Prevalence of Cytomegalovirus Antibodies in Croatian Childbearing-Aged and Pregnant Women: A Ten-Year Retrospective Study (2015–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Methods
2.3. Statistical Analysis
3. Results
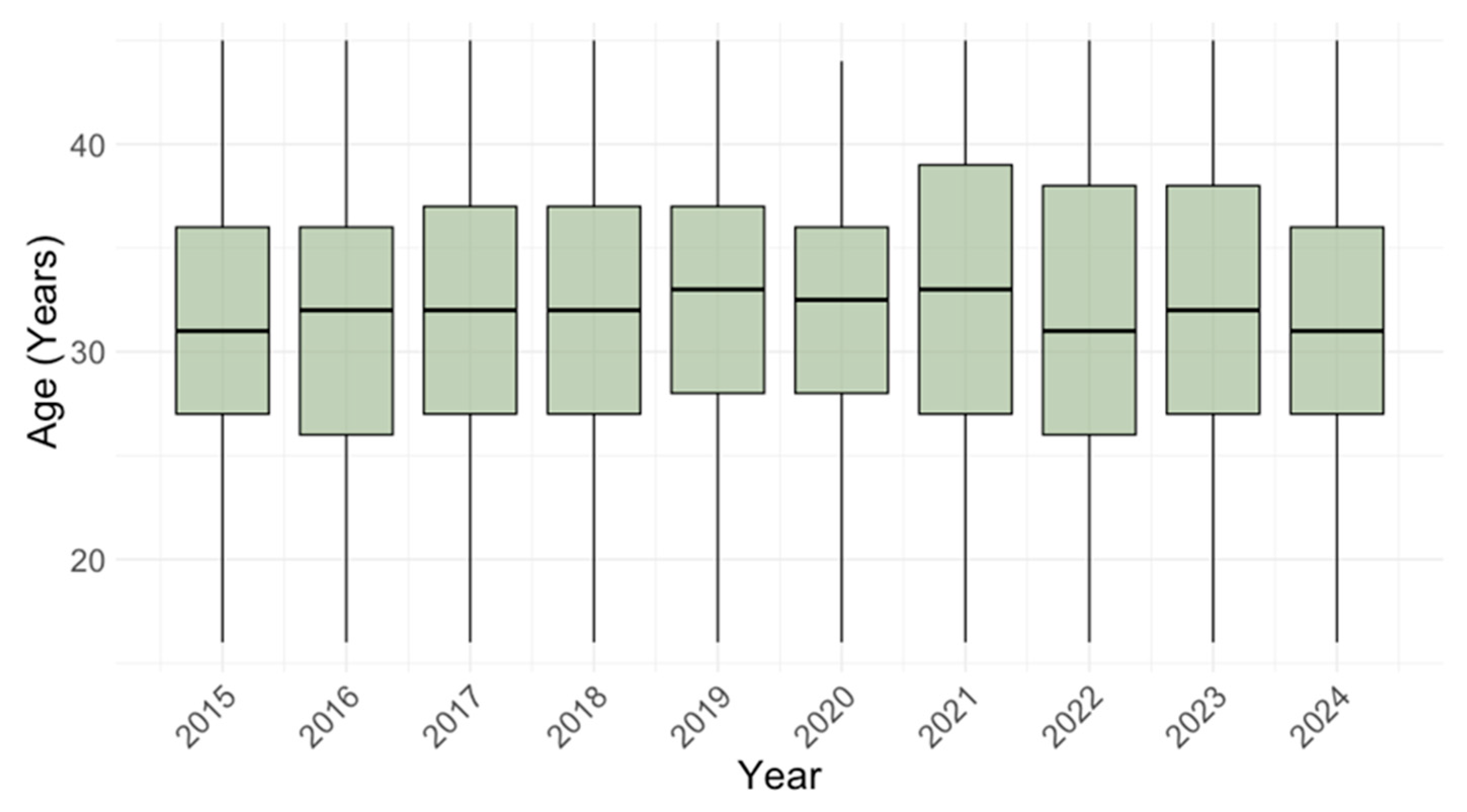
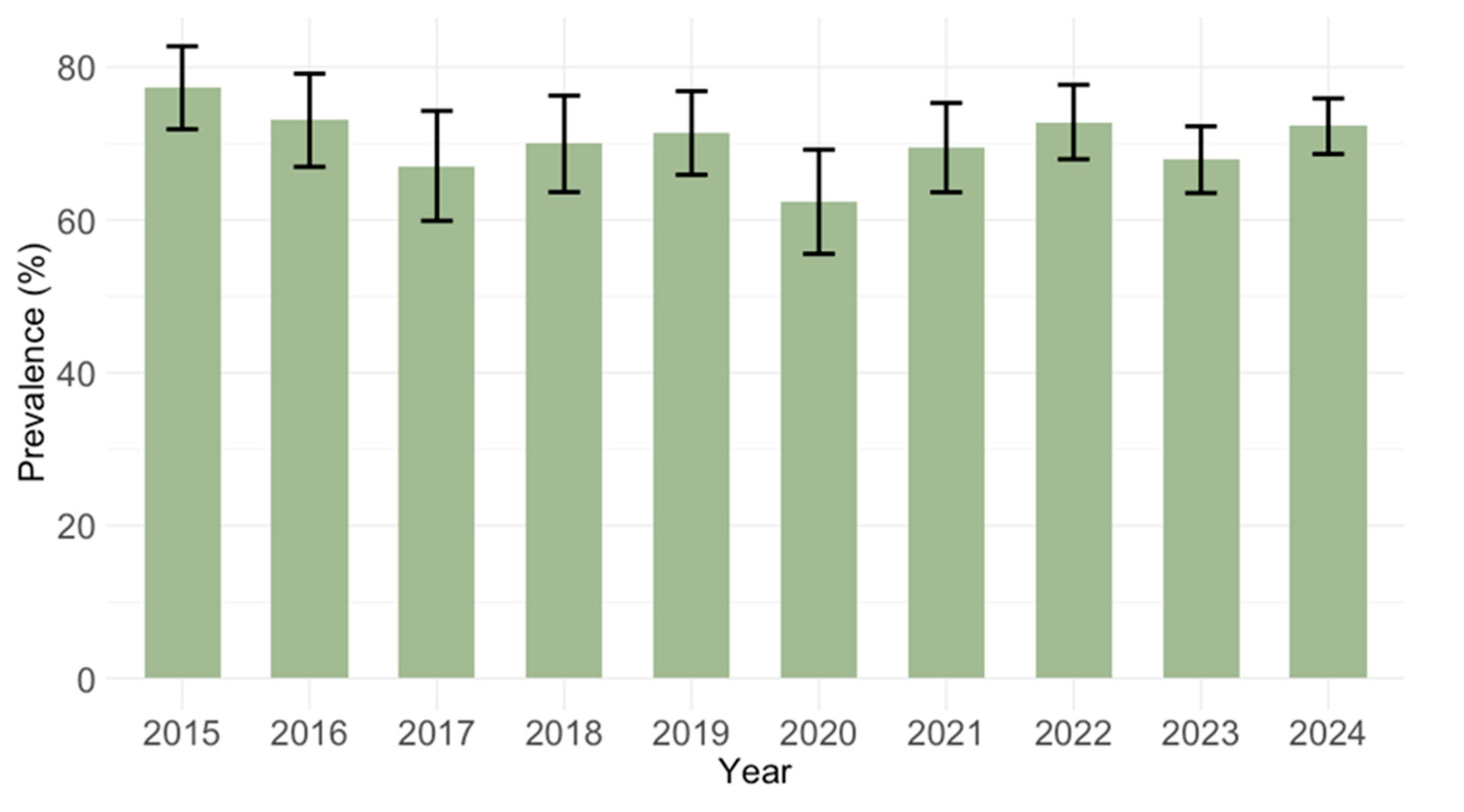
3.1. Temporal Trends of Cytomegalovirus IgG and IgM Seroprevalence
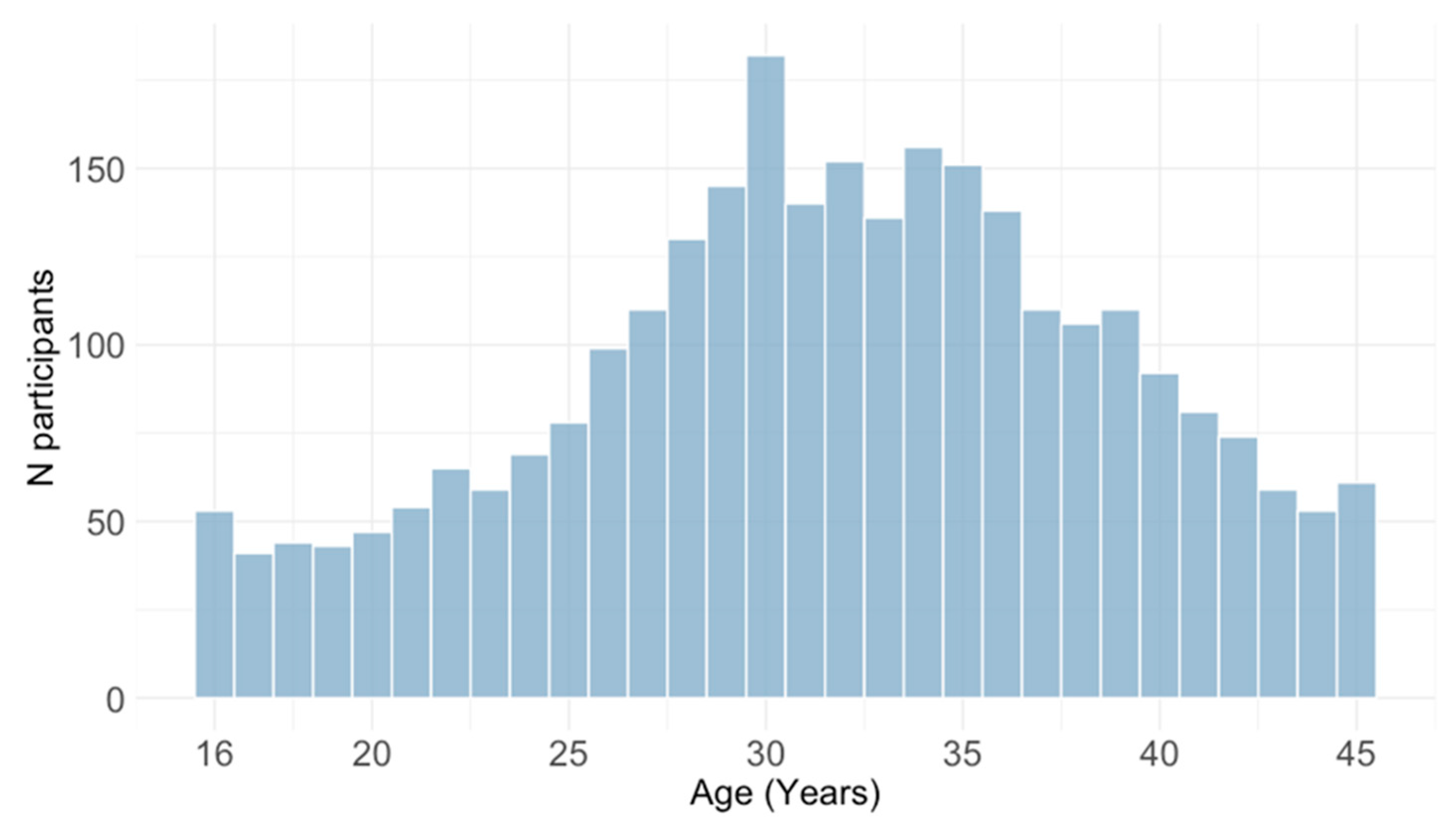
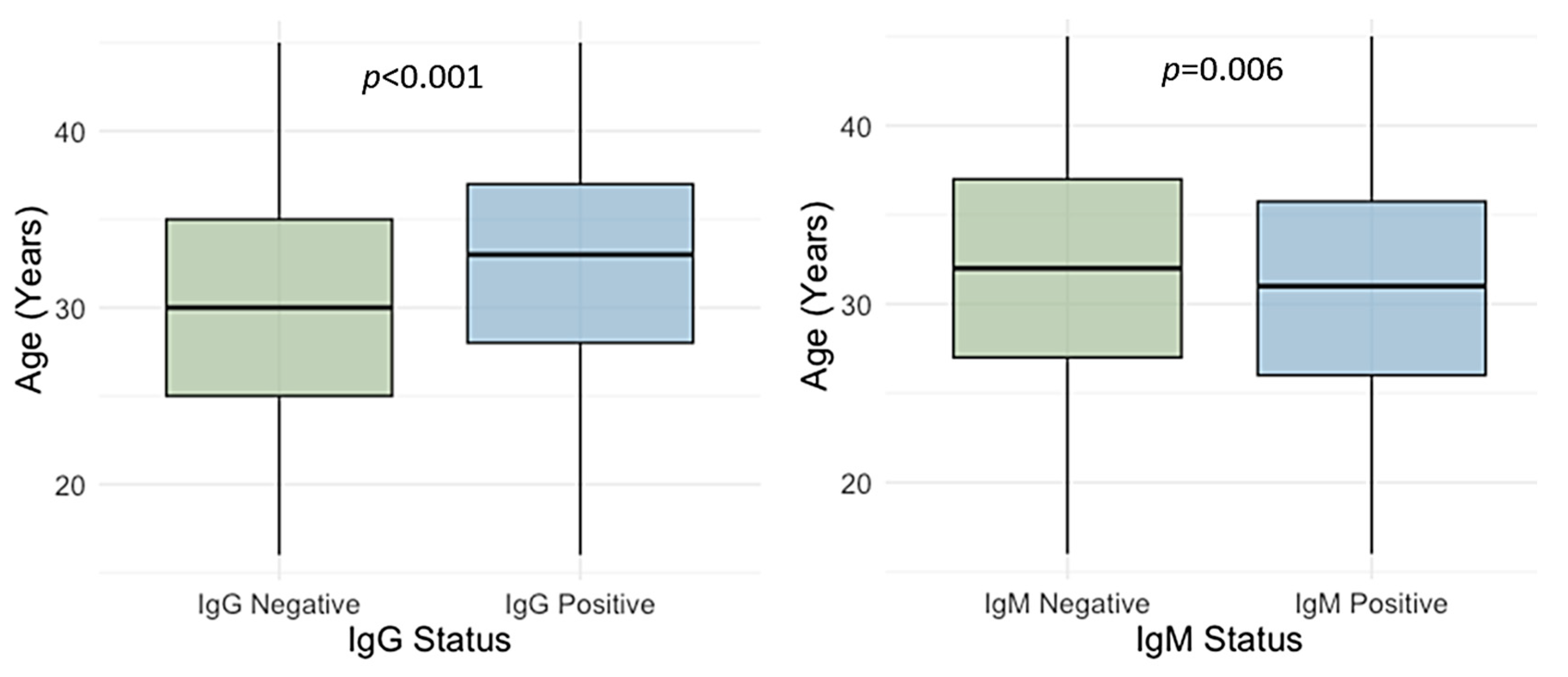
3.2. Age-Related Trends of Cytomegalovirus IgG and IgM Seroprevalence
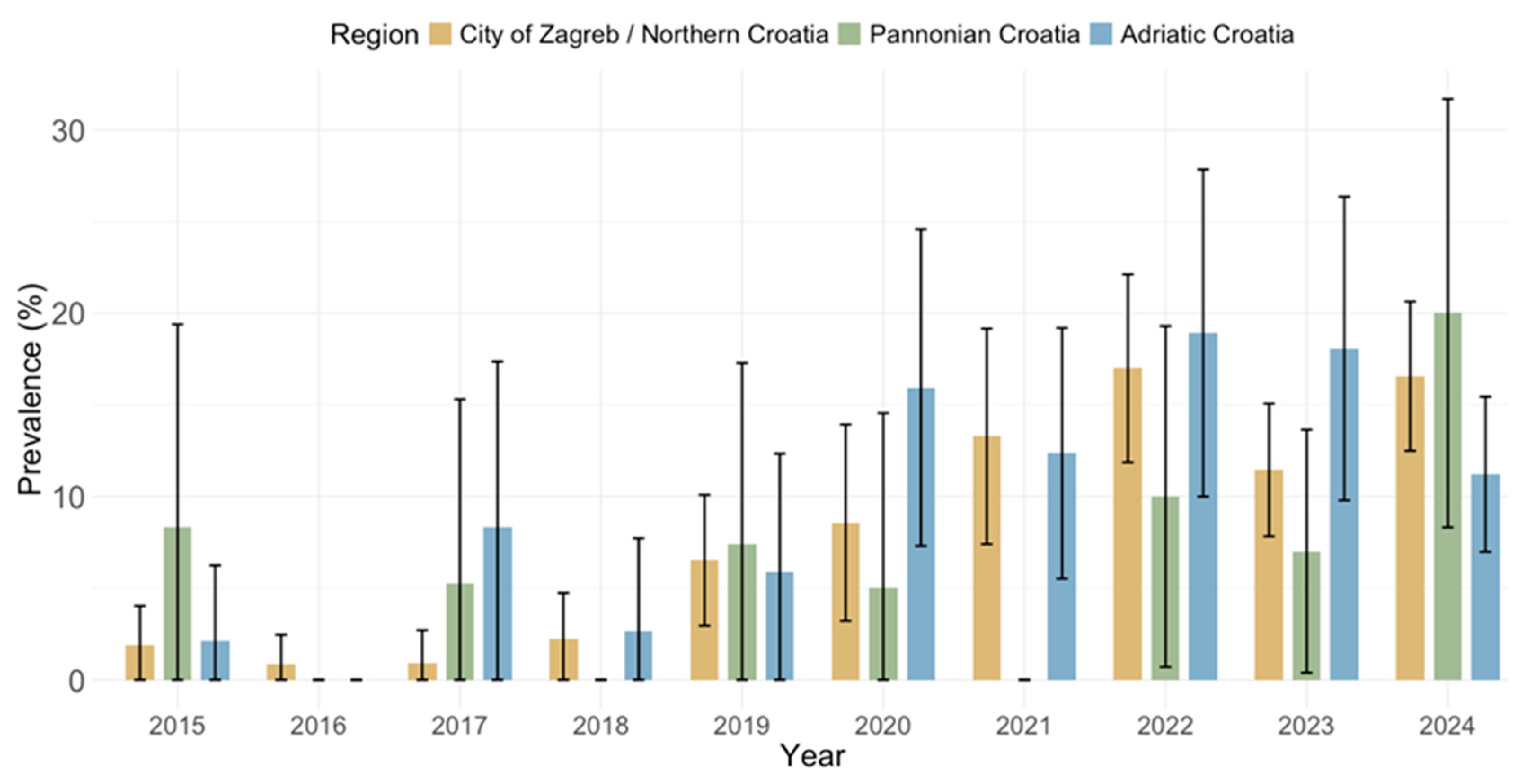
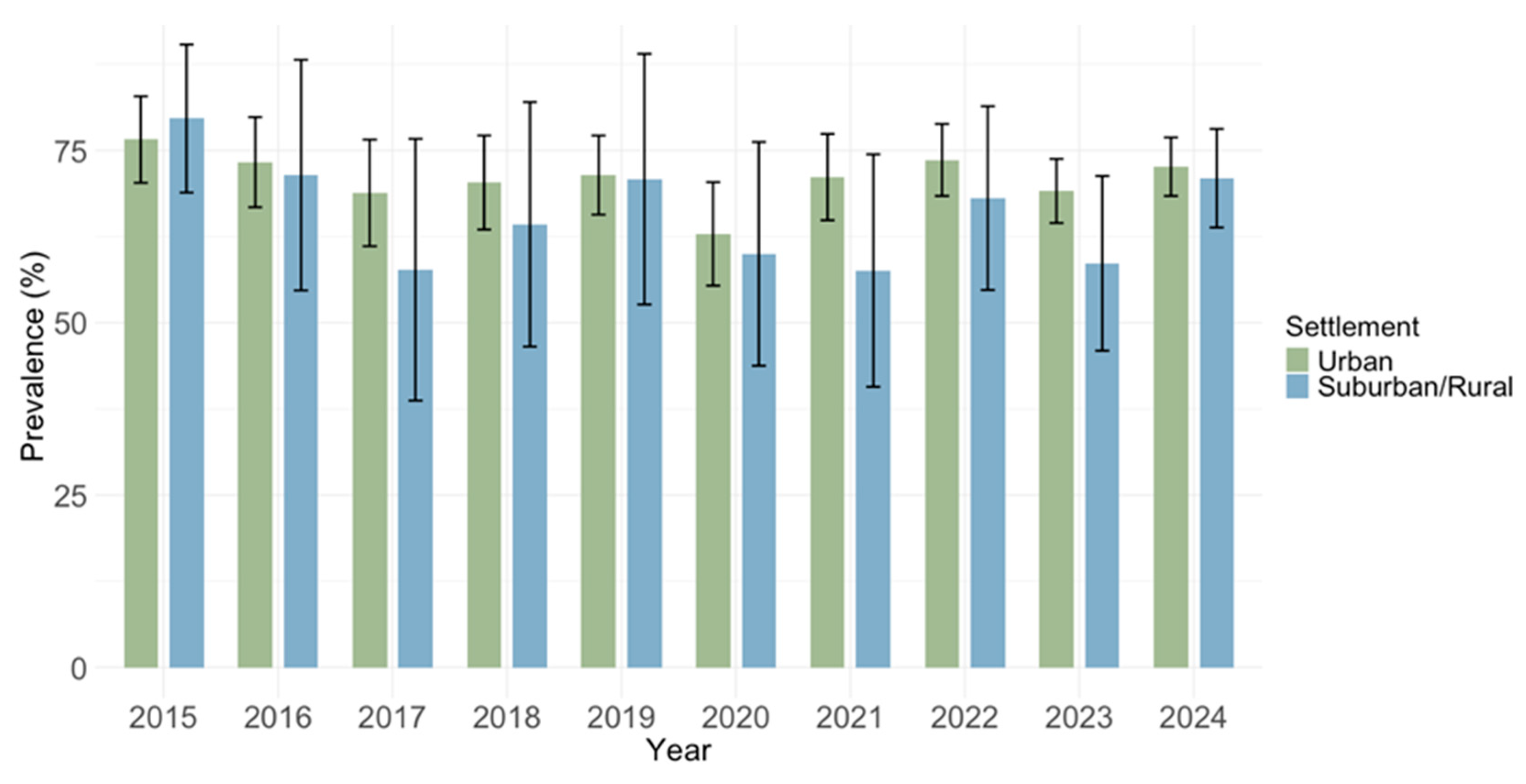
3.3. Spatial Trends of Cytomegalovirus IgG and IgM Seroprevalence
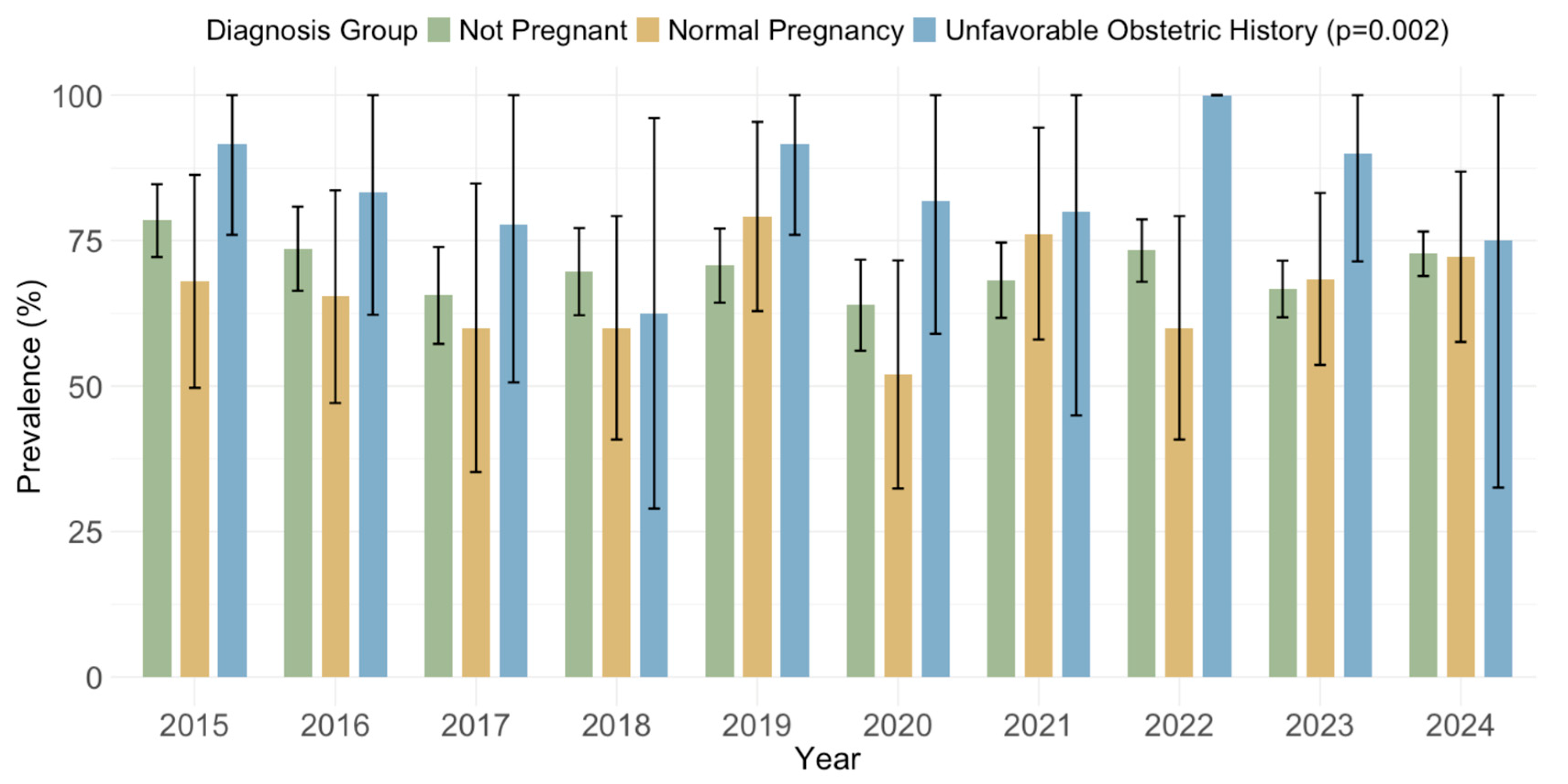
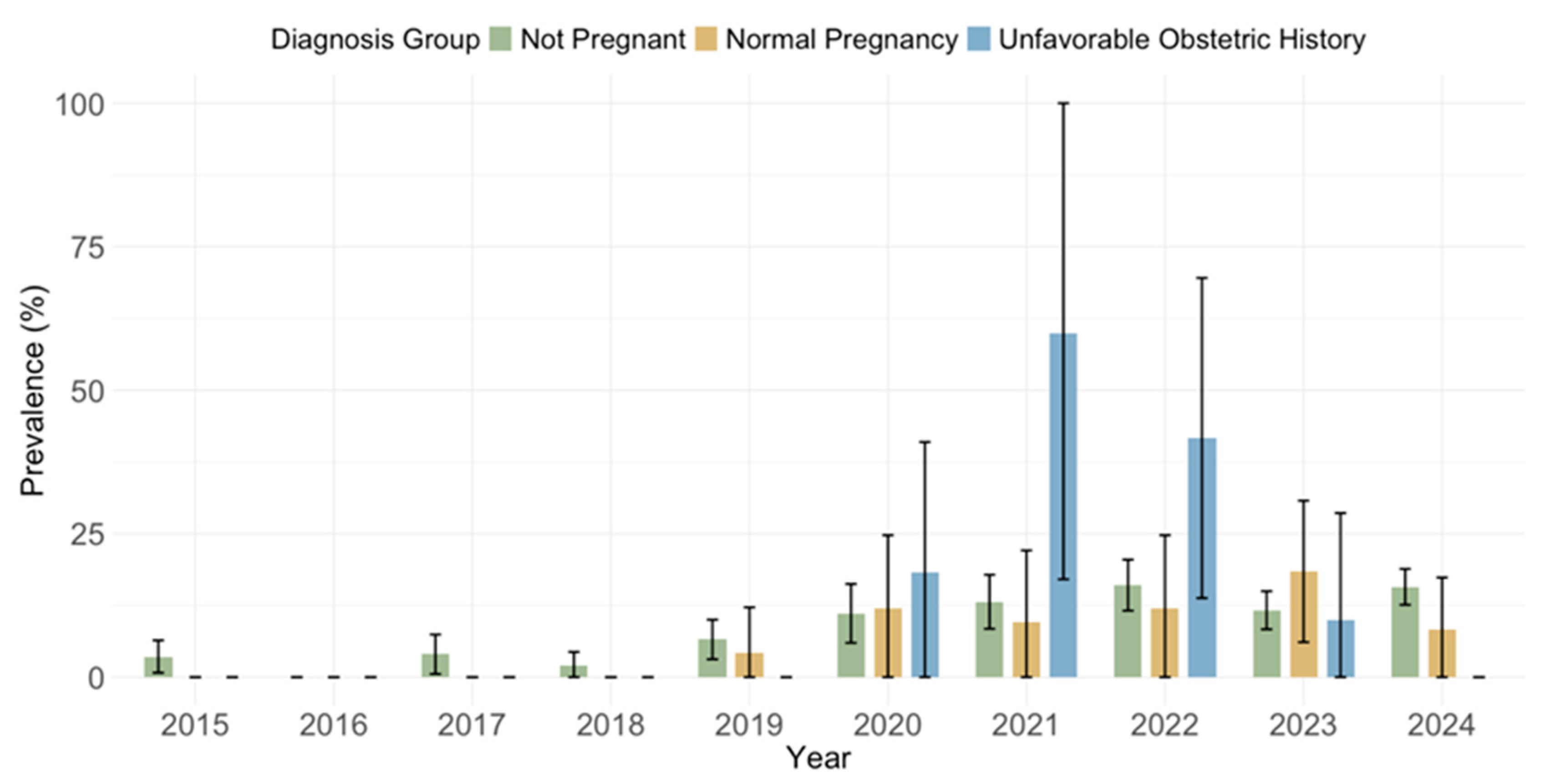
3.4. Cytomegalovirus IgG and IgM Seroprevalence by Obstetric History
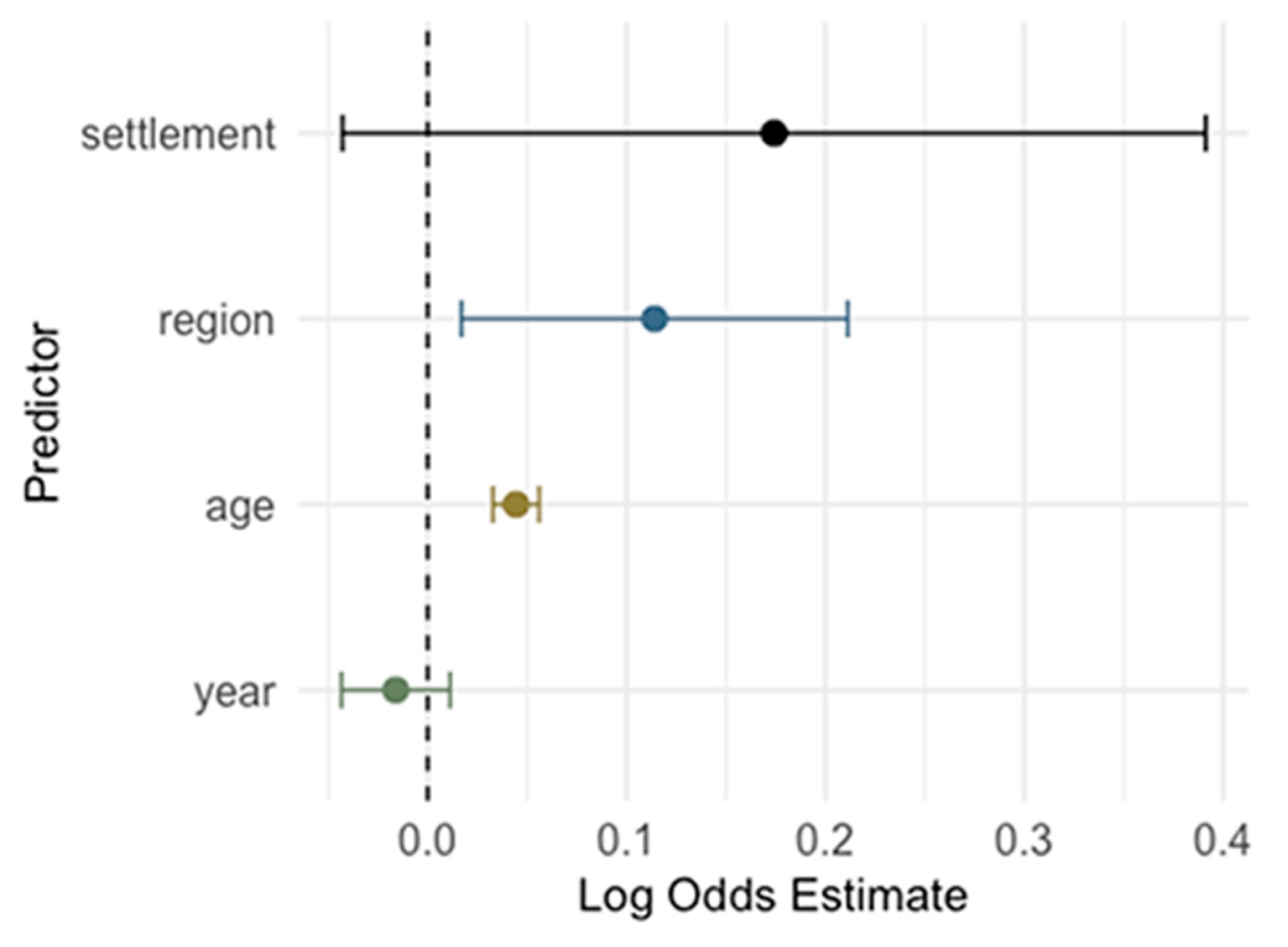
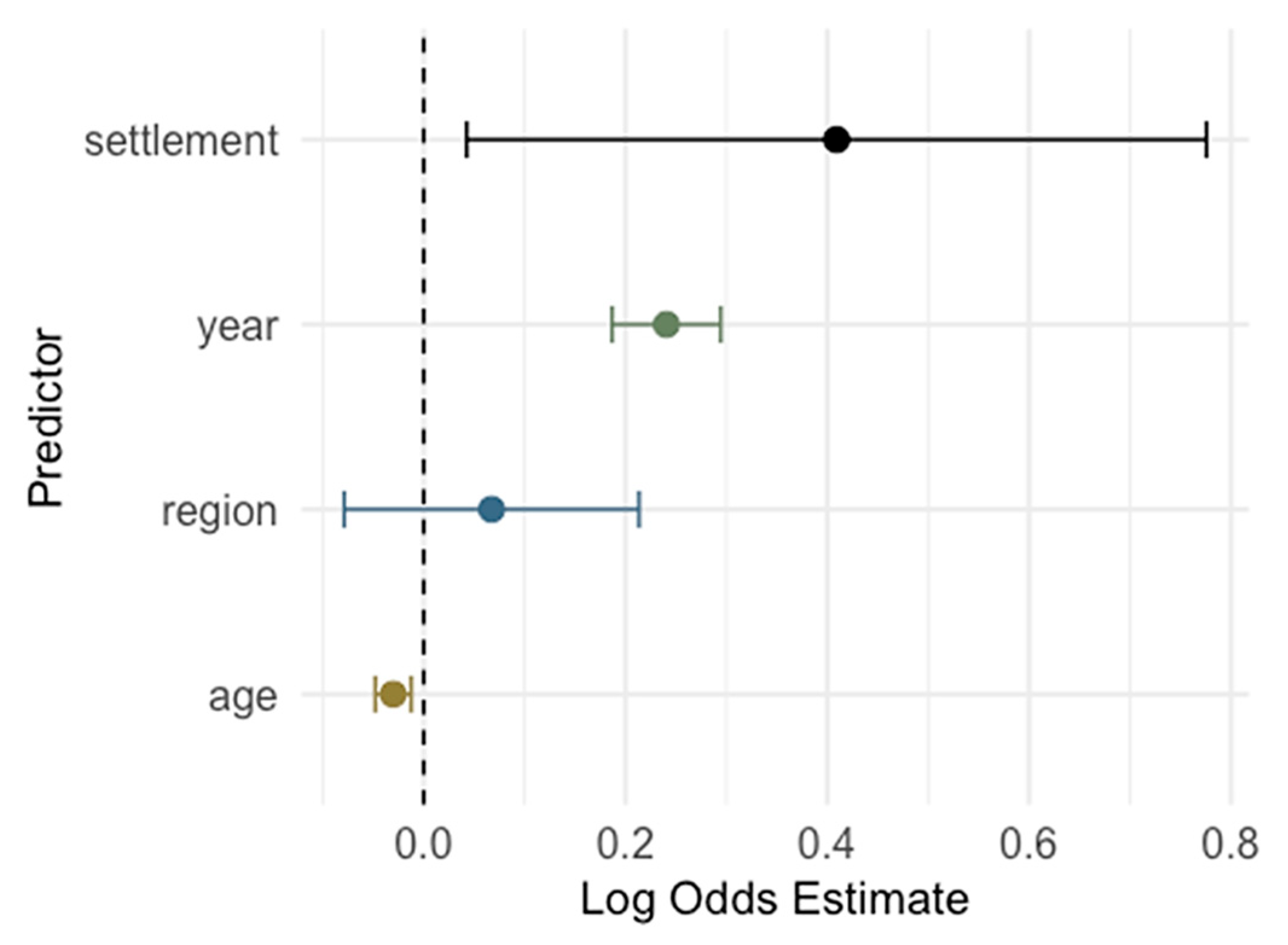
3.5. Risk Analysis for Cytomegalovirus IgG and IgM Seropositivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMV | Cytomegalovirus |
