Immunization with mRNA-LNPs Encoding Ornithodoros Argasid Tick Antigens Induces Humoral Immune Responses and Tick Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Ticks and Tick Material
2.2. Antigen Selection and Formulation of mRNA-Lipid Nanoparticles (mRNA-LNPs)
2.3. Generation of Recombinant Proteins
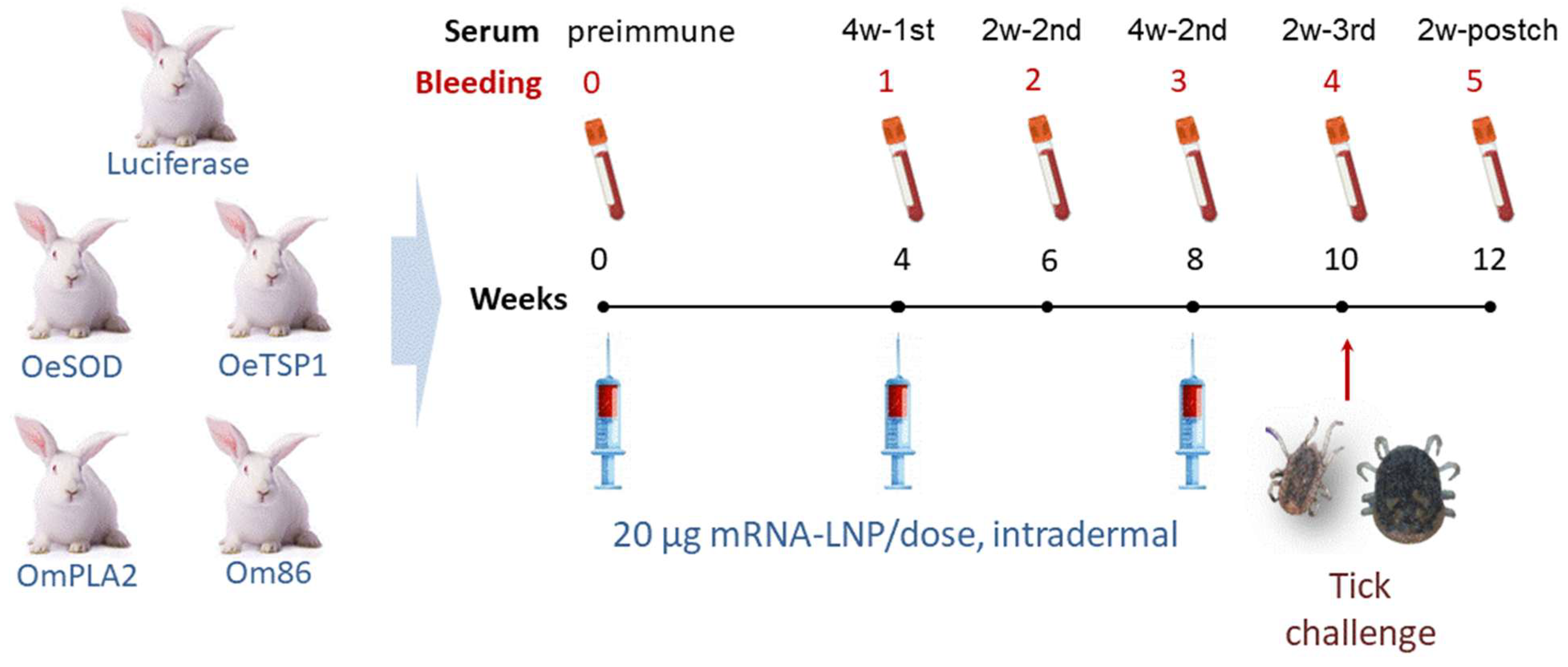
2.4. Immunisation of Rabbits
2.5. Humoral Response Analysis
2.6. Tick Challenge and Evaluation of Vaccine Efficacy
3. Results
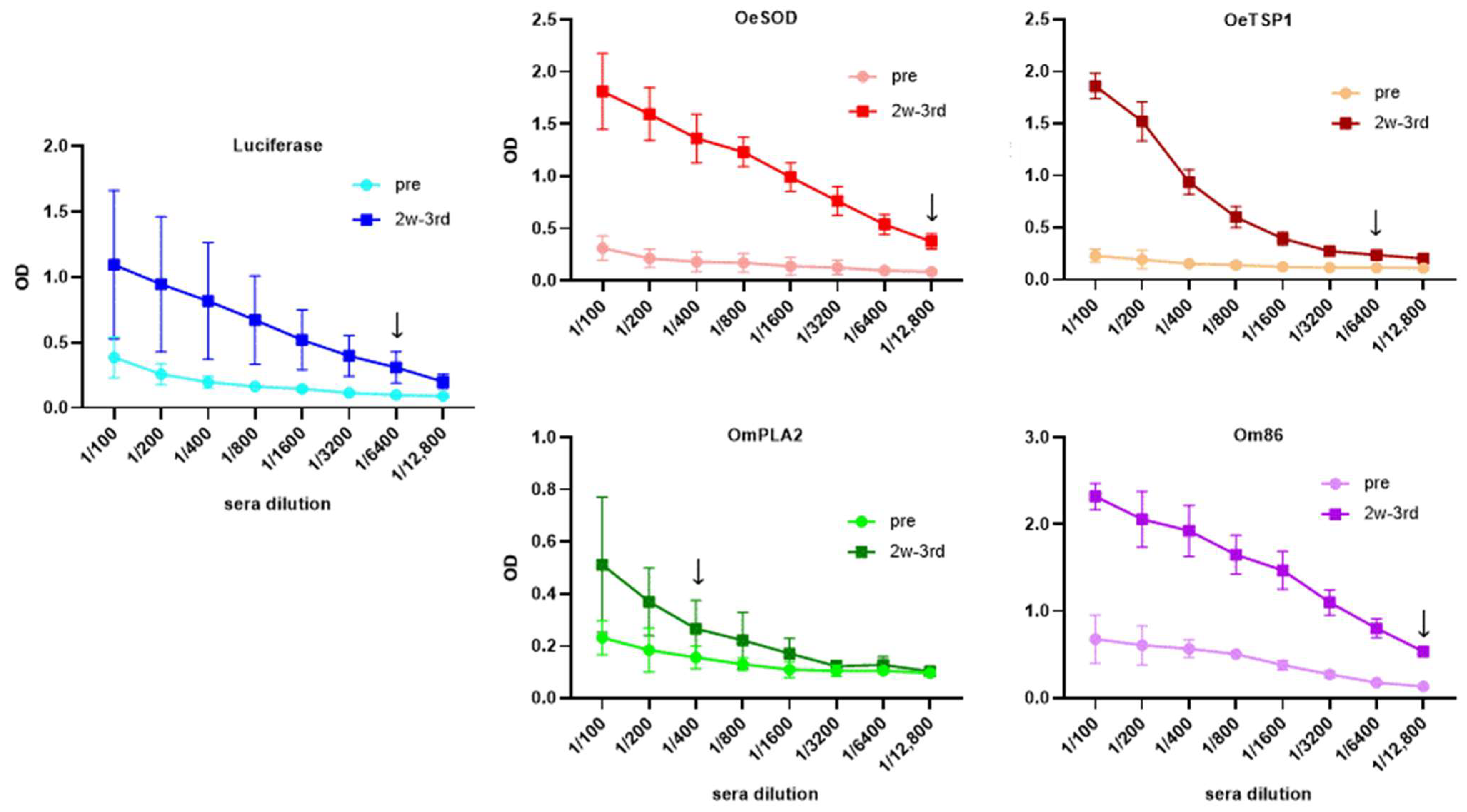
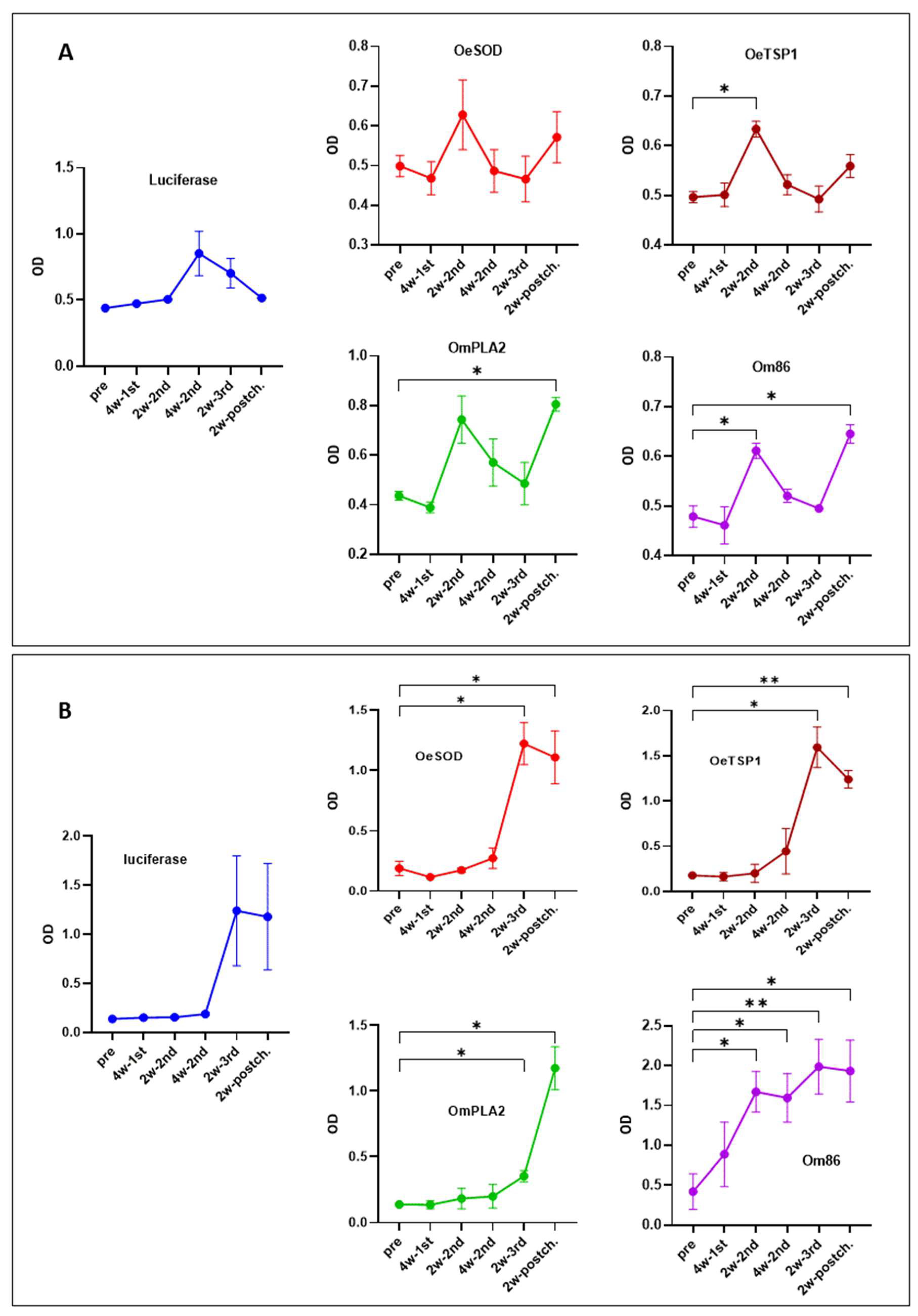
3.1. mRNA-LNPs Encoding Ornithodoros Antigens and Firefly Luciferase Induce Humoral Immune Responses in Rabbits
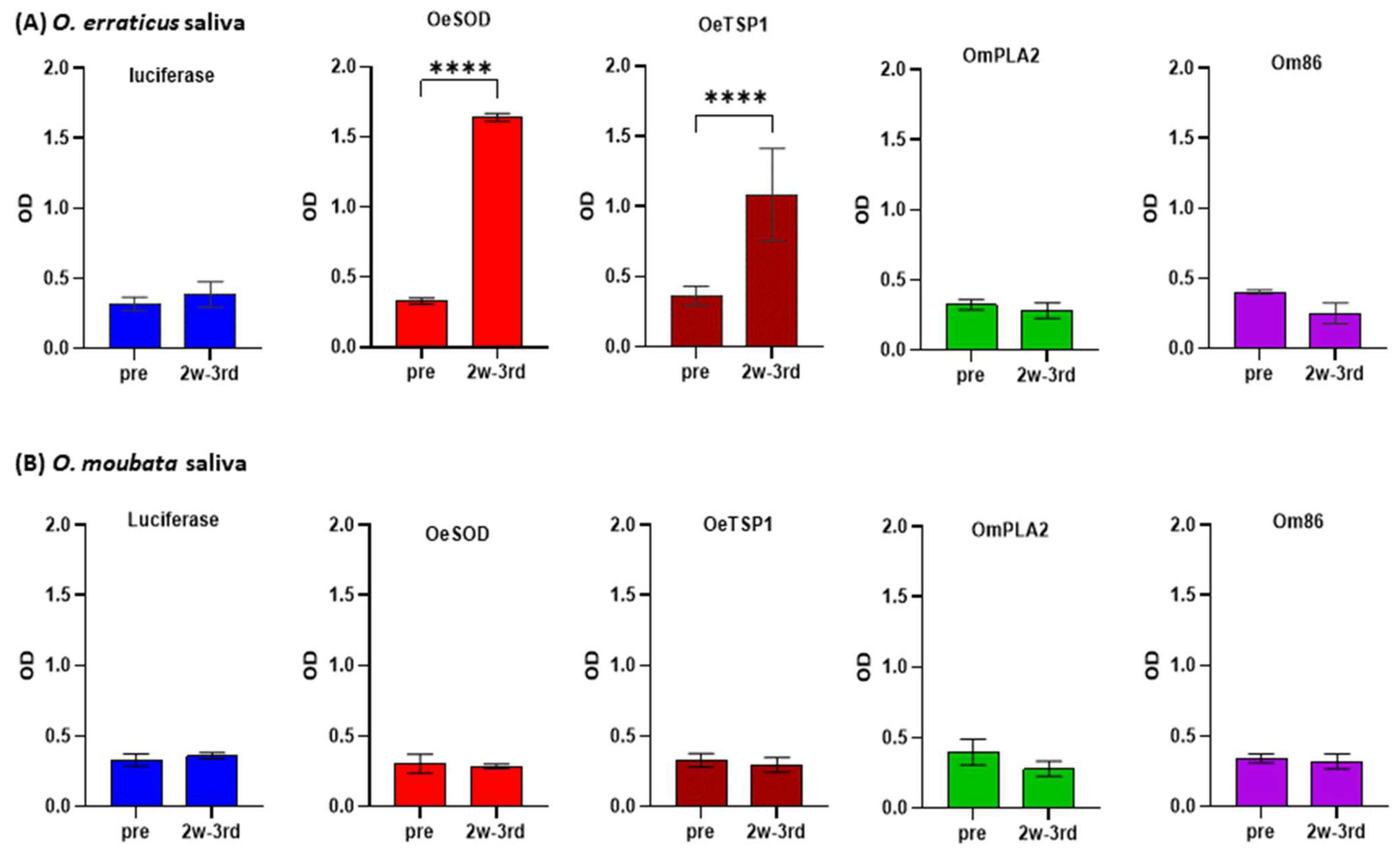
3.2. Humoral Responses Elicited by mRNA-LNPs Recognize Native Orthologous Proteins in Tick Saliva and Midgut Extracts
3.3. Protective Effects of the Immune Response on the Ticks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonenshine, D.E.; Roe, R.M. Overview: Ticks, People and Animals. In Biology of Ticks; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: Oxford, UK, 2014; Volume 1, pp. 3–16. [Google Scholar]
- Rashid, M.; Rashid, M.I.; Akbar, H.; Ahmad, L.; Hassan, M.A.; Ashraf, K.; Saeed, K.; Gharbi, M. A systematic review on modelling approaches for economic losses studies caused by parasites and their associated diseases in cattle. Parasitology 2019, 146, 129–141. [Google Scholar] [CrossRef]
- Cutler, S. Relapsing fever—A forgotten disease revealed. J. Appl. Microbiol. 2010, 108, 1115–1122. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing fevers: Neglected tick-borne diseases. Front. Cell. Infect. Microbiol. 2018, 8, 98. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Laddomada, A.; Martínez Avilés, M. Editorial: African Swine Fever. Front. Veter. Sci. 2021, 7, 632292. [Google Scholar] [CrossRef] [PubMed]
