.F. Host Densities as Determinants of Abundance in Parasite Communities. Proc. R. Soc. B Biol. Sci. 1998, 265, 1283–1289. [Google Scholar] [CrossRef]
- Ardia, D.R.; Schat, K.A. Ecoimmunology. In Avian Immunology; Davison, F., Kaspers, B., Schat, K.A., Eds.; Academic Press: London, UK, 2008; pp. 421–441. [Google Scholar]
- Wascher, C.A.F.; Bauer, A.C.; Holtmann, A.R.; Kotrschal, K. Environmental and Social Factors Affecting the Excretion of Intestinal Parasite Eggs in Graylag Geese. Behav. Ecol. 2012, 23, 1276–1283. [Google Scholar] [CrossRef]
- Poulin, R. Modification of Host Social Networks by Manipulative Parasites. Behaviour. 2018, 155, 671–688. [Google Scholar] [CrossRef]
- Stockmaier, S.; Ulrich, Y.; Albery, G.F.; Cremer, S.; Lopes, P.C. Behavioural Defences against Parasites across Host Social Structures. Funct. Ecol. 2023, 37, 809–820. [Google Scholar] [CrossRef]
- Taborsky, M.; Cant, M.A.; Komdeur, J. The Evolution of Social Behaviour; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Beauchamp, G. The Evolution of Communal Roosting in Birds: Origin and Secondary Losses. Behav. Ecol. 1999, 10, 675–687. [Google Scholar] [CrossRef]
- Janousek, W.M.; Marra, P.P.; Kilpatrick, A.M. Avian Roosting Behavior Influences Vector-Host Interactions for West Nile Virus Hosts. Parasites Vectors. 2014, 7, 399. [Google Scholar] [CrossRef]
- Laughlin, A.J.; Hall, R.J.; Taylor, C.M. Ecological Determinants of Pathogen Transmission in Communally Roosting Species. Theor. Ecol. 2019, 12, 225–235. [Google Scholar] [CrossRef]
- Briard, L.; Ezenwa, V.O. Parasitism and Host Social Behaviour: A Meta-Analysis of Insights Derived from Social Network Analysis. Anim. Behav. 2021, 172, 171–182. [Google Scholar] [CrossRef]
- Silk, J.B. The Adaptive Value of Sociality in Mammalian Groups. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 539–559. [Google Scholar] [CrossRef] [PubMed]
- Wascher, C.A.F.; Canestrari, D.; Baglione, V. Affiliative Social Relationships and Coccidian Oocyst Excretion in a Cooperatively Breeding Bird Species. Anim. Behav. 2019, 158, 121–130. [Google Scholar] [CrossRef]
- Wascher, C.A.F. Association between Social Factors and Gastrointestinal Parasite Product Excretion in a Group of Non-Cooperatively Breeding Carrion Crows. Behav. Ecol. Sociobiol. 2021, 75, 30. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Red-Billed Chough Pyrrhocorax pyrrhocorax. Available online: https://datazone.birdlife.org/species/factsheet/red-billed-chough-pyrrhocorax-pyrrhocorax (accessed on 23 July 2025).