| TORCH | Toxoplasma, other, rubella, cytomegalovirus, herpes simplex virus |
| HIV | Human immunodeficiency virus |
| AIDS | Acquired immunodeficiency syndrome |
| AI | Avidity index |
| CI | Confidence interval |
| IQR | Interquartile range |
References
- International Committee on Taxonomy of Viruses (ICTV). Cytomegalovirus. Available online: https://ictv.global/report/chapter/orthoherpesviridae/orthoherpesviridae/cytomegalovirus (accessed on 15 July 2025).
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the Worldwide Seroprevalence of Cytomegalovirus: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef]
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A Systematic Literature Review of the Global Seroprevalence of Cytomegalovirus: Possible Implications for Treatment, Screening, and Vaccine Development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef]
- Tzialla, C.; Salomè, S.; Mondì, V. Clinical Manifestations of Non-Congenital CMV Infection in Infants and Immunocompetent Children: Review of Cases from the Past Decade. Microorganisms 2025, 13, 772. [Google Scholar] [CrossRef]
- Gupta, M.; Shorman, M. Cytomegalovirus Infections. In StatPearls; Internet; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459185/ (accessed on 15 July 2025).
- Ljungman, P.; Chemaly, R.F.; Khawaja, F.; Alain, S.; Avery, R.; Badshah, C.; Boeckh, M.; Fournier, M.; Hodowanec, A.; Komatsu, T.; et al. Consensus Definitions of Cytomegalovirus (CMV) Infection and Disease in Transplant Patients Including Resistant and Refractory CMV for Use in Clinical Trials: 2024 Update from the Transplant Associated Virus Infections Forum. Clin. Infect. Dis. 2024, 79, 787–794. [Google Scholar] [CrossRef]
- Pontes, K.F.M.; Nardozza, L.M.M.; Peixoto, A.B.; Werner, H.; Tonni, G.; Granese, R.; Araujo Júnior, E. Cytomegalovirus and Pregnancy: A Narrative Review. J. Clin. Med. 2024, 13, 640. [Google Scholar] [CrossRef]