- Jori, F.; Bastos, A.; Boinas, F.; Van Heerden, J.; Heath, L.; Jourdan-Pineau, H.; Martinez-Lopez, B.; Pereira de Oliveira, R.; Pollet, T.; Quembo, C.; et al. An updated review of Ornithodoros ticks as reservoirs of African swine fever in Sub-Saharan Africa and Madagascar. Pathogens 2023, 12, 469. [Google Scholar] [CrossRef] [PubMed]
- Naddaf, S.R.; Ghazinezhad, B.; Bahramali, G.; Cutler, S.J. Phylogenetic analysis of the spirochete Borrelia microti, a potential agent of relapsing fever in Iran. J. Clin. Microbiol. 2012, 50, 2873–2876. [Google Scholar] [CrossRef]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 2: Borrelia Relapsing Fever Group and Unclassified Borrelia. Biology 2021, 10, 1117. [Google Scholar] [CrossRef]
- Buysse, M.; Duhayon, M.; Cantet, F.; Bonazzi, M.; Duron, O.; Guo, W.-P. Vector competence of the African argasid tick Ornithodoros moubata for the Q fever agent Coxiella burnetii. PLoS Neglected Trop. Dis. 2021, 15, e0009008. [Google Scholar] [CrossRef]
- Abbas, M.N.; Jmel, M.A.; Mekki, I.; Dijkgraaf, I.; Kotsyfakis, M. Recent Advances in Tick Antigen Discovery and Anti-Tick Vaccine Development. Int. J. Mol. Sci. 2023, 24, 4969. [Google Scholar] [CrossRef]
- Obaid, M.K.; Islam, N.; Alouffi, A.; Khan, A.Z.; da Silva Vaz Jr, I.; Tanaka, T.; Ali, A. Acaricides resistance in ticks: Selection, diagnosis, mechanisms, and mitigation. Front. Cell. Infect. Microbiol. 2022, 12, 941831. [Google Scholar] [CrossRef] [PubMed]
- Ndawula, C., Jr.; Tabor, A.E. Cocktail anti-tick vaccines: The unforeseen constraints and approaches toward enhanced efficacies. Vaccines 2020, 8, 457. [Google Scholar] [CrossRef]