- Blanco, G.; Merino, S.; Tella, J.L.; Fargallo, J.A.; Gajón, A. Hematozoa in Two Populations of the Threatened Red-Billed Chough in Spain. J. Wildl. Dis. 1997, 33, 642–645. [Google Scholar] [CrossRef][Green Version]
- Bignal, E.; Bignal, S.; Still, E. Gapeworm Infection in Choughs. Ringing Migr. 1987, 8, 56–58. [Google Scholar] [CrossRef]
- Meyer, R.M.; Simpson, V.R. Gapeworm Infection in Choughs Pyrrhocorax pyrrhocorax: Further Evidence. Bird Study 1988, 35, 223–225. [Google Scholar] [CrossRef]
- Cuevas, J.A.; Blanco, G. Chova Piquirroja—Pyrrhocorax pyrrhocorax. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Morales, M.B., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2015. [Google Scholar]
- Pennycott, T.; Bignal, E.; McCracken, D. Significant Helminthiasis, Ocular Defects and Other Disorders in an Endangered Population of Red-Billed Choughs (Pyrrhocorax pyrrhocorax): A Descriptive Observational Study. Vet. Rec. 2025, 196, e4961. [Google Scholar] [CrossRef]
- Reid, J.M.; Bignal, E.; Bignal, S.; McCracken, D.I.; Fenn, S.R.; Trask, A.E.; Monaghan, P. Integrating Advances in Population and Evolutionary Ecology with Conservation Strategy through Long-Term Studies of Red-Billed Choughs. J. Anim. Ecol. 2021, 91, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Fargallo, J.A.; Tella, J.L.; Cuevas, J.A. Role of Buildings as Nest-Sites in the Range Expansion and Conservation of Choughs Pyrrhocorax pyrrhocorax in Spain. Biol. Conserv. 1997, 79, 117–122. [Google Scholar] [CrossRef]
- Blanco, G. La Chova Piquirroja en Edificios Históricos de Segovia: Un Modelo de Conservación del Patrimonio Natural y Artístico; Caja Segovia: Segovia, Spain, 2003. [Google Scholar]
- Blanco, G.; Sánchez-Marco, A.; Negro, J.J. Night Capture of Roosting Cave Birds by Neanderthals: An Actualistic Approach. Front. Ecol. Evol. 2021, 9, 733062. [Google Scholar] [CrossRef]
- Long, P.L.; Millard, B.J.; Joyner, L.P.; Norton, C.C. A Guide to Laboratory Techniques Used in the Study and Diagnosis of Avian Coccidiosis. Folia Vet. Lat. 1976, 6, 201–217. [Google Scholar] [PubMed]
- Thienpont, D.; Rochette, F.; Vanparijs, O.F.G. Diagnosing Helminthiasis by Coprological Examination; Janssen Research Foundation: Beerse, Belgium, 1986. [Google Scholar]
- Levecke, B.; Behnke, J.M.; Ajjampur, S.S.R.; Albonico, M.; Ame, S.M.; Charlier, J.; Geiger, S.M.; Hoa, N.T.V.; Ngassam, R.I.K.; Kotze, A.C.; et al. A Comparison of the Sensitivity and Fecal Egg Counts of the McMaster Egg Counting and Kato-Katz Thick Smear Methods for Soil-Transmitted Helminths. PLoS Neglected Trop. Dis. 2011, 5, e1201. [Google Scholar] [CrossRef]
- Joyner, L.P. The Identification and Diagnosis of Avian Coccidiosis. In Avian Coccidiosis; Long, P.L., Boorman, K.N., Freeman, B.M., Eds.; British Poultry Science: Edinburgh, UK, 1978; pp. 29–49. [Google Scholar]
- Duszynski, D.W.; Wilber, P.G. A Guideline for the Preparation of Species Descriptions in the Eimeriidae. J. Parasitol. 1997, 83, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Keckeisen, C.; Šujanová, A.; Himmel, T.; Matt, J.; Nedorost, N.; Chagas, C.R.F.; Weissenböck, H.; Harl, J. Isospora and Lankesterella Parasites (Eimeriidae, Apicomplexa) of Passeriform Birds in Europe: Infection Rates, Phylogeny, and Pathogenicity. Pathogens 2024, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic Species Co-Occurrence Analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Jovani, R.; Tella, J.L. Parasite Prevalence and Sample Size: Misconceptions and Solutions. Trends Parasitol. 2006, 22, 214–218. [Google Scholar] [CrossRef]
- Greiner, E.C. Isospora, Atoxoplasma and Sarcocystis. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 108–119. [Google Scholar]