- Revello, M.G.; Lazzarotto, T.; Guerra, B.; Spinillo, A.; Ferrazzi, E.; Kustermann, A.; Guaschino, S.; Vergani, P.; Todros, T.; Frusca, T.; et al. A Randomized Trial of Hyperimmune Globulin to Prevent Congenital Cytomegalovirus. N. Engl. J. Med. 2014, 370, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Romero Starke, K.; Kofahl, M.; Freiberg, A.; Schubert, M.; Groß, M.L.; Schmauder, S.; Hegewald, J.; Kämpf, D.; Stranzinger, J.; Nienhaus, A.; et al. The Risk of Cytomegalovirus Infection in Daycare Workers: A Systematic Review and Meta-Analysis. Int. Arch. Occup. Environ. Health 2020, 93, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Schattner, A. The Wide Spectrum of Presentations of Cytomegalovirus Infection in Immunocompetent Hosts: An Exhaustive Narrative Review. Pathogens 2024, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Salomè, S.; Corrado, F.R.; Mazzarelli, L.L.; Maruotti, G.M.; Capasso, L.; Blazquez-Gamero, D.; Raimondi, F. Congenital Cytomegalovirus Infection: The State of the Art and Future Perspectives. Front. Pediatr. 2023, 11, 1276912. [Google Scholar] [CrossRef]