- van Oosterwijk, J.G.; Wikel, S.K. Resistance to ticks and the path to anti-tick and transmission blocking vaccines. Vaccines 2021, 9, 725. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Contreras, M. Tick vaccines: Current status and future directions. Expert Rev. Vaccines 2015, 14, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Contreras, M. Additional considerations for anti-tick vaccine research. Expert Rev. Vaccines 2022, 21, 1019–1021. [Google Scholar] [CrossRef]
- Hart, T.M.; Cui, Y.; Telford, S.R.; Marín-López, A.; Calloway, K.; Dai, Y.; Matias, J.; DePonte, K.; Jaycox, J.; DeBlasio, M.; et al. Tick feeding or vaccination with tick antigens elicits immunity to the Ixodes scapularis exoproteome in guinea pigs and humans. Sci. Transl. Med. 2025, 17, eads9207. [Google Scholar] [CrossRef]
- Oleaga, A.; Obolo-Mvoulouga, P.; Manzano-Román, R.; Pérez-Sánchez, R. Functional annotation and analysis of the Ornithodoros moubata midgut genes differentially expressed after blood feeding. Ticks Tick-Borne Dis. 2017, 8, 693–708. [Google Scholar] [CrossRef]
- Oleaga, A.; Obolo-Mvoulouga, P.; Manzano-Román, R.; Pérez-Sánchez, R. De novo assembly and analysis of midgut transcriptome of the argasid tick Ornithodoros erraticus and identification of genes differentially expressed after blood feeding. Ticks Tick-Borne Dis. 2018, 9, 1537–1554. [Google Scholar] [CrossRef]
- Oleaga, A.; Soriano, B.; Llorens, C.; Pérez-Sánchez, R.; Caimano, M.J. Sialotranscriptomics of the argasid tick Ornithodoros moubata along the trophogonic cycle. PLoS Neglected Trop. Dis. 2021, 15, e0009105. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, R.; Carnero-Morán, Á.; Soriano, B.; Llorens, C.; Oleaga, A. RNA-seq analysis and gene expression dynamics in the salivary glands of the argasid tick Ornithodoros erraticus along the trophogonic cycle. Parasites Vectors 2021, 14, 170. [Google Scholar] [CrossRef]
- Díaz-Martín, V.; Manzano-Román, R.; Oleaga, A.; Pérez-Sánchez, R. New salivary anti-haemostatics containing protective epitopes from Ornithodoros moubata ticks: Assessment of their individual and combined vaccine efficacy. Veter. Parasitol. 2015, 212, 336–349. [Google Scholar] [CrossRef]