- Levine, N.D. Perkinsus Gen. N. and Other New Taxa in the Protozoan Phylum Apicomplexa. J. Parasitol. 1978, 64, 549. [Google Scholar] [CrossRef]
- Poon, S.K.; Chew, W.K. Isospora corvi Ray, Shivnani, Oommen and Bhaskaran, 1952 from the Common House Crow (Corvus splendens Vieillot) of Selangor, Peninsular Malaysia. Folia Parasitol. 1991, 38, 201–207. [Google Scholar]
- Upton, S.J.; Langen, T.A.; Wright, T.F. A New Species of Isospora Schneider, 1881 (Apicomplexa: Eimeriidae) from the White-Throated Magpie Jay Calocitta Formosa (Passeriformes: Corvidae) from Costa Rica. Syst. Parasitol. 1995, 31, 195–199. [Google Scholar] [CrossRef]
- Liu, D.; Brice, B.; Elliot, A.; Ryan, U.; Yang, R. Isospora coronoideae n. sp. (Apicomplexa: Eimeriidae) from the Australian Raven (Corvus coronoides) (Passeriformes: Corvidae) (Linnaeus, 1758) in Western Australia. Parasitol. Res. 2019, 118, 2399–2408. [Google Scholar] [CrossRef]
- Dolnik, O.V.; Dolnik, V.R.; Bairlein, F. The Effect of Host Foraging Ecology on the Prevalence and Intensity of Coccidian Infection in Wild Passerine Birds. Ardea 2010, 98, 97–103. [Google Scholar] [CrossRef]
- Blanco, G.; Cuevas, J.A.; Frías, Ó.; del Barrio, J.L.G. A Shot in the Dark: Sport Hunting of Declining Corvids Promotes the Inadvertent Shooting of Threatened Red-Billed Choughs. J. Nat. Conserv. 2019, 52, 125739. [Google Scholar] [CrossRef]
- Still, E.; Monaghan, P.; Bignal, E. Social Structuring at a Communal Roost of Choughs Pyrrhocorax pyrrhocorax. Ibis 1987, 129, 398–403. [Google Scholar] [CrossRef]
- Svobodová, M. Isospora, Caryospora and Eimeria (Apicomplexa) from Passeriform Birds from Czech Republic. Acta Protozool. 1994, 33, 101–108. [Google Scholar]
- Parsa, F.; Bayley, S.; Bell, F.; Dodd, S.; Morris, R.; Roberts, J.; Wawman, D.; Clegg, S.R.; Dunn, J.C. Epidemiology of Protozoan and Helminthic Parasites in Wild Passerine Birds of Britain and Ireland. Parasitology 2023, 150, 297–310. [Google Scholar] [CrossRef]
- Gill, H.; Paperna, I. Proliferative Visceral Isospora (Atoxoplasmosis) with Morbid Impact on the Israeli Sparrow Passer domesticus biblicus Hartert, 1904. Parasitol. Res. 2008, 103, 493–499. [Google Scholar] [CrossRef]
- Terio, K.A.; Adkesson, M.J. Systemic Isosporosis in Passerine Birds. In Fowler’s Zoo and Wild Animal Medicine Current Therapy; Miller, R.E., Lamberski, N., Calle, P.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 9, pp. 454–458. [Google Scholar]
- Amaral, C.I.; de Oliveira, A.R.; Oliveira, E.S.; Lopes, M.C.; Lacerda, M.d.S.C.; de Carvalho, M.P.N.; Ecco, R. Systemic Isosporosis in Three Species of Passerine Birds. Vet. Res. Commun. 2025, 49, 222. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.T.; Thomas, N.J.; Hunter, D.B. Parasitic Diseases of Wild Birds; Wiley-Blackwell: Ames, IA, USA, 2009. [Google Scholar]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed.; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- McLaughlin, J.D. Cestodes. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 261–276. [Google Scholar]
- Banda, E.; Blanco, G. Strict Mate Fidelity and Reduced Breeding Dispersal of Widowed Red-Billed Choughs. Bird Study 2014, 61, 371–377. [Google Scholar] [CrossRef]
- Bignal, E.; Bignal, S.; McCracken, D. The Social Life of the Chough. Br. Wildl. 1997, 8, 373–383. [Google Scholar]
- Blanco, G.; Tella, J.L.; Torre, I. Traditional Farming and Key Foraging Habitats for Chough Pyrrhocorax pyrrhocorax Conservation in a Spanish Pseudosteppe Landscape. J. Appl. Ecol. 1998, 35, 232–239. [Google Scholar] [CrossRef]