- Akpan, U.S.; Pillarisetty, L.S. Congenital Cytomegalovirus Infection. In StatPearls; Internet; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK541003/ (accessed on 15 July 2025).
- Enders, G.; Daiminger, A.; Bäder, U.; Exler, S.; Enders, M. Intrauterine Transmission and Clinical Outcome of 248 Pregnancies with Primary Cytomegalovirus Infection in Relation to Gestational Age. J. Clin. Virol. 2011, 52, 244–246. [Google Scholar] [CrossRef]
- Świątkowska-Freund, M.; Bednarek, S.; Sasak-Cieślar, N.; Kocięcka, N.; Powroźnik, P.; Waldman, A. Cytomegalovirus Seroprevalence in Northern Poland in the Population Planning Pregnancy and Pregnant Women. Viruses 2025, 17, 537. [Google Scholar] [CrossRef]
- Pass, R.F.; Fowler, K.B.; Boppana, S.B.; Britt, W.J.; Stagno, S. Congenital Cytomegalovirus Infection Following First Trimester Maternal Infection: Symptoms at Birth and Outcome. J. Clin. Virol. 2006, 35, 216–220. [Google Scholar] [CrossRef]
- Chatzakis, C.; Ville, Y.; Makrydimas, G.; Dinas, K.; Zavlanos, A.; Sotiriadis, A. Timing of Primary Maternal Cytomegalovirus Infection and Rates of Vertical Transmission and Fetal Consequences. Am. J. Obstet. Gynecol. 2020, 223, 870–883.e11. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Ljubin-Sternak, S.; Ban, M.; Kolaric, B.; Sviben, M.; Mlinaric-Galinovic, G. Seroprevalence of TORCH Infections in Women of Childbearing Age in Croatia. J. Matern.-Fetal Neonatal Med. 2011, 24, 280–283. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Kolaric, B.; Belamaric, M.; Sviben, M.; Ferenc, T.; Navolan, D.; Bekic, V.; Milasincic, L.; Antolasic, L.; Vilibic, M.; et al. Screening for TORCH Antibodies in Croatian Childbearing-Aged Women, 2014–2023. Antibodies 2024, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Croatian Bureau of Statistics. The NUTS Classification in Croatia. Available online: https://dzs.gov.hr/highlighted-themes/prostorne-klasifikacije-i-subnacionalne-statistike-2-694/the-nuts-classification-in-croatia/699 (accessed on 15 July 2025).