- Obolo-Mvoulouga, P.; Oleaga, A.; Manzano-Román, R.; Pérez-Sánchez, R. Evaluation of the protective efficacy of Ornithodoros moubata midgut membrane antigens selected using omics and in silico prediction algorithms. Ticks Tick-Borne Dis. 2018, 9, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, R.; Manzano-Román, R.; Obolo-Mvoulouga, P.; Oleaga, A. In silico selection of functionally important proteins from the mialome of Ornithodoros erraticus ticks and assessment of their protective efficacy as vaccine targets. Parasites Vectors 2019, 12, 508. [Google Scholar] [CrossRef]
- Carnero-Morán, Á.; Oleaga, A.; Cano-Argüelles, A.L.; Pérez-Sánchez, R. Function-guided selection of salivary antigens from Ornithodoros erraticus argasid ticks and assessment of their protective efficacy in rabbits. Ticks Tick-Borne Dis. 2023, 14, 102218. [Google Scholar] [CrossRef]
- Cano-Argüelles, A.L.; Oleaga, A.; González-Sánchez, M.; Vizcaíno-Marín, R.; Pérez-Sánchez, R. Vaccinomics-driven selection and validation of protective salivary antigens from the argasid tick Ornithodoros moubata. Ticks Tick-Borne Dis. 2025, 16, 102483. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Linares-Fernández, S.; Lacroix, C.; Exposito, J.-Y.; Verrier, B. Tailoring mRNA Vaccine to Balance Innate/Adaptive Immune Response. Trends Mol. Med. 2020, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020, 65, 14–20. [Google Scholar] [CrossRef]
- Kon, E.; Elia, U.; Peer, D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 2022, 73, 329–336. [Google Scholar] [CrossRef]
- Rouf, N.Z.; Biswas, S.; Tarannum, N.; Oishee, L.M.; Muna, M.M. Demystifying mRNA vaccines: An emerging platform at the forefront of cryptic diseases. RNA Biol. 2022, 19, 386–410. [Google Scholar] [CrossRef]