- Blanco, G.; Tella, J.L. Temporal, Spatial and Social Segregation of Red-Billed Choughs between Two Types of Communal Roost: A Role for Mating and Territory Acquisition. Anim. Behav. 1999, 57, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Laiolo, P.; Fargallo, J.A. Linking Environmental Stress, Feeding Shifts and the ‘Island Syndrome’: A Nutritional Challenge Hypothesis. Popul. Ecol. 2014, 56, 203–216. [Google Scholar] [CrossRef]
- Trask, A.E.; Bignal, E.M.; McCracken, D.I.; Monaghan, P.; Piertney, S.B.; Reid, J.M.; Eizaguirre, C. Evidence of the Phenotypic Expression of a Lethal Recessive Allele under Inbreeding in a Wild Population of Conservation Concern. J. Anim. Ecol. 2016, 85, 879–891. [Google Scholar] [CrossRef]
- Morinha, F.; Bastos, R.; Carvalho, D.; Travassos, P.; Santos, M.; Blanco, G.; Bastos, E.; Cabral, J.A. A Spatially-Explicit Dynamic Modelling Framework to Assess Habitat Suitability for Endangered Species: The Case of Red-Billed Chough under Land Use Change Scenarios in Portugal. Biol. Conserv. 2017, 210, 96–106. [Google Scholar] [CrossRef][Green Version]
- Morinha, F.; Milá, B.; Dávila, J.A.; Fargallo, J.A.; Potti, J.; Blanco, G. The Ghost of Connections Past: A Role for Mainland Vicariance in the Isolation of an Insular Population of the Red-Billed Chough (Aves: Corvidae). J. Biogeogr. 2020, 47, 2567–2583. [Google Scholar] [CrossRef]
- Wenzel, M.A.; Webster, L.M.I.; Blanco, G.; Burgess, M.D.; Kerbiriou, C.; Segelbacher, G.; Piertney, S.B.; Reid, J.M. Pronounced Genetic Structure and Low Genetic Diversity in European Red-Billed Chough (Pyrrhocorax pyrrhocorax) Populations. Conserv. Genet. 2012, 13, 1213–1230. [Google Scholar] [CrossRef]
| Capillaria sp. | Other Nematodes | Cestodes | ||
|---|---|---|---|---|
| Roosting Site | n | Positive Rate (95% CI) HPG (Range) | Positive Rate (95% CI) HPG (Range) | Positive Rate (95% CI) HPG (Range) |
| Southern Plateau | ||||
| Aranjuez | 25 | 0.0 (0.0–10.9) | 16.0 (5.4–33.0) 48.0 ± 50.5 (7–134), n = 4 | 0.0 (0.0–10.9) |
| La Aldehuela | 23 | 0.0 (0.0–11.7) | 4.3 (0.2–18.4) 5.0, n = 1 | 0.0 (0.0–11.7) |
| Huerta de Valdecarábanos | 15 | 0.0 (0.0–17.1) | 6.7 (0.4–26.6) 6.0, n = 1 | 20.0 (5.7–43.1) 58.0 ± 18.8 (28–74), n = 3 |
| Barajas de Melo | 4 | 0.0 (0.0–45.1) | 0.0 (0.0–45.1) | 0.0 (0.0–45.1) |
| Northern Plateau | ||||
| Catedral de Segovia | 49 | 4.1 (0.7–12.3) 40.0 ± 28.3 (20–60), n = 2 | 0.0 (0.0–5.8) | 0.0 (0.0–5.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, G.; Garijo-Toledo, M.M.; González del Barrio, J.L.; Frías, Ó.; Montoya Ayala, R.; Palacios-Martínez, I. Sociability Linked to Reproductive Status Affects Intestinal Parasite Infections in the Red-Billed Chough. Pathogens 2025, 14, 915. https://doi.org/10.3390/pathogens14090915
Blanco G, Garijo-Toledo MM, González del Barrio JL, Frías Ó, Montoya Ayala R, Palacios-Martínez I. Sociability Linked to Reproductive Status Affects Intestinal Parasite Infections in the Red-Billed Chough. Pathogens. 2025; 14(9):915. https://doi.org/10.3390/pathogens14090915
Chicago/Turabian StyleBlanco, Guillermo, Maria M. Garijo-Toledo, José Luis González del Barrio, Óscar Frías, Raymundo Montoya Ayala, and Iñigo Palacios-Martínez. 2025. "Sociability Linked to Reproductive Status Affects Intestinal Parasite Infections in the Red-Billed Chough" Pathogens 14, no. 9: 915. https://doi.org/10.3390/pathogens14090915
APA StyleBlanco, G., Garijo-Toledo, M. M., González del Barrio, J. L., Frías, Ó., Montoya Ayala, R., & Palacios-Martínez, I. (2025). Sociability Linked to Reproductive Status Affects Intestinal Parasite Infections in the Red-Billed Chough. Pathogens, 14(9), 915. https://doi.org/10.3390/pathogens14090915