- Puhakka, L.; Sarvikivi, E.; Lappalainen, M.; Surcel, H.M.; Saxen, H. Decrease in Seroprevalence for Herpesviruses among Pregnant Women in Finland: Cross-Sectional Study of Three Time Points 1992, 2002 and 2012. Infect. Dis. 2016, 48, 406–410. [Google Scholar] [CrossRef]
- Gorun, F.; Motoi, S.; Malita, D.; Navolan, D.B.; Nemescu, D.; Olariu, T.R.; Craina, M.; Vilibic-Cavlek, T.; Ciohat, I.; Boda, D.; et al. Cytomegalovirus Seroprevalence in Pregnant Women in the Western Region of Romania: A Large-Scale Study. Exp. Ther. Med. 2020, 20, 2439–2443. [Google Scholar] [CrossRef]
- Leuridan, E.; Ieven, M.; Hens, N.; Van Damme, P. High Susceptibility to Cytomegalovirus Infection of Pregnant Women in Flanders, Belgium. Facts Views Vis. Obgyn 2012, 4, 76–81. [Google Scholar] [PubMed]
- Hoehl, S.; Berger, A.; Ciesek, S.; Rabenau, H.F. Thirty Years of CMV Seroprevalence—A Longitudinal Analysis in a German University Hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1095–1102. [Google Scholar] [CrossRef]
- Pavia, G.; Licata, F.; Marascio, N.; Giancotti, A.; Tassone, M.T.; Costa, C.; Scarlata, G.G.M.; Prestagiacomo, L.E.; Gigliotti, S.; Trecarichi, E.M.; et al. Seroprevalence and Age-Related Susceptibility of TORCH Infections in Childbearing Age Women: A 5-Year Cross-Sectional Retrospective Study and a Literature Review. J. Infect. Public Health 2024, 17, 102537. [Google Scholar] [CrossRef]
- Radoi, C.L.; Zlatian, O.; Balasoiu, M.; Dragomir, T.L.; Sorop, M.I.; Bagiu, I.C.; Boeriu, E.; Susan, M.; Sorop, B.; Oprisoni, L.A.; et al. Seroprevalence of Anti-Cytomegalovirus Antibodies in Pregnant Women from South-West Romania. Microorganisms 2024, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Coste-Mazeau, P.; Hamon, M.; Ribot, É.; Hantz, S.; Alain, S. Mise en Place du Dépistage de l’Infection Congénitale à Cytomégalovirus dans une Maternité Française de Type 3 [Implementation of Screening for Cytomegalovirus Congenital Infection in a French Type 3 Maternity]. Gynecol. Obstet. Fertil. Senol. 2024, 52, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Viviani, S.; Montomoli, E.; Marchi, S. Seroprevalence of Antibodies to Cytomegalovirus in Pregnant Women in the Apulia Region (Italy). J. Prev. Med. Hyg. 2021, 62, E372–E376. [Google Scholar] [CrossRef]
- Palazzotto, E.; Bonura, F.; Calà, C.; Capra, G.; Pistoia, D.; Mangione, D.; Mascarella, C.; Minì, G.; Enea, M.; Giammanco, G.M.; et al. Serological Status for TORCH in Women of Childbearing Age: A Decade-Long Surveillance (2012–2022) in Italy. J. Med. Microbiol. 2023, 72, 001733. [Google Scholar] [CrossRef]
- Stoykova, Z.D.; Ivanova, L.I.; Todorova, T.T. The Role of Cytomegalovirus in Congenital and Early Postnatal Infections in Northeastern Bulgaria. Folia Medica 2017, 59, 298–302. [Google Scholar] [CrossRef]
- Arapović, J.; Rajič, B.; Pati, S.; Brizić, I.; Azinović, I.; Šušak, B.; Ostojić, M.; Tutiš, B.; Raguž, A.B.; Tomić, V.; et al. Cytomegalovirus Seroprevalence and Birth Prevalence of Congenital CMV Infection in Bosnia and Herzegovina: A Single-Center Experience. Pediatr. Infect. Dis. J. 2020, 39, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Pribakovic, J.A.; Katanic, N.; Radevic, T.; Tasic, M.S.; Kostic, M.; Stolic, B.; Radulovic, A.; Minic, V.; Bojovic, K.; Katanic, R. Serological Status of Childbearing-Aged Women for Toxoplasma gondii and Cytomegalovirus in Northern Kosovo and Metohija. Rev. Soc. Bras. Med. Trop. 2019, 52, e20170313. [Google Scholar] [CrossRef]
- Terzi, N.; Papanagiotou, A.; Prifti, E.; Angeletou, A.; Paftounou, A.; Koumbi, I.; Kazani, M.; Chaidopoulos, D.; Adamis, G.; Tzanetou, K. Seroprevalence and Susceptibility to Primary Cytomegalovirus Infection among Childbearing Women. Int. J. Infect. Dis. 2014, 21, 458. [Google Scholar] [CrossRef][Green Version]
- de la Calle, M.; Rodríguez-Molino, P.; Romero Gómez, M.P.; Baquero-Artigao, F. Cytomegalovirus Seroprevalence in Pregnant Women in Madrid: First Step for a Systematic Screening. Enferm. Infecc. Microbiol. Clin. 2023, 41, 55–56. [Google Scholar] [CrossRef]
- Barlinn, R.; Dudman, S.G.; Trogstad, L.; Gibory, M.; Muller, F.; Magnus, P.; Rollag, H. Maternal and Congenital Cytomegalovirus Infections in a Population-Based Pregnancy Cohort Study. APMIS 2018, 126, 899–906. [Google Scholar] [CrossRef]
- Antona, D.; Lepoutre, A.; Fonteneau, L.; Baudon, C.; Halftermeyer-Zhou, F.; Le Strat, Y.; Lévy-Bruhl, D. Seroprevalence of Cytomegalovirus Infection in France in 2010. Epidemiol. Infect. 2017, 145, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Siennicka, J.; Dunal-Szcepaniak, M.; Trzcińska, A.; Godzik, P.; Rosińska, M. High Seroprevalence of CMV among Women of Childbearing Age Implicates High Burden of Congenital Cytomegalovirus Infection in Poland. Pol. J. Microbiol. 2017, 65, 425–432. [Google Scholar] [CrossRef]
- Kahraman Kilbas, E.P.; Ciftci, I.H.; Kilbas, I.; Toptan, H. Seroprevalence of TORCH Viral Agents in Pregnant Women in Turkey: Systematic Review and Meta-Analysis. Pathogens 2025, 14, 37. [Google Scholar] [CrossRef]
- Bahçeci, İ.; Karaca, E.; Duran, Ö.F.; Aksoy, D.; İbik, Y.E.; Kırcı, U.B. Seroprevalence of Toxoplasma, Rubella and Cytomegalovirus in Women of Fertility Age in Our Region. Turkiye Parazitol. Derg. 2023, 47, 11–15. [Google Scholar] [CrossRef]