- Matias, J.; Kurokawa, C.; Sajid, A.; Narasimhan, S.; Arora, G.; Diktas, H.; Lynn, G.E.; DePonte, K.; Pardi, N.; Valenzuela, J.G.; et al. Tick immunity using mRNA, DNA and protein-based Salp14 delivery strategies. Vaccine 2021, 39, 7661–7668. [Google Scholar] [CrossRef] [PubMed]
- Sajid, A.; Matias, J.; Arora, G.; Kurokawa, C.; DePonte, K.; Tang, X.; Lynn, G.; Wu, M.-J.; Pal, U.; Strank, N.O.; et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci. Transl. Med. 2021, 13, eabj9827. [Google Scholar] [CrossRef]
- Matias, J.; Cui, Y.; Tang, X.; Sajid, A.; Arora, G.; Wu, M.-J.; DePonte, K.; Muramatsu, H.; Tam, Y.K.; Narasimhan, S.; et al. Specific mRNA lipid nanoparticles and acquired resistance to ticks. Vaccine 2023, 41, 4996–5002. [Google Scholar] [CrossRef] [PubMed]
- Matias, J.; Cui, Y.; Lynn, G.E.; DePonte, K.; Mesquita, E.; Muramatsu, H.; Alameh, M.G.; Dwivedi, G.; Tam, Y.K.; Pardi, N.; et al. mRNA vaccination of rabbits alters the fecundity, but not the attachment, of adult Ixodes scapularis. Sci. Rep. 2024, 14, 496. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; de la Fuente, J. Control of Ixodes ricinus and Dermacentor reticulatus tick infestations in rabbits vaccinated with the Q38 Subolesin/Akirin chimera. Vaccine 2016, 34, 3010–3013. [Google Scholar] [CrossRef]
- Nawaz, M.; Malik, M.I.; Zhang, H.; Hassan, I.A.; Cao, J.; Zhou, Y.; Hameed, M.; Hussain Kuthu, Z.; Zhou, J. Proteomic analysis of exosome-like vesicles isolated from saliva of the tick Haemaphysalis longicornis. Front. Cell. Infect. Microbiol. 2020, 10, 542319. [Google Scholar] [CrossRef]
- Zhou, W.; Tahir, F.; Wang, J.C.-Y.; Woodson, M.; Sherman, M.B.; Karim, S.; Neelakanta, G.; Sultana, H. Discovery of exosomes from tick saliva and salivary glands reveals therapeutic roles for CXCL12 and IL-8 in wound healing at the tick-human skin interface. Front. Cell Dev. Biol. 2020, 8, 554. [Google Scholar] [CrossRef]
- de la Fuente, J.; Kopáček, P.; Lew-Tabor, A.; Maritz-Olivier, C. Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 2016, 38, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Bishop, L.J.; Stutzer, C.; Maritz-Olivier, C. More than three decades of Bm86: What we know and where to go. Pathogens 2023, 12, 1071. [Google Scholar] [CrossRef]
- Parizi, L.F.; Githaka, N.W.; Logullo, C.; Zhou, J.; Onuma, M.; Termignoni, C.; da Silva Vaz, I., Jr. Universal tick vaccines: Candidates and remaining challenges. Animals 2023, 13, 2031. [Google Scholar] [CrossRef]
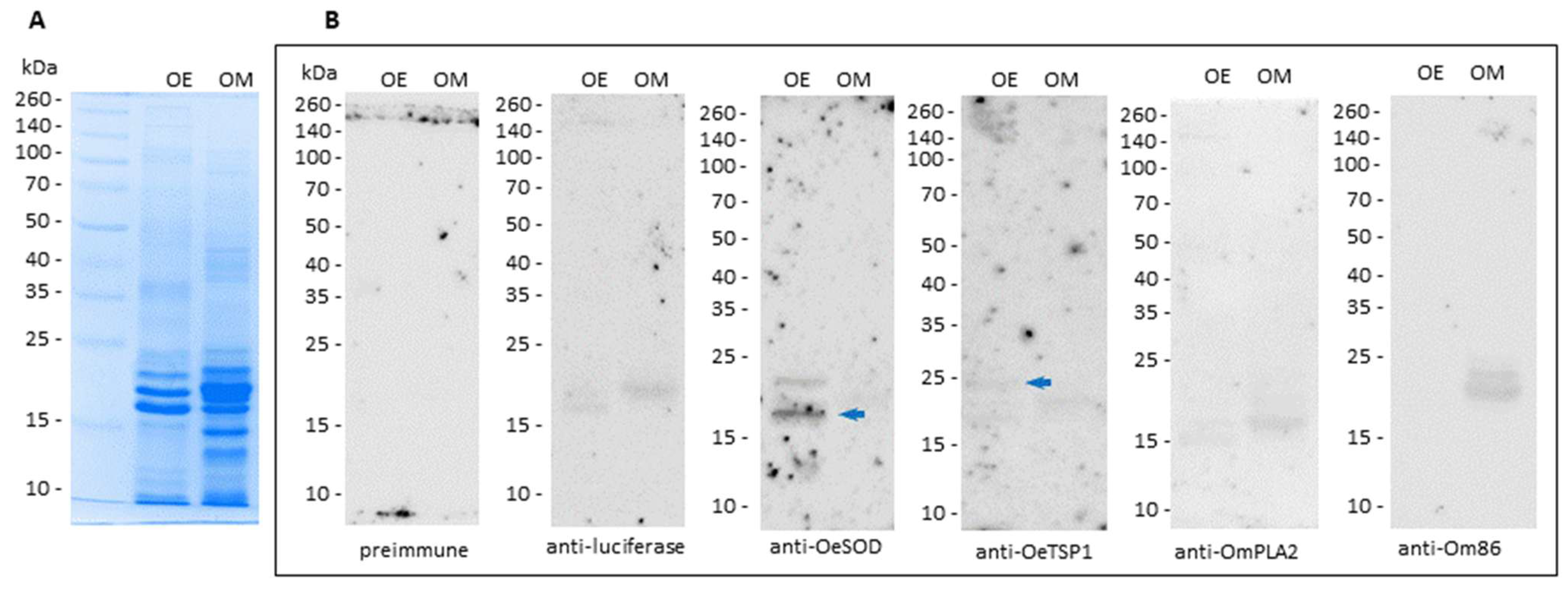
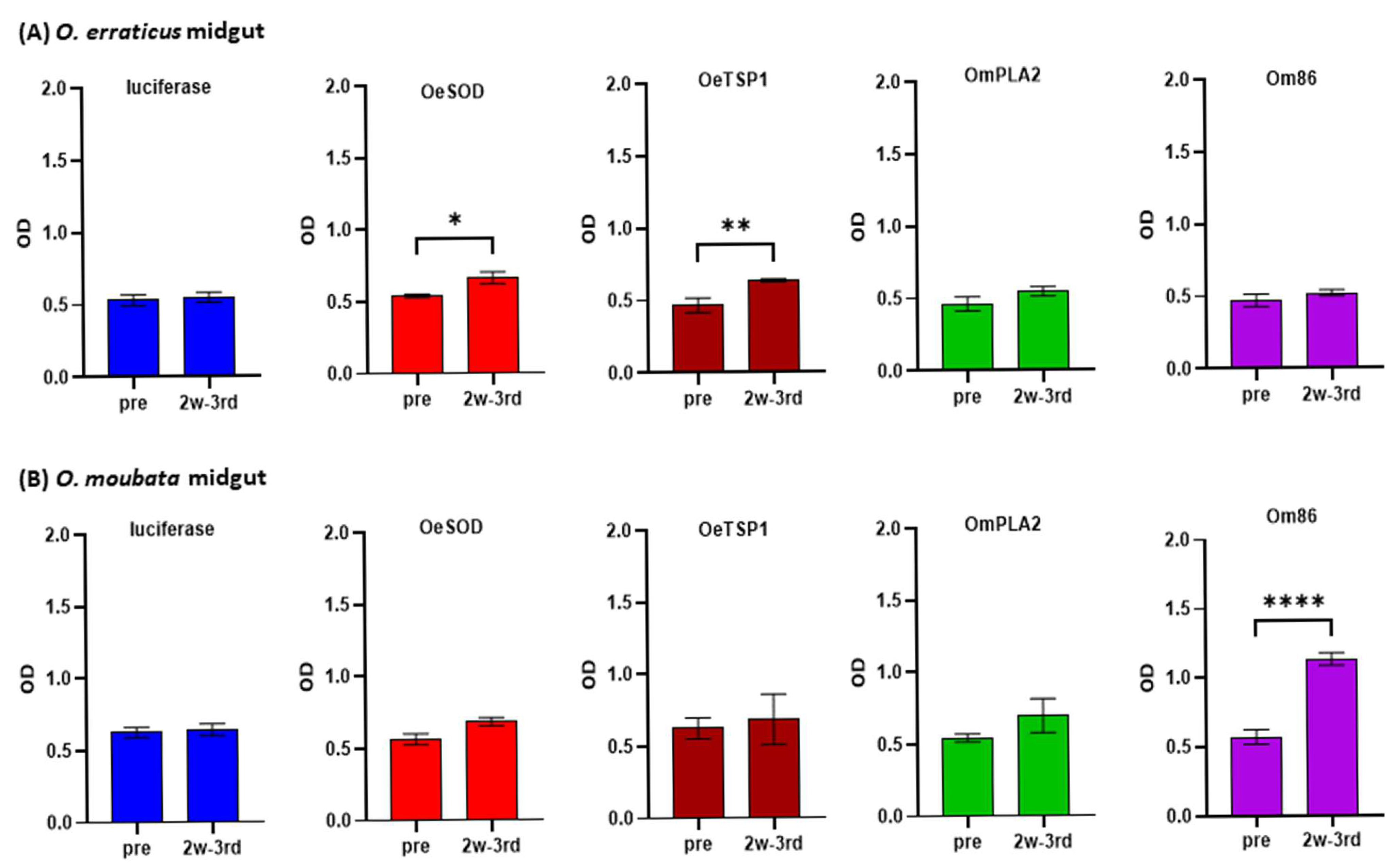
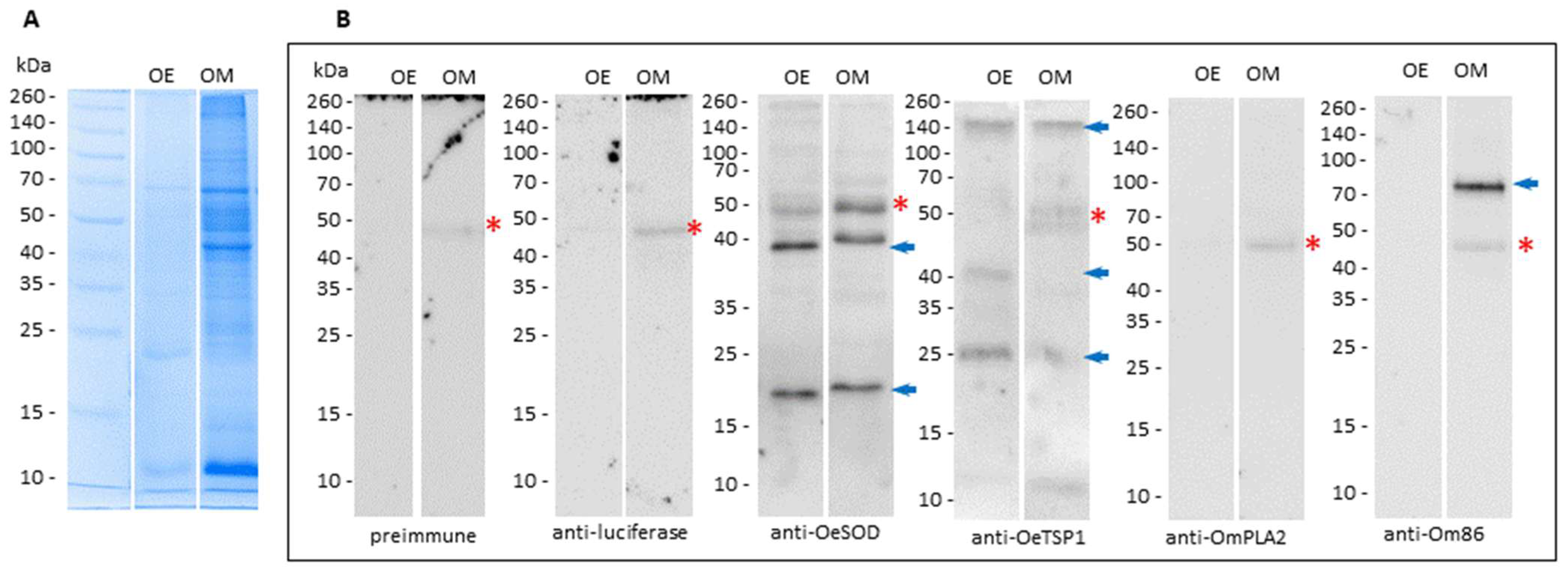

| Antigen Name | Protein Description | GenBank/ Uniprot Code | Species | Organ | % E O. erraticus | %E O. moubata | References |
|---|---|---|---|---|---|---|---|
| OeSOD | Superoxide dismutase | MBZ4008920.1 | O. erraticus | SG | 54.3 | 3.1 | [24] |
| OeTSP1 | Tetraspanin | A0A293MYE4 | O. erraticus | MG | 56.0 | 11.1 | [23] |
| OmPLA2 | Phospholipase A2 | AGJ90343.1 | O. moubata | SG | ND | 44.2 | [21] |
| Om86 | Bm86 antigen homologue | A0AA96JYS1 | O. moubata | MG | 0 | 7 | [22] |
| Luc | Luciferase | BAF48396.1 | P. pyralis | - | - | - | [34] |
| Parameter | Developmental Stage | LUC (Control) | OeSOD (% Reduction) | OeTSP1 (% Reduction) | OmPLA2 (% Reduction) | Om86 (% Reduction) |
|---|---|---|---|---|---|---|
| Ingested blood (mg) | Males | 3.8 ± 0.2 | 3.0 ± 1.0 (20.1) | 3.3 ± 0.2 (11.8) | 3.0 ± 0.1 (20.7) * | 3.5 ± 0.7 (7.8) |
| Females | 13.0 ± 0.8 | 13.6 ± 1.9 | 11.1 ± 1.1 (14.1) | 13.8 ± 1.2 | 11.5 ± 0.3 (11.7) * | |
| Nymphs-3 | 2.6 ± 0.1 | 2.4 ± 0.3 (5.9) | 2.2 ± 0.1 (14.6) * | 2.4 ± 0.3 (5.9) | 2.6 ± 0.3 | |