- Pembrey, L.; Raynor, P.; Griffiths, P.; Chaytor, S.; Wright, J.; Hall, A.J. Seroprevalence of Cytomegalovirus, Epstein Barr Virus and Varicella Zoster Virus among Pregnant Women in Bradford: A Cohort Study. PLoS ONE 2013, 8, e81881. [Google Scholar] [CrossRef]
- Knowles, S.J.; Grundy, K.; Cahill, I.; Cafferkey, M.T.; Geary, M. Low Cytomegalovirus Seroprevalence in Irish Pregnant Women. Ir. Med. J. 2005, 98, 210–212. [Google Scholar] [PubMed]
- Gaj, Z.; Rycel, M.; Wilczyński, J.; Nowakowska, D. Seroprewalencja Zakazeń Cytomegalowirusem w Populacji Polskich Kobiet Ciężarnych [Seroprevalence of Cytomegalovirus Infection in the Population of Polish Pregnant Women]. Ginekol. Pol. 2012, 83, 337–341. [Google Scholar]
- Vilibic-Cavlek, T.; Kolaric, B.; Beader, N.; Vrtar, I.; Tabain, I.; Mlinaric-Galinovic, G. Seroepidemiology of Cytomegalovirus Infections in Croatia. Wien. Klin. Wochenschr. 2017, 129, 129–135. [Google Scholar] [CrossRef]
- Aljumaili, Z.K.; Alsamarai, A.M.; Najem, W.S. Cytomegalovirus Seroprevalence in Women with Bad Obstetric History in Kirkuk, Iraq. J. Infect. Public Health 2014, 7, 277–288. [Google Scholar] [CrossRef]
- Fatima, T.; Siddiqui, H.; Ghildiyal, S.; Baluni, M.; Singh, D.V.; Zia, A.; Dhole, T.N. Cytomegalovirus Infection in Pregnant Women and Its Association with Bad Obstetric Outcomes in Northern India. Microb. Pathog. 2017, 113, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.G.; Stoian, D.L.; Daescu, A.-M.C.; Motofelea, A.C.; Ciohat, I.M.; Navolan, D.B.; Vilibic-Cavlek, T.; Bogdanic, M.; Nemescu, D.; Tomescu, L.; et al. The Impact of Latent Cytomegalovirus Infection on Spontaneous Abortion History and Pregnancy Outcomes in Romanian Pregnant Women. Microorganisms 2024, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Pitfalls in the Serological Evaluation of Maternal Cytomegalovirus Infection as a Potential Cause of Fetal and Neonatal Involvements: A Narrative Literature Review. J. Clin. Med. 2022, 11, 5006. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | N (%) Tested | |
|---|---|---|
| Age group | 16–20 years | 228 (8.0) |
| 21–25 years | 325 (11.5) | |
| 26–30 years | 666 23.5) | |
| 31–35 years | 735 (25.9) | |
| 36–40 years | 556 (19.6) | |
| 41–45 years | 328 (11.5) | |
| Geographic region | City of Zagreb/Northern Croatia | 1761 (62.1) |
| Pannonian Croatia | 303 (10.7) | |
| Adriatic Croatia | 759 (26.7) | |
| Missing | 15 (0.5) | |
| Settlement | Urban | 2335 (82.3) |
| Suburban/rural | 488 (17.2) | |
| Missing | 15 (0.5) | |
| Obstetric history | Non-pregnant | 2261 (79.8) |
| Pregnant | 260 (9.2) | |
| Unfavorable obstetric history | 95 (3.3) | |
| Missing | 219 (7.7) | |
| Age Group | Tested | CMV IgG | p | CMV IgM | p | ||
|---|---|---|---|---|---|---|---|
| N | N (%) | 95% CI | N (%) | 95% CI | |||
| 16–20 years | 228 | 113 (49.6) | 43.1–56.0 | <0.001 | 31 (13.6) | 9.7–11.7 | 0.090 |
| 21–25 years | 325 | 216 (66.5) | 61.2–71.4 | 38 (11.7) | 8.6–16.5 | ||
| 26–30 years | 666 | 457 (68.6) | 65.0–72.0 | 67 (10.1) | 8.0–12.6 | ||
| 31–35 years | 735 | 537 (73.1) | 69.7–76.1 | 72 (9.8) | 7.9–12.2 | ||
| 36–40 years | 556 | 432 (77.5) | 73.9–80.8 | 48 (8.6) | 6.6–11.3 | ||
| 41–45 years | 328 | 251 (76.5) | 71.6–80.8 | 22 (6.7) | 4.5–9.9 | ||
| Total | 2838 | 2006 (70.6) | 68.9–72.3 | 278 (9.8) | 8.7–10.9 | ||
| Year | CMV IgG Odds Estimate | p | CMV IgM Odds Estimate | p |
|---|---|---|---|---|
| 2015 | Ref. | Ref. | ||
| 2016 | −0.266 | 0.263 | −14.066 | 0.961 |
| 2017 | −0.618 | 0.011 | 0.130 | 0.831 |
| 2018 | −0.499 | 0.033 | −0.557 | 0.435 |
| 2019 | −0.285 | 0.203 | 0.743 | 0.135 |
| 2020 | −0.719 | 0.001 | 1.476 | 0.001 |
| 2021 | −0.434 | 0.051 | 1.685 | <0.001 |
| 2022 | −0.250 | 0.242 | 1.896 | <0.001 |
| 2023 | −0.519 | 0.009 | 1.543 | <0.001 |
| 2024 | −0.256 | 0.186 | 1.800 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilibić-Čavlek, T.; Barbić, K.; Ježek, T.; Navolan, D.; Sanković, A.; Sviben, M.; Glavaš, S.; Mureșan, D.; Pirtea, L.; Bogdanić, M. Prevalence of Cytomegalovirus Antibodies in Croatian Childbearing-Aged and Pregnant Women: A Ten-Year Retrospective Study (2015–2024). Pathogens 2025, 14, 916. https://doi.org/10.3390/pathogens14090916
Vilibić-Čavlek T, Barbić K, Ježek T, Navolan D, Sanković A, Sviben M, Glavaš S, Mureșan D, Pirtea L, Bogdanić M. Prevalence of Cytomegalovirus Antibodies in Croatian Childbearing-Aged and Pregnant Women: A Ten-Year Retrospective Study (2015–2024). Pathogens. 2025; 14(9):916. https://doi.org/10.3390/pathogens14090916
Chicago/Turabian StyleVilibić-Čavlek, Tatjana, Klara Barbić, Tadej Ježek, Dan Navolan, Ana Sanković, Mario Sviben, Sara Glavaš, Daniel Mureșan, Laurentiu Pirtea, and Maja Bogdanić. 2025. "Prevalence of Cytomegalovirus Antibodies in Croatian Childbearing-Aged and Pregnant Women: A Ten-Year Retrospective Study (2015–2024)" Pathogens 14, no. 9: 916. https://doi.org/10.3390/pathogens14090916
APA StyleVilibić-Čavlek, T., Barbić, K., Ježek, T., Navolan, D., Sanković, A., Sviben, M., Glavaš, S., Mureșan, D., Pirtea, L., & Bogdanić, M. (2025). Prevalence of Cytomegalovirus Antibodies in Croatian Childbearing-Aged and Pregnant Women: A Ten-Year Retrospective Study (2015–2024). Pathogens, 14(9), 916. https://doi.org/10.3390/pathogens14090916