| Survival (%) | Males | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 99.9 ± 1 (0.1) |
| Females | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | |
| Nymph-3 | 99.0 ± 1.0 | 98.6 ± 1.0 (0.4) | 100 ± 0 | 99.3 ± 1.0 | 99.3 ± 1.0 | |
| Moulting (%) | Nymphs-3 | 98.6 ± 2.4 | 90.2 ± 6.4 (8.4) | 89.8 ± 14.4 (8.8) | 92.6 ± 6.5 (6.0) | 90.3 ± 5.3 (8.3) |
| Oviposition (eggs/female) | Females | 52.1 ± 2.4 | 36.9 ± 4.1 (29.2) * | 39.6 ± 2.7 (24.0) * | 42.5 ± 6.1 (18.4) * | 31.4 ± 9.1 (39.7) ** |
| Fertility (larvae/female) | Females | 44.2 ± 1.4 | 28.7 ± 4.2 (35.1) ** | 36.4 ± 2.8 (17.6) * | 35.2 ± 3.4 (20.4) * | 28.4 ± 8.5 (35.7) ** |
| Efficacy (%) | 40 ± 11.9 | 23.1 ± 15.5 | 24.1 ± 10.9 | 41.6 ± 13.6 |
| Parameter | Developmental Stage | LUC (Control) | OeSOD (% Reduction) | OeTSP1 (% Reduction) | OmPLA2 (% Reduction) | Om86 (% Reduction) |
|---|---|---|---|---|---|---|
| Ingested blood (mg) | Males | 32.1 ± 1.8 | 33.1 ± 3.4 | 28.0 ± 2.0 (11.8) | 29.2 ± 3.0 (9.0) | 29.2 ± 1.2 (8.9) |
| Females | 216.2 ± 1.1 | 187.6 ± 40.8 (13.2) | 201.6 ± 1.0 (6.8) | 230.5 ± 20.4 | 213.4 ± 18.7 (1.3) | |
| Nymphs-3 | 12.3 ± 3.1 | 12.3 ± 2.4 | 13.5 ± 0.9 | 15.5 ± 1.4 | 12.3 ± 1.6 | |
| Survival (%) | Males | 96.4 ± 3.6 | 92.2 ± 8.7 (4.2) | 92.6 ± 3.3 (3.8) | 96.5 ± 0.2 | 96.3 ± 0.1 (0.1) |
| Females | 96.7 ± 03.3 | 88.9 ± 8.3 (4.5) | 80 ± 1.0 (16.7) * | 91.1 ± 8.3 (5.6) | 77.8 ± 8.3 (18.9) ** | |
| Nymph-3 | 98.9 ± 1.1 | 93.3 ± 5.0 (5.6) | 97.0 ± 1.0 (1.9) | 94.0 ± 7.1 (4.9) | 90.5 ± 6.3 (8.4) | |
| Moulting (%) | Nymphs-3 | 97.9 ± 0.1 | 96.5 ± 62.2 (1.4) | 96.9 ± 1.5 (1.0) | 93.5 ± 3.8 (4.4) * | 97.1 ± 1.1 (0.8) |
| Oviposition (eggs/female) | Females | 258.0 ± 0.7 | 196.8 ± 34.9 (23.7) ** | 228.6 ± 13.8 (11.4) | 219.0 ± 9.6 (15.1) ** | 221.7 ± 17.2 (14.1) ** |
| Fertility (larvae/female) | Females | 228.8 ± 0.41 | 184.0 ± 28.7 (19.6) ** | 222.4 ± 2.3 (2.8) | 204.1 ± 12.2 (10.8) * | 203.3 ± 9.9 (11.1) * |
| Efficacy (%) | 32.1 ± 7.2 | 21.9 ± 1.0 | 23.5 ± 12.3 | 34.6 ± 10.4 |
| Antigen Name | Protein Description | Protective Efficacy Against O. erraticus | Protective Efficacy Against O. moubata | ||
|---|---|---|---|---|---|
| Recombinant | mRNA-LNP | Recombinant | mRNA-LNP | ||
| OeSOD | Superoxide dismutase | 54.3% | 40%  | 3.1% | 32.1%  |
| OeTSP1 | Tetraspanin | 56.0% | 23.1%  | 11.1% | 21.9%  |
| OmPLA2 | Phospholipase A2 | not determined | 24.1% | 44.2% | 23.5%  |
| Om86 | Bm86 antigen homologue | 0% | 41.6%  | 7% | 34.6%  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleaga, A.; Cano-Argüelles, A.L.; González-Sánchez, M.; Vizcaíno-Marín, R.; Pérez-Sánchez, R. Immunization with mRNA-LNPs Encoding Ornithodoros Argasid Tick Antigens Induces Humoral Immune Responses and Tick Resistance. Pathogens 2025, 14, 914. https://doi.org/10.3390/pathogens14090914
Oleaga A, Cano-Argüelles AL, González-Sánchez M, Vizcaíno-Marín R, Pérez-Sánchez R. Immunization with mRNA-LNPs Encoding Ornithodoros Argasid Tick Antigens Induces Humoral Immune Responses and Tick Resistance. Pathogens. 2025; 14(9):914. https://doi.org/10.3390/pathogens14090914
Chicago/Turabian StyleOleaga, Ana, Ana Laura Cano-Argüelles, María González-Sánchez, Rocío Vizcaíno-Marín, and Ricardo Pérez-Sánchez. 2025. "Immunization with mRNA-LNPs Encoding Ornithodoros Argasid Tick Antigens Induces Humoral Immune Responses and Tick Resistance" Pathogens 14, no. 9: 914. https://doi.org/10.3390/pathogens14090914
APA StyleOleaga, A., Cano-Argüelles, A. L., González-Sánchez, M., Vizcaíno-Marín, R., & Pérez-Sánchez, R. (2025). Immunization with mRNA-LNPs Encoding Ornithodoros Argasid Tick Antigens Induces Humoral Immune Responses and Tick Resistance. Pathogens, 14(9), 914. https://doi.org/10.3390/pathogens14090914









